Clinical effectiveness of diquafosol ophthalmic solution 3% in Korean patients with dry eye disease: a multicenter prospective observational study
Youngsub Eom, Hyo Myung Kim
1Department of Ophthalmology, Korea University College of Medicine, Seoul 02841, Republic of Korea
2Department of Ophthalmology, Korea University Ansan Hospital, Gyeonggi-do 15355, Republic of Korea
3Department of Ophthalmology, Korea University Anam Hospital, Seoul 02841, Republic of Korea
Abstract
● KEYWORDS: dry eye disease; diquafosol ophthalmic solution; routine clinical practice
INTRODUCTION
Dry eye disease is a multifactorial condition of tear film instability which produces a range of discomforting ocular symptoms and/or visual impairment with potentially damaging effects on the ocular surface[1-4]. Estimates of the worldwide prevalence of dry eye disease range from about 5%to 50%, with discrepancies likely reflecting differences in nonstandardized diagnostic criteria[5-6]. In Korea, the prevalence of diagnosed dry eye disease in the general population is 8.0%[7],although the prevalence is considerably higher in the elderly population (30.3%)[8].
Dry eye workshop II (DEWS II) proposes diagnostic tests to examine dry eye disease, including subjective symptoms,tear breakup time (TBUT), tear osmolarity, and ocular surface staining. It also proposes to evaluate abnormal lipids,meibomian gland dysfunction (MGD), and tear volume as subtype classification tests[4]. Those tests are valid diagnostic approaches to diagnose dry eye disease and divide its subtypes,respectively. Good therapeutic agents for dry eye disease must be able to improve the parameters of both diagnostic and subtype classification tests.

Figure 1 Korean Corneal Disease Study Group guidelines[1] If there is a discrepancy between the level of symptoms and signs, the severity level is determined according to the level of signs. If there is a discrepancy among the level of signs, the severity level is determined following the Oxford grading scheme.
Therapeutic options for treating dry eye disease include artificial tears, anti-inflammatory therapy, secretagogues,and tear retention treatment[9]. Artificial tears include viscosity agents such as hyaluronate, polyvinyl alcohol, and carboxymethyl cellulose[10], anti-inflammatory agents such as topical cyclosporine A and corticosteroids[11], and the secretagogue diquafosol are becoming increasingly popular for dry eye management[9,12-13]. Diquafosol is a purinergic P2Y2 receptor agonist currently approved in Japan, Republic of Korea, Indonesia, Malaysia, Philippines, Thailand, Vietnam,Cambodia, and China for treatment of dry eye. Available as a 3% ophthalmic solution, diquafosol stimulates tear fluid and mucin secretion[12-13].
The clinical efficacy of diquafosol in dry eye disease has been demonstrated in multiple randomized controlled trials[14-20], case series[21-23], case-control studies[24-25], and a non-interventional observational study[26]with a good safety profile[16,20,26]. A large Japanese survey of 3196 patients with dry eye demonstrated the benefit of diquafosol in routine clinical practice setting[26].Currently, there is insufficient clinical data on routine use of diquafosol in Korea. Thus, this prospective observational study tried to investigate the clinical effects of diquafosol in dry eye patients treated with other medicines in a “real-world”clinical setting. Patients included those who visited the study institute for the first time and were treated with existing drugs in combination with diquafosol, solely diquafosol, or existing drugs replaced by diquafosol.
SUBJECTS AND METHODS
Ethical Approval This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.All subjects provided voluntary written informed consent for study participation, which was reviewed by the Institutional Review Board of Korea University Anam Hospital (IRB No.2015AN0156) and each institution.
Study Design This prospective observational study of Korean patients with dry eye disease in a real-world clinical setting was conducted between 20 October 2015 and 1 November 2016 at 20 institutions and examined the effectiveness and safety of diquafosol ophthalmic solution 3% (Diquas?; Santen Pharmaceutical Co., Ltd., Osaka, Japan) administered for 8wk.Three groups of patients were analyzed: patients who added diquafosol ophthalmic solution 3% to existing eye drops (Add group); patients who discontinued all existing medications and were prescribed diquafosol ophthalmic solution 3% only,or new patients prescribed diquafosol alone (Monotherapy group); and patients who discontinued part of their existing medication and received diquafosol ophthalmic solution 3%instead (Switch group).
Inclusion and Exclusion Criteria Inclusion criteria were adults aged 19 years or older diagnosed with dry eye Level I,II, or III, as defined by Korean Corneal Disease Study Group(KCDSG) guidelines (Figure 1)[1], who had not used diquafosol ophthalmic solution 3% for 1mo prior to study participation.Dry eye level IV was defined with ocular surface staining grade of 4 or more (marked or severe) in the Oxford grading scheme[27], tear film break up immediately after eye opening,and/or daily life limited symptoms according to KCDSG guidelines[1]. Main exclusion criteria were patients diagnosed with dry eye level IV as defined in KCDSG guidelines[1];patients with Sj?gren’s syndrome, severe blepharitis (meibum quality or meibum expressibility grade 3)[28-29]or severe eye inflammation/infection; patients who underwent eye surgery within 3mo prior to study participation; patients receiving eye drops for a purpose other than dry eye treatment (e.g.glaucoma or allergy); patients with known hypersensitivity to diquafosol; pregnant or breast-feeding women; and subjects who were determined to be ineligible by the study investigator.
