Fluorinated TiO2 Hollow Photocatalysts for Photocatalytic Applications
Jiabi Li, Xi Wu, Shengwei Liu
School of Environmental Science and Engineering, Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology, Sun Yat-sen University, Guangzhou 510006, China.
Abstract: Recently, extensive studies have been carried out to synthesize spherical microassemblies with hollow interiors and specific surface functionalizations, which usually exhibit fascinating enhanced or emerging properties and have promising applications in catalysis, photocatalysis, energy conversion and storage, biomedical applications, etc. With particular emphasis on the results obtained mainly by the authors’ research group, this review provides a brief summary of the recent progress on the fabrication and potential photocatalytic applications of fluorinated TiO2 porous hollow microspheres (FTiO2 PHMs). The synthesis strategies for F-TiO2 PHMs include a simplified two-step templating method and template-free method based on the fluoride-mediated self-transformation (FMST) mechanism. Compared to the two-step templating method, the template formation, coating, and removal steps for the FMST method are programmatically proceeded in“black-box”-like one-pot reactions without additional manual steps. The four underlying steps involved in the fabrication of F-TiO2 PHMs through the FMST pathway, nucleation, self-assembly, surface recrystallization, and self-transformation, are presented. By controlling these four steps in the FMST pathway, F-TiO2 PHMs can be successfully fabricated with a high yield by a simple one-pot hydrothermal treatment. The multi-level microstructural characteristics (including the interior cavity and hierarchical porosity) and compositions of hollow TiO2 microspheres as well as the primary building blocks can be well tailored. The unique superstructures of the F-TiO2 PHM photocatalysts provide advantages for photocatalytic applications by improving the light harvesting, mass transfer, and membrane antifouling. In addition, the in situ-introduced surface fluorine species during the formation of F-TiO2 PHMs provide significant surface fluorination effects, which are not only favorable for the adsorption and activation of reactant molecules, but also beneficial for surface trapping and interfacial transfer of photo-excited electrons and holes. Moreover, the porous hollow superstructures exhibit considerably better compatibility and tolerance to guest modifications, and thus the photocatalytic performances of F-TiO2 PHMs can be increased by synergetic host and guest modifications, such as ion doping, group functionalization, and nanoparticle loading.The light-harvesting range and intensity can be increased, the charge recombination can be reduced, mass transfer and adsorption can be promoted, and the surface reactivity can be tuned by introducing specific surface functionalities or nanoparticular cocatalysts. Consequently, the entire photocatalytic process can be systematically modulated to optimize the overall photocatalytic performance. The as-prepared F-TiO2 PHMs typically integrate the merits of interior cavity,hierarchical porosity, and surface fluorination and are open to synergetic host-guest modifications, which provides abundant compositional/structural parameters and specific physicochemical properties for systematically modulating the interconnected photocatalytic processes and promising potential photocatalytic applications.
Key Words: TiO2; Hollow; Microspheres; Hierarchical porosity; Surface fluorination
1 Introduction
Advanced photocatalytic technique is one of the most promising strategies in employing solar energy to solve increasingly serious energy crisis and environmental pollution1,2. To this end, exploiting advanced semiconductor photocatalysts is a primary step to develop practical and efficient photocatalytic techniques. Among various semiconductor photocatalysts, titania has proven to be one of the most suitable,due largely to its superior catalytic activity, long-term stability and low cost3. Unfortunately, most of the currently available titania photocatalysts are still characteristic of narrow lightresponse range and low quantum efficiency, which greatly restrict their practical applications3. In the past decades, various strategies, including bulk engineering and surface/interfacial modification of titania photocatalysts, have been developed with an attempt to modify the photocatalytic processes and to improve the photocatalytic efficiency3–22.