Drug Administration One drop per eye of diquafosol ophthalmic solution 3% (30 mg/mL) was administered 6 times daily for 8wk. When co-administered with other eye drops, at least 5min elapsed between application of diquafosol ophthalmic solution 3% and any other ophthalmic drug.
Patient Evaluations Demographic data and medical, surgical,and medication histories were recorded and a physical examination was conducted at baseline. Measurement of visual acuity and intraocular pressure were conducted at baseline and week 8. All subjects completed a subjective symptom questionnaire to evaluate subjective symptoms and underwent ophthalmic examination to evaluate objective signs at baseline and weeks 4 and 8. Assessment of meibum quality and expressibility were performed only at baseline and week 8.Subjective symptoms of dry eye were evaluated using the 12-item Ocular Surface Disease Index (OSDI) questionnaire with severity of symptoms for each item scored from 0 (none) to 4(always). Patient Reported Outcomes (PRO) were assessed at week 8 after study participation with subjective improvement during the 8-week treatment period assessed by two questions:1) How do you assess your current dry eye symptoms compared to 8wk ago? Responses were graded from 1 to 5 (1,much better; 2, better; 3, no change; 4, worse; and 5, much worse); 2) What is the most discomforting part about dry eye?Response options (1, irritation; 2, foreign body sensation; 3,sore eye or pain; 4, blurred vision; and 5, other).
TBUT was conducted using standard methods following application of a fluorescein-based dye to the eye under cobalt blue illumination and a yellow barrier filter[30]. A stopwatch was used to measure TBUT three times up to a tenth of a second, and the average value was obtained. Cornea and conjunctival staining was based on National Eye Institute/Industry (NEI) scoring scheme[31]. For evaluation, the cornea and conjunctiva were divided into 5 and 6 zones, respectively.After grading each zone, the sum of corneal scores and the sum of conjunctival scores were recorded. MGD was evaluated by assessing meibum quality and expressibility. Both upper and lower eyelids were gently pressed with a cotton swab to assess meibum quality and expressibility. Meibum quality of the central glands was graded from 0 to 3 (clear fluid, cloudy fluid, cloudy particulate fluid and inspissated like toothpaste),and meibum expressibility was graded from 0 to 3 according to the number of expressible glands among the eight central glands (all glands expressible, 3-4 gland expressible, 1-2 gland expressible, and no glands expressible)[28-29].
Clinical Effectiveness Endpoints The primary effectiveness endpoint was comparison of TBUT variation between baseline and week 8. Secondary effectiveness endpoints were comparisons of: cornea and conjunctival staining score variation between baseline and weeks 4 and 8, OSDI variation between baseline and weeks 4 and 8, meibum quality and expressibility variation between baseline and week 8, and evaluation of PRO results at week 8.
Safety Endpoints The incidence of adverse events (AEs) by treatment group was compared, and the association between AEs and diquafosol ophthalmic solution 3% was evaluated.
Statistical Analysis Descriptive statistics for all subject data were obtained using Statistical Package for the Social Sciences(SPSS) version 20.0 (IBM Corp., Armonk, NY, USA). Oneway analysis of variance (ANOVA) was used to compare parameters at baseline among the three groups. Repeated measures ANOVA with the Bonferroni correction was used to compare parameters between baseline, week 4, and week 8.Normality of each parameter variations from baseline to week 8 in each group was assessed using Kolmogorov-Smirnov and Shapiro-Wilk’s tests. For data that were not normally distributed, the non-parametric Kruskal-Wallis test was used for comparison of all three groups. The results of tests and observations were recorded as mean, standard deviation(SD), or percentage, as applicable. Results were considered statistically significant if theP<0.05.

Figure 2 Flow diagram of patient selection process FAS: Full analysis set; PPS: Per-protocol set; SS: Safety set.
RESULTS
Patient Characteristics A total of 450 subjects were enrolled and formed the safety analysis set in this study. Of these,86 subjects were excluded due to dropout and 7 subjects were excluded due to protocol violation identified after the end of the study. Subjects dropped out for the following reasons: lost to follow-up (n=23), adverse events (n=21),consent withdrawal (n=17), lack of source document (n=15),unmet inclusion/exclusion criteria (n=4), use of prohibited concomitant eye drop (n=3), not applicable observation cohort(n=2), and eye drop prescription change (n=1). Reasons for the 7 protocol violations were as follows: unmet inclusion/exclusion criteria (n=3), non-applicable observation cohort(n=2), and eye drop prescription change (n=2; Figure 2).
A total of 357 subjects were included in the clinical effectiveness analysis. The mean±SD age was 49.5±16.5y(range, 20 to 85y), and 284 subjects (79.6%) were female.Table 1 shows baseline clinical characteristics of the study participants and eyes analyzed in each group. There were no significant differences in mean corneal and conjunctival staining score, OSDI score, and meibum quality at baseline among the three groups. However, statistically significant differences among the three groups were identified for TBUT and meibum expressibility. The switch group showed less severe values for TBUT and meibum expressibility than the other two groups (Table 1).