Especially, it has been demonstrated that the morphological and textural characteristics of titania photocatalysts strongly influence the light harvesting, transportation and transfer of photoexcited electrons and holes, diffusion and adsorption of reactant molecules, which are crucial factors determining the photocatalytic performances15–26. While various TiO2nanostructures have been synthesized and investigated in the previous studies, the controllable fabrication and photocatalytic applications of TiO2hollow structures merits special attention18.It is expected that higher photocatalytic reactivity and energy conversion efficiency could be achieved using TiO2hollow structures as photocatalysts, due to their flexibly designed multileveled micro-/nanostructures, readily available compatible and synergetic guest modification, porous frameworks and high specific surface area, larger substrateadsorption capacities as well as stronger light-harvesting intensities17,18. Up to now, a rich variety of methods has already been exploited for fabricating hollow TiO2nanostructures27–32.In particular, template-directed approaches, including the direct or inverse replication of preformed structures or organized reaction environments, have been used extensively to create hollow TiO2nanostructures. Typically, conventional templating methods involve three steps27, as shown in Fig. 1. Especially,the preformed specific template core with uniform size and specific surface functionalization is usually necessary, which directing the following coating step and determining the cavity size of final hollow products. Unfortunately, the cost for producing and consuming preformed templates is usually high,and the multiple steps may cause more complexity and less reproducibility. To overcome the drawbacks associated with conventional templating method, the authors developed three modified strategies to simplify the synthesis procedures of aforementioned templating methods, which will be elaborated later (Fig. 1). Notably, one common point is that all the three strategies developed by the authors to produce hollow TiO2nanostructures involve the participation of specific fluoride as either precursors or structure-controlling agents17,33–42. Due to the subtle interaction between fluoride and titania and versatile role of fluoride playing, the multi-level microstructural characteristics of hollow TiO2nanostructures can be well tailored, including the size, shape, cavity size, shell thickness and porosity of the hollow framework as well as the size, shape and surface chemistry of the primary nanobuilding blocks27.Moreover, the fluoride-mediated synthesis processes generally result in thein situsurface fluorination of synthesized hollow titania nanostructures34. Significantly, surface fluorination of titania photocatalysts are not only favorable for the adsorption and activation of reactant molecules, but are also beneficial for surface trapping and interfacial transfer of photoexcited electrons and holes, which together contribute to reducing the recombination probabilities of photoexcited electrons and holes and improving the surface photocatalytic redox reaction efficiencies30,36,37. Taken together, the attractive physicochemical properties of the hollow architectures in combination with the unique surface fluorination effects make fluorinated TiO2hollow nanostructures emerge as potential candidates for various photocatalytic applications. In addition,the porous framework of fluorinated TiO2hollow nanostructures endow them with diverse compatible guest modifications with further synergetic effects in modulating photocatalytic processes and performances. In this feature article, we briefly summarize recent progress regarding the fabrication and potential photocatalytic applications of fluorinated TiO2hollow nanostructures, with particular emphasis on the results obtained by the authors.
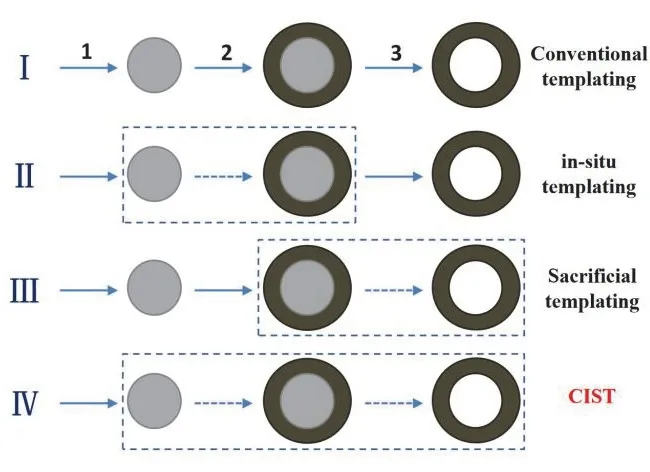
Fig. 1 Four typical routes for general synthesis of hollow nanomaterials: (I) conventional templating; (II) in situ templating;(III) sacrificial templating; (IV) chemically induced self-assembly and transformation (CIST). The dashed boxes denote the combined steps.
2 Synthetic strategies
2.1 General strategies
Until now, the most versatile approach for general preparation of hollow structures is still the conventional templating method(as illustrated in Fig. 1 (I)). The conventional templating methods usually involved the use of various hard templates,typically, monodispersed silica and polymer latex colloids.These templates are characteristic of narrow size distribution,ready availability in relatively large amounts, availability in a wide range of sizes from commercial sources, and simplicity of their synthesis using well-known procedures. In general,conventional templating methods involve the following three major steps27,28: (1) formation of monodispersed template; (2)uniform coating the templates with desired materials or their precursors; and (3) selective removal of the templates to obtain hollow structures. Note that, in most cases, in order to achieve uniform coating in step 2, modification of the template with specific functional groups and/or surface characteristics,including surface charges and porosity, are carried out in advance. The conventional templating method is versatile for the synthesis of a great variety of inorganic and composite hollow nanomaterials. Moreover, it is still the most effective approach in controlling the uniformity in morphology, cavity size, shell thickness and porosity of the resulting hollow creatures.Unfortunately, the rupture or even collapse of the hollow shells occurred commonly during the template removal process (step 3). Worse, the cost for producing and consuming preformed templates is usually high, and the multiple steps may cause more complexity and less reproducibility. To overcome the drawbacks associated with the aforementioned conventional templating method, three advanced strategies were continuously developed to simplify the synthesis procedures. Basically, the simplified synthesis protocols to generate hollow nanomaterials are referred to a combination of two or more consecutive steps mentioned in Fig. 1 (I). Afterwards, three simplified synthesis strategies developed by the authors to synthesize fluorinated hollow TiO2photocatalysts are illustrated in detail. A specific fluoride is usually present in the synthesis system as either precursor or structure-controlling agent to control the production of hollow TiO2nanostructures.
2.2 Simplified strategies
2.2.1 Self-templating method
The first one is termed as ‘in situtemplating’ method (Fig. 1(II)), in which the involved templates arein situformed in the synthesis system and are coupled together with the following coating step. In other words, on the basis of the different reaction activity, the core template and the shell materials are formed sequentially in a homogeneous solution in one-pot reaction system, and the first step for preformed template core is avoided.Most of the soft template arein situformed, and thus most soft template-based strategies can be also regarded asin situtempalting methods. For examples, gas bubble is a typical soft template formedin situin the reaction systems, which is stabilized by the shell materials (or their precursors) and directs the assembly the shell materials around the gas bubble27,28. In addition to a variety of soft templates, hard templates can also bein situintroduced into the synthesis system. A typical example is the versatile carbonaceous polysaccharide sphere template that isin situtransformed from saccharide starting materials43. This case is demonstrated by using (NH4)2TiF6as titania precursor together with glucose as precursors forin situformation of Cbased template (Fig. 2a)44. Hydrothermal treatment of their mixed solution at 180 °C for 12 h results in composite solid microspheres consisting of amorphous TiO2nanoparticles intercalating among the skeleton of porous carbonaceous polysaccharide microspheres. Subsequent calcination of these composite solid microspheres in air above 400 °C gives rise to multi-shelled F-TiO2PHMs with a drastic shrinkage in particle size. Note that the combustion of plentiful carbonaceous polysaccharide within the solid microspheres in air induced phase transition from anatase to rutile as below as 400 °C44. This is less common for fluorinated TiO2particles that are normally thermally stable against phase transition from anatase to rutile even above 600 °C3. Another notable feature of the present FTiO2PHMs is the formation of multi-shells, which is possibly due to the heterogeneous contraction as a consequence of nonequilibrium sintering processes45.
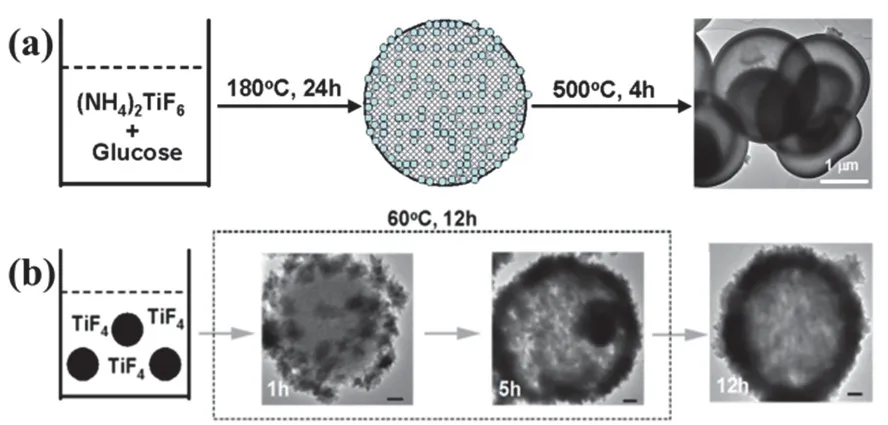
Fig. 2 Simplified two-step templating method for fluorinated TiO2 porous hollow microspheres. (a) Self-templating: Coupled templatesynthesis with shell-coating, Adapted from J. Phys Chem. Solids.,Elsevier publisher 44. (b) Sacrificial templating: Coupled shell-coating with template-removal (Note: The black dots denote preformed SiO2 microspheres), Adapted from Cryst. Growth Des., ACS publisher 38.