Eye drops or ointments used with diquafosol ophthalmic solution 3% to treat dry eye disease in the Add and Switch groups include hyaluronic acid (0.1, 0.15, 0.18, and 0.3%;n=159), steroids (fluorometholone, loteprednol, prednisolone,and rimexolone;n=51), 0.05% cyclosporine (n=41),carbomer-based lipid-containing artificial tear (n=31),carboxymethylcellulose (n=31), antibiotics (n=21), lid hygiene(n=12), solcoseryl eye gel (n=11), lanolin eye ointment(n=9), antibiotic-dexamethasone combination (n=5), and nonsteroidal antiinflammatory drugs (NSAIDs) (n=2).
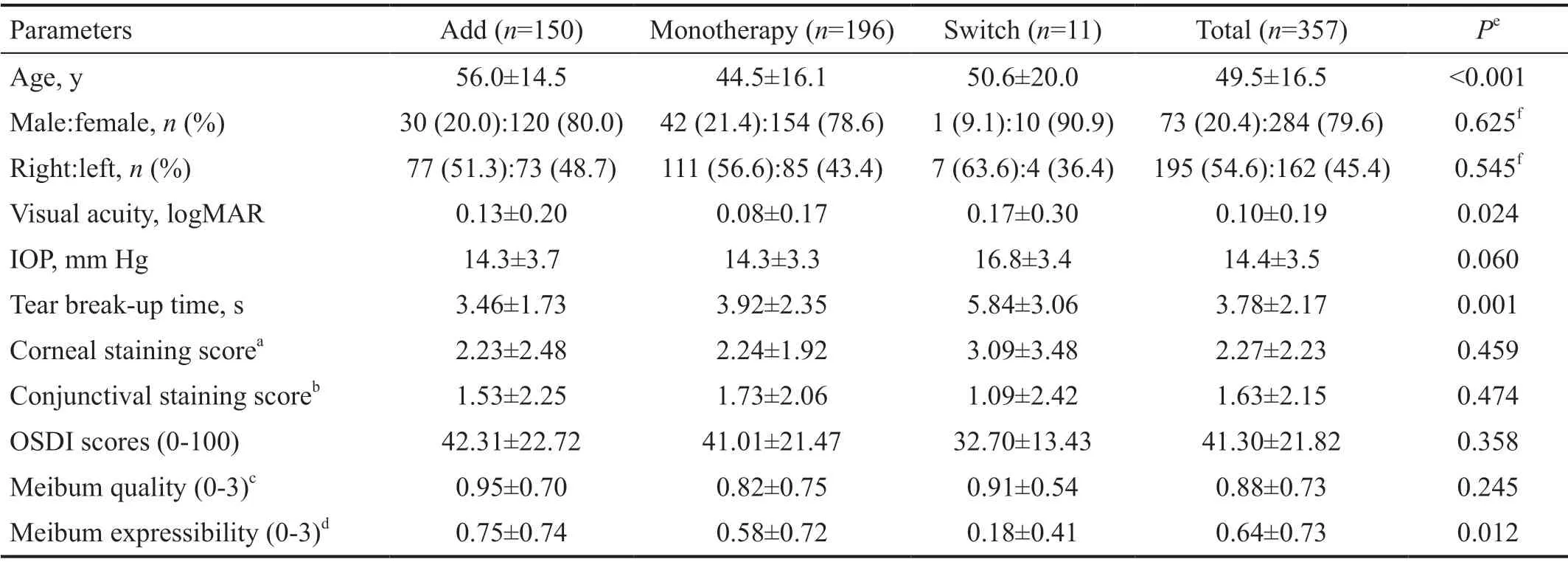
Table 1 Baseline characteristics of study population and eyes n=357
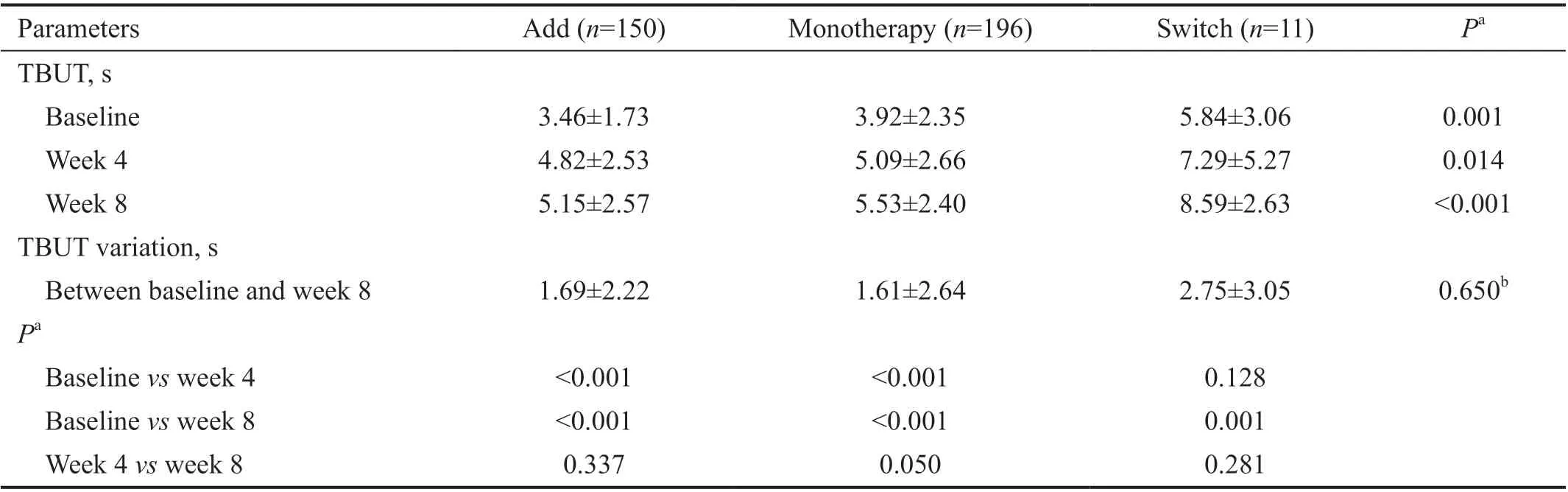
Table 2 Comparison of TBUT before and after treatment with diquafosol ophthalmic solution 3% in Add, Monotherapy, and Switch groups in Korean patients with dry eye disease n=357, mean±SD
Effectiveness Evaluation The mean TBUT increased by 1.68s from baseline (3.78s) to week 8 (5.46s). The Add and Monotherapy groups showed a significant increase in TBUT(from 3.46±1.73s to 4.82±2.53s in the Add group and from 3.92±2.35s to 5.09±2.66s in the Monotherapy group) after 4wk of treatment and showed an additional increasing TBUT tendency after 8wk of treatment (5.15±2.57s and 5.53±2.40s,respectively; Table 2). The Switch group showed a significant increase in mean TBUT of 2.75±3.05s from baseline to week 8 with no significant difference in mean TBUT variation compared to the Add and Monotherapy groups (1.69±2.22s and 1.61±2.64s, respectively; Kruskal-Wallis test,P=0.650).Mean corneal staining scores showed a significant decrease over time in the Add and Monotherapy groups (from 2.23±2.48 at baseline, to 1.35±1.86 at week 4, and 0.85±1.24 at week 8 in Add group and from 2.24±1.92 at baseline, to 1.37±1.34 at week 4, and 0.97±1.16 at week 8 in Monotherapy group), with a decrease in mean corneal staining score from baseline to week 8 found in the Switch group (from 3.09±3.47 at baseline to 1.64±2.69 at week 8; Table 3). No significant differences in corneal staining variation between baseline and week 8 were found among three groups (P=0.862). Mean conjunctival staining scores decreased over time in the Add andMonotherapy groups (from 1.53±2.25 at baseline, to 0.81±1.45 at week 4, and 0.54±0.99 at week 8 in the Add group and from 1.73±2.06 at baseline, to 0.97±1.48 at week 4, and 0.72±1.26 at week 8 in the Monotherapy group), but not in the Switch group (Table 3).
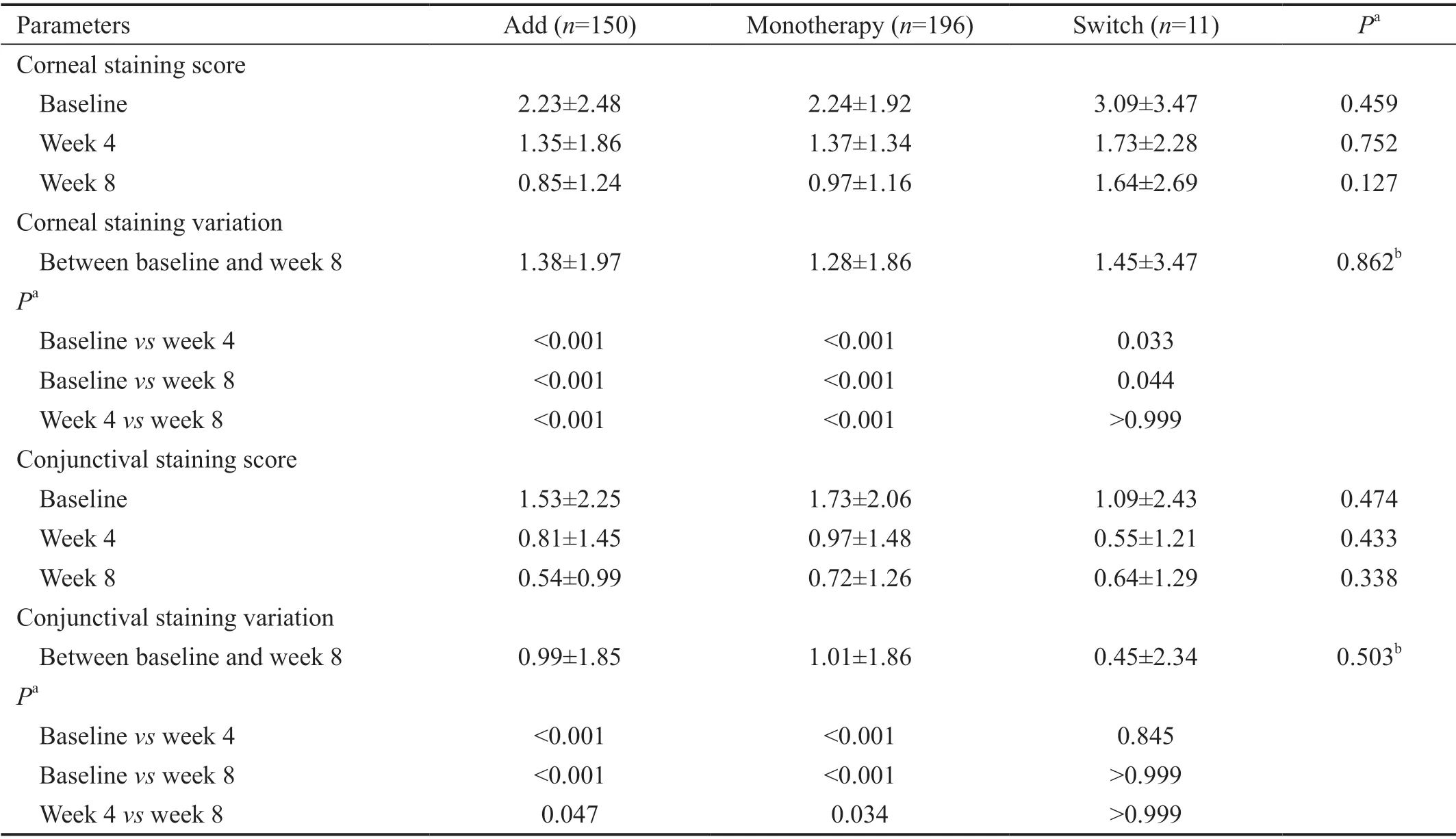
Table 3 Comparison of corneal and conjunctival staining scores before and after treatment with diquafosol ophthalmic solution 3% in Add, Monotherapy, and Switch groups in Korean patients with dry eye disease n=357, mean±SD
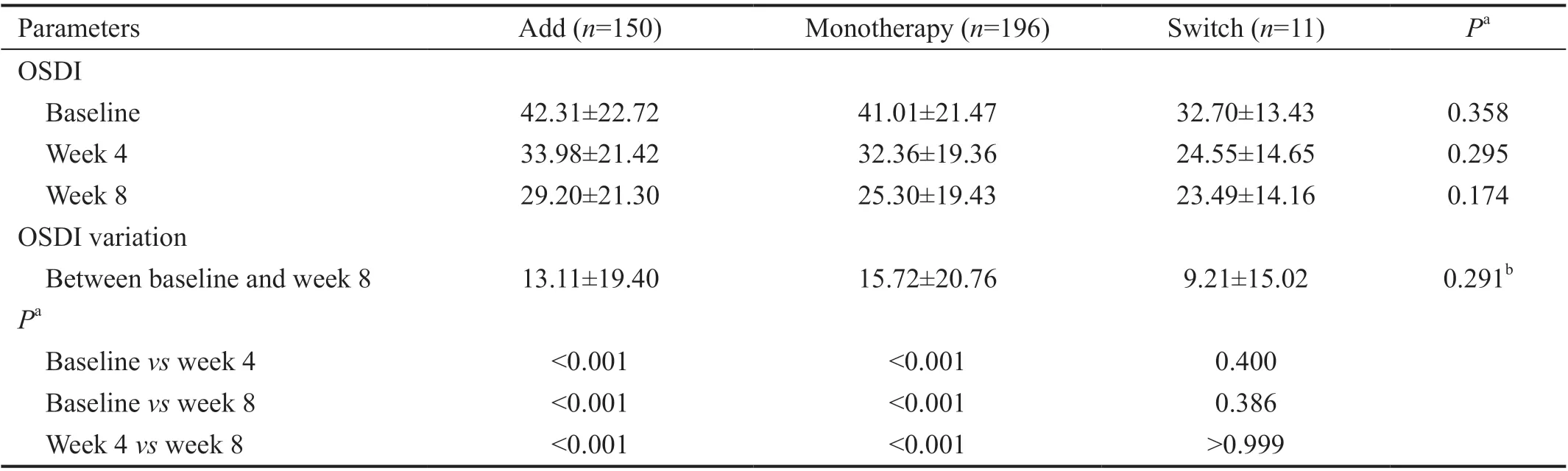
Table 4 Comparison of OSDI before and after treatment with diquafosol ophthalmic solution 3% in Add, Monotherapy, and Switch groups in Korean patients with dry eye disease n=357, mean±SD
Analysis of the OSDI questionnaire of subjective symptoms of dry eye found a consistent decrease in mean OSDI scores over time in the Add and Monotherapy groups, but not in the Switch group (Table 4). No significant between-group differences in OSDI variation were observed between baseline and week 8(P=0.291).