2.2.2 Sacrificial templating method
The second one is termed as ‘sacrificial templating’ method(Fig. 1 (III)), in which the coating step occurs accompanied by the simultaneous removal of the template core, that is, preformed template is automatically removed during the coating step, and step 3 are saved. A classical sacrificial templating method is selftemplating (including hollowing process based on interfacial reactions driven by Kirkendall diffusion, galvanic replacement,ion exchangeetc.), in which the preformed templates are not only the scaffolds directing the formation of shell materials, but are also the precursors towards shell materials27,28. The sacrificial templating method for fabricating F-TiO2PHMs is demonstrated by using preformed SiO2microspheres as templates and TiF4as titania precursor (Fig. 2b)38. After keeping the mixture at 60 °C for 12 h, uniform F-TiO2PHMs are readily formed. The cavity size, shell thickness and particle size of aspreparedF-TiO2PHMs can be adjusted to some extent by controlling the experimental parameters, including the size of the SiO2template, the concentration of the TiF4precursor, reaction temperature and time,etc. For example, by tuning the concentration of the TiF4precursor, the wall thickness of F-TiO2PHMs can be easily tuned. At a low TiF4concentration (0.01 mol·L?1), due to the limited TiO2deposited and low HF generated, the surface of the SiO2spherical template cannot be completely coated and the SiO2template still partially remain in the products. With an increase of the TiF4concentration (0.02–0.06 mol·L?1), the shell of TiO2hollow spheres become thicker.However, further increase to 0.10 mol·L?1, many ill-defined aggregates are generated because of a high supersaturation.Time-dependent evolution experiments clearly indicated the progressive increase in shell thickness of TiO2layer along with the decrease in diameter of SiO2template core, suggesting the concurrent template-directed TiO2deposition andin situSiO2template-sacrificial dissolution. This is understandable taking account of the etching effect of HF byproduct towards SiO2core,during the template directed deposition of TiO2from hydrolysis of TiF4precursor as shown in the equation38:

Similar strategy was also successfully extended to get porous TiO2nanotubes using preformed VOxnanobelts as templates and TiF4as titania precursor39.
2.2.3 Template-free method: fluoride-mediated selftransformation (FMST)
The last one, Template-free Method, is proposed by the authors as “chemically induced self-assembly and transformation” (CIST, Fig. 1 (IV))33, in which the template formation, coating and removal are programmatically proceeded in “blackbox”-like one-pot reactions without any additional manual steps taken part in conventional templating procedures.The CIST methods towards formation of hollow structures are also often referred as template-free method, although in which all the three steps 1–3 are indeed existed and are recorded.Needless to say, the CIST methods are highly desirable, which shall significantly simplify the synthesis procedures and reduce the production cost, making the scale-up of synthesis feasible and cost-effective. Taking account of the central roles of fluoride in the formation of fluorinated TiO2hollow microspheres, the synthesis protocols are also usually named as fluoride mediated self-transformation (FMST) pathway.
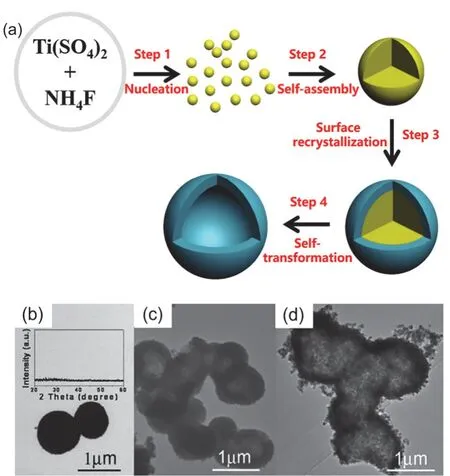
Fig. 3 (a) Fluoride-mediated self-transformation (FMST) pathway to fluorinated TiO2 porous hollow microspheres, Adapted from Nanostructured Photocatalysts (Book), Springer publisher 46. (b–d) TEM images of TiO2 microspheres prepared with RF = 1 at 200 °C for varying hydrothermal time: (b) 30 min; (c) 9 h; (d) 36 h during FMST pathway. Inset in (b) shows the corresponding XRD pattern, Adapted from J. Catal., Elsevier publisher 17.