Mean meibum quality and expressibility at baseline and week 8 is shown in Table 5. In the Add group, mean meibum quality decreased by 0.28±0.68 from baseline to week 8, with similar mean reductions in the Monotherapy group (0.25±0.68). Mean variation of meibum quality in the Switch group showed a small increase of 0.09 (Table 5). There was no significant difference in mean meibum quality variation among groups(P=0.132). Mean meibum expressibility decreased from 0.75±0.74 at baseline to 0.56±0.68 at week 8 in the Add group and from 0.58±0.72 at baseline to 0.44±0.61 at week 8 in the Monotherapy group but increased (0.27±0.65) in the Switch group (Table 5). There was no significant difference in mean meibum expressibility variation among groups (P=0.087).Of 350 subjects who responded to the PRO questionnaire, 259(74.0%) responded better or much better improvement of dry eye symptoms. Only 4 subjects (1.1%) responded worsening of dry eye symptoms compared to 8wk before (Figure 3A).In all three groups, the most discomforting part about dry eye was foreign body sensation (36.0%), followed by sore eye or pain (26.6%; Figure 3B). There were 3 patients who reported a sticky sensation due to an increase in mucin. There were no significant differences in answers to PRO Question 1 (P=0.154) or Question 2 (P=0.753) among the three groups(Figure 3A).
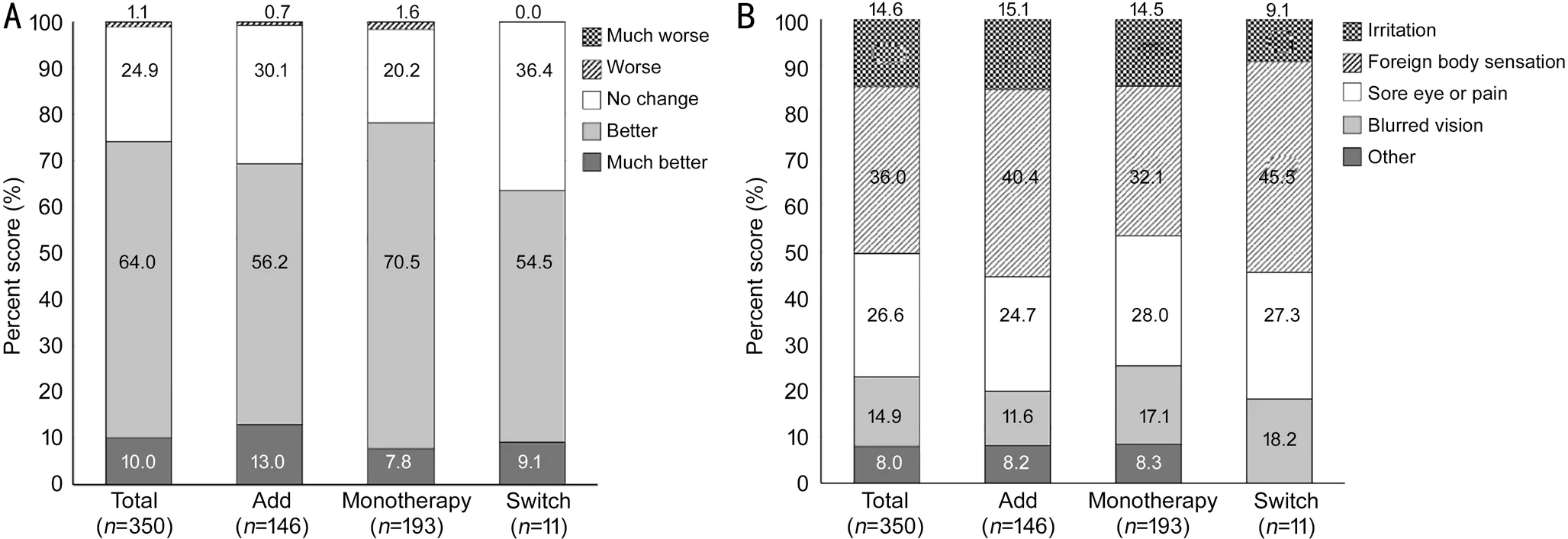
Figure 3 Assessments by patient reported outcomes questionnaire A: Question 1-How do you assess your current dry eye symptoms compared to 8wk ago? B: Question 2-What is the most discomforting part about dry eye?
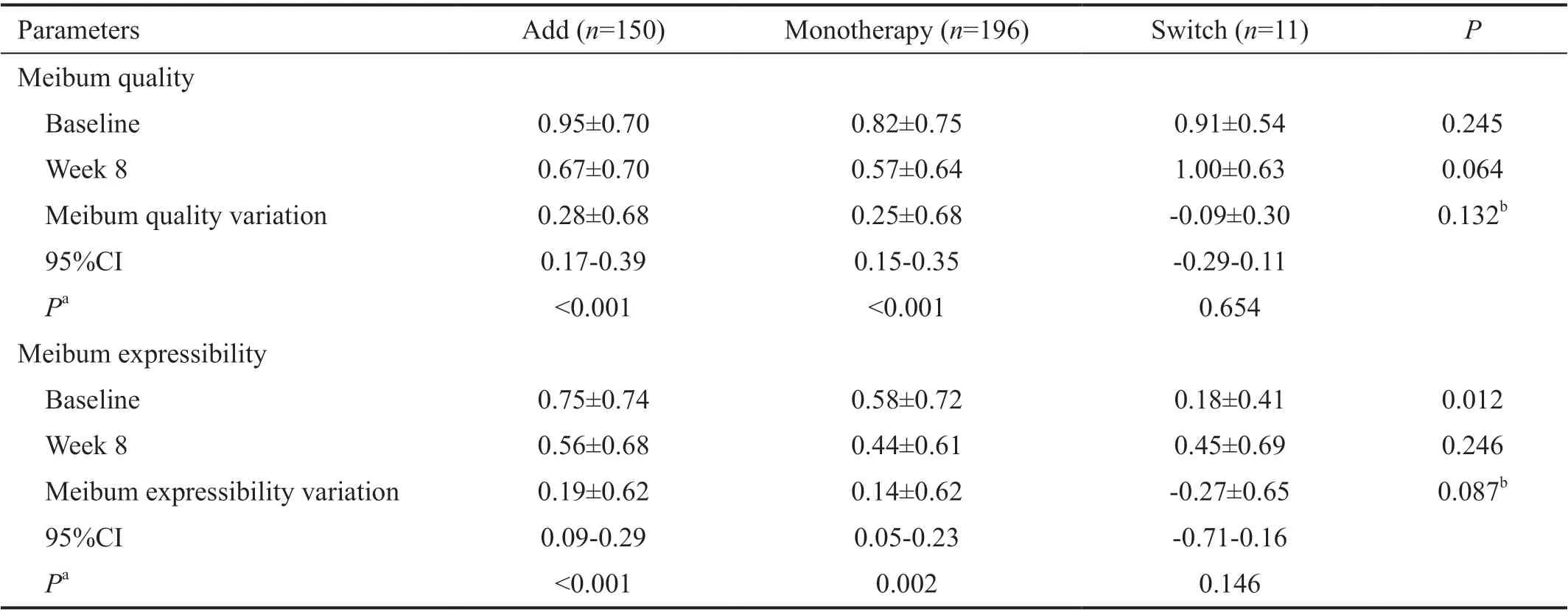
Table 5 Comparison of meibum quality and expressibility before and after treatment with diquafosol ophthalmic solution 3% in Add,Monotherapy, and Switch groups in Korean patients with dry eye disease n=357, mean±SD
Safety Evaluation Of 450 subjects enrolled in this study, 132 subjects (29.3%;n=54 in Add group,n=73 in Monotherapy group, andn=5 in Switch group, respectively) experienced 193 AEs, and the vast majority (97%) were mild or moderate.Six subjects (1.33%) reported 12 severe AEs that required hospitalization or prolonged hospitalization. However, all AEs were considered unlikely to be related to the study drug.
DISCUSSION
This prospective observational study of Korean patients with dry eye disease treated with diquafosol ophthalmic solution 3% for 8wk evaluated the effectiveness of diquafosol on subjective dry eye symptoms and objective signs in three groups. All subjective symptoms and objective signs applied to evaluate curative effects on dry eye disease improved in the Add and Monotherapy groups at 4 and 8wk after treatment. In the Switch group, TBUT and corneal staining scores improved at 8wk following treatment, but this group had a small sample size.
In the Add group, all test parameters of dry eye disease were ameliorated at 4 and 8wk after treatment with existing drugs in combination with diquafosol, which may be attributed to the improvement effect of diquafosol on the three main components of tear films. Hyaluronic acid improves ocular surface staining and the aqueous layer, and increases conjunctival goblet cells[32]. In dry eye disease including Sj?gren’s syndrome, steroids and cyclosporine improve symptoms and signs by suppressing inflammation[33-35]. In particular, cyclosporine is effective in increasing the aqueous layer[36]. Carbomer-based eye drops can ameliorate subjective symptoms and TBUT in evaporative dry eye disease with short TBUT by supplementing lipids to the tear layer[37]. Solcoseryl eye drops can improve corneal erosions and show curative effects by augmenting humidity of the ocular surface in dry eye disease[38]. Conversely, diquafosol improves all three main tear components of mucin, aqueous, and lipid layers[39-40], as well as obstructive MGD[23]. Thus, it seems that dry eye patients could show additional improvements in symptoms and signs when diquafosol eye drops were added to existing dry eye treatment in this study. Consistent with our results, a previous study revealed additional curative effects of diquafosol eye drop in dry eye patients who were already treated with hyaluronic acid eye drops[17]. Another study showed that diquafosol more rapidly improved subjective symptoms and corneal staining scores compared to 0.05% cyclosporine in dry eye patients treated with artificial tears in combination with diquafosol or 0.05% cyclosporin[41].
DEWS II proposes evaluation of subjective symptoms, TBUT,tear osmolarity, ocular surface staining, abnormal lipid, MGD,and tear volume for dry eye disease diagnosis and subtype classification[4]. Previous study showed that TBUT and corneal staining scores, especially foreign body sensation (ocular discomfort experienced the most in dry eye patients in this study), were significantly ameliorated in dry eye patients treated solely with diquafosol 6 times a day[42]. A previous study reported that values of the Schirmer test significantly increased and tear osmolarity tended to decrease compared to before diquafosol treatment; however, these changes were not statistically significant[43]. No studies present evidence to prove significant improvement in tear osmolarity with diquafosol,possibly because altered tear osmolarity does not fully reflect changes in other dry eye disease parameters[44]. In addition,previous studies have shown that application of diquafosol eye drops significantly improved quality of vision and lipid secretion in patients with obstructive MGD[16,23]. In line with previous studies, subjective symptoms, TBUT, ocular surface staining, and MGD were more improved in the Add (treated with existing drugs and diquafosol added) and Monotherapy(treated solely with diquafosol without other drugs) groups following diquafosol treatment compared to those baseline values. Thus, diquafosol can be preferentially selected as a therapeutic agent for dry eye disease since it improves most parameters of diagnostic and subtype classification tests.
A systematic review of 8 randomized controlled trials involving 1516 patients with dry eye concluded that diquafosol was a safe therapeutic option for treating dry eye disease.No severe AEs were reported with diquafosol concentrations ranging from 0.5% to 5%[45]. Similarly, although 6 subjects reported severe AEs in this study, all were considered unlikely to be related to diquafosol. The results of this study, obtained in real-world clinical practice, are in good general agreement with those obtained in previous clinical studies of diquafosol ophthalmic solution.
The present study has some limitations. Subjects were not randomly allocated to each study group. This observational study was conducted in dry eye patients treated with existing medicines or in patients who first visited our institutes in real-world settings. In addition, patients from tertiary care university hospitals were recruited into this study. Since patients with more severe symptoms or who are not well treated with existing drugs are more likely to be referred to university hospitals, it is thought that the drugs were added rather than replaced. Thus, there are great differences between populations in each group. In particular, the Switch group (11 subjects) was too small for statistical analysis. This finding that sample size in the Switch group is too small seems to represent the real-world clinical practice pattern seen in tertiary hospitals. Despite the small population, TBUT and corneal staining scores significantly improved in the Switch group at 8wk after diquafosol treatment. To assess the clinical effects of diquafosol in patients encountered in real-world clinical settings, an observational study should be conducted in patients admitted to such settings.
In conclusion, this study showed significant improvement in subjective symptoms and objective signs of dry eye disease treated with existing medicines in combination with diquafosol eye drop. Improvements were also seen in patients treated solely with diquafosol. Thus, diquafosol can be used as an effective therapeutic agent or applied to dry eye patients who do not respond to existing drugs or did not exhibit sufficient curative effects with previous treatments in real-world clinical settings.
ACKNOWLEDGEMENTS
The authors thank the following professors for data collection:Hung Won Tchah, University of Ulsan College of Medicine,Republic of Korea; Hyung Joon Kim, College of Medicine,Catholic University of Daegu, Republic of Korea; Shi Hwan Choi, Chungnam National University School of Medicine,Republic of Korea; Woo Chan Park, Dong-A University College of Medicine, Republic of Korea; Jong Soo Lee, Pusan National University College of Medicine, Republic of Korea;Sang-Bumm Lee, Yeungnam University College of Medicine,Republic of Korea; Hyun Seung Kim, College of Medicine,The Catholic University of Korea, Republic of Korea; Kyoung Yul Seo, Yonsei University College of Medicine, Republic of Korea; Joon Young Hyon, Seoul National University College of Medicine, Republic of Korea; Mee Kum Kim, Seoul National University College of Medicine, Republic of Korea;Hyung Keun Lee, Yonsei University College of Medicine,Republic of Korea; Jong Suk Song, Korea University College of Medicine, Republic of Korea; Hong Kyun Kim, School of Medicine, Kyungpook National University, Republic of Korea;In Cheon You, Chonbuk National University Medical School,Republic of Korea; Myoung Joon Kim, University of Ulsan College of Medicine, Republic of Korea; Kyung Chul Yoon,Chonnam National University Medical School, Republic of Korea; Young Keun Han, Seoul National University College of Medicine, Republic of Korea; Jin Hyoung Kim, Inje University College of Medicine, Republic of Korea; Tae-Young Chung,Sungkyunkwan University School of Medicine, Republic of Korea; So Hyang Chung, College of Medicine, The Catholic University of Korea, Republic of Korea.
Medical writing support was provided by Content Ed Net under the direction of the authors with funding from Santen Pharmaceutical Co., Ltd., Korea.
Foundation:Supported by Santen Pharmaceuticals, Co., Ltd.
Conflicts of Interest:Eom Y, None; Kim HM, None.
 International Journal of Ophthalmology2021年10期
International Journal of Ophthalmology2021年10期
- International Journal of Ophthalmology的其它文章
- lmpact of intraocular pressure fluctuations on progression of normal tension glaucoma
- Effective treatment for secondary angle-closure glaucoma caused by traumatic lens subluxation:phacoemulsification with capsular-tension-ring implantation combined with ophthalmic endoscopecontrolled goniosynechialysis
- Efficacy and safety of newly developed preservativefree latanoprost 0.005% eye drops versus preserved latanoprost 0.005% in open angle glaucoma and ocular hypertension: 12-week results of a randomized,multicenter, controlled phase III trial
- Progressive restrictive strabismus in an infant
- Association of peripheral anterior synechia, intraocular pressure, and glaucomatous optic neuropathy in primary angle-closure diseases
- Protective effect of LIF-huMSCs on the retina of diabetic model rats