Fabrication of F-TiO2PHMs by FMST pathway is one-pot template-free method. The basic formation processes are illustrated in Fig. 3a34,35,46. Typically, four underlying steps are involved. Step 1, nucleation: rapid production of mass metastable TiO2nanoclusters. Step 2, self-assembly:spontaneous assembly of the incipient TiO2nanoclusters into amorphous solid aggregates. Step 3, surface recrystallization:heterogeneous nucleation and crystallization on the surface of amorphous microspheres. Step 4, self-transformation:preferential dissolution of the amorphous interior and concurrent thickening the porous crystalline external shell to produce hollow microspheres. By controlling the four steps in FMST pathway, the multi-level microstructural characteristics of hollow TiO2nanostructures can be flexibly tailored, including the size, shape, cavity size, shell thickness and porosity of the hollow framework as well as the size, shape and surface chemistry of the primary nanobuilding blocks17,38(Fig. 3b–d).
Based on this FMST strategy, we have successfully fabricated F-TiO2PHMs in high yield by simple hydrothermal treatment of TiOSO4or Ti(SO4)2aqueous solution containing certain fluoride(e.g. NH4F, NH4HF2, CF3COOH)17,33–37. The cavity size, shell thickness and particle size can be easily tuned to great extent by changing experimental parameters, including reactant sources(titanium precursors and fluoride), molar ratio of fluorine to titanium (RF), hydrothermal temperature and time, and dopant,etc. Fig. 4a shows the typical scanning electron microscopy(SEM) image of the F-TiO2PHMs obtained by hydrothermal hydrolysis of Ti(SO4)2in the presence of NH4F (RF= 1) at 200°C for 9 h17. The particles were relatively uniform in diameter (900± 100 nm). Some of the hollow architectures are broken with open mouth. The porous shell walls are composed of closely packed nanoparticles with a roughened exterior. Transmission electron microscopy (TEM) images (Fig. 4b) confirmed the presence of the hollow structure and indicated a shell wall thickness of 100–200 nm. The selected area electron diffraction(SAED) indicated that the shell wall consisted of a polycrystalline aggregate of anatase nanoparticles (Fig. 4b, inset;d-spacings and {hkl} values: 0.351 (101), 0.238 (004), 0.189(200), 0.169 (105), and 0.166 nm (211); space group:I41/amd(141);a= 0.3785 nm,c= 0.9514 nm, JCPDS No. 21-1272).
Unfortunately, during the formation of F-TiO2PHMs, a large fraction of dispersed nanoparticles is commonly produced simultaneously, and the spherical products were usually illshaped and fragile. To overcome this major drawback and from well-defined hollow TiO2microspheres, urea was introduced as a base catalyst to optimize Step 2 (self-assembly, Fig. 3a) in the synthetic protocols35. Clearly, the pH value of the reaction system greatly affects the progress of the hydrolysis and condensation process. Since urea is thermally decomposed during the reaction, and the pH value of reaction system will be fine-tuned in real-time, which is beneficial for tuning nucleation and self-assembly processes. First, by urea modulation, the pH value of reaction system is increased to some extent, the nucleation rate is faster, providing sufficient metastable TiO2nanoclusters for subsequent aggregation. Second, by urea modulation, the pH value of reaction system is closer to the isoelectric point of TiO2, electrostatic repulsion between primary TiO2nanoparticles is weakening, favoring for aggregation.Besides, the TiO2condensation reaction is promoted in a basecatalyzed synthetic system. Therefore, urea tunes the nucleation dynamics and surface states of the elementary TiO2building blocks, which together promote the formation of uniform metastable solid spherical micro-aggregates. Subsequently, their transformation into hollow microspheres accompany with less randomly dispersed nanoparticles (Fig. 4c).
As to the mass flow in the Step 4 (self-transformation, Fig.3a), the hollowing process can also be classified into two different types47,48. The first type, “outward ripening”, refers to the mass transport starting from the center of starting aggregate;the second type, “inward ripening”, where the mass relocation starts from the surface region of starting aggregate. The inward versus outward hollowing process can be controlled by the solvent composition49. Normally, in pure water system, outward hollowing process occurred with a gradual and progressive decrease in the shell thickness17. In contrast, in a mixed ethanolwater solvent, an inward hollowing process occursviaa spherein-shell intermediate49. The ethanol added seems to affect the diffusion and adsorption of active fluorine species, being responsible for the varied dissolution behaviors of the interior and the different formation processes and structures of F-TiO2PHMs. Furthermore, the shape of shell-building nanoparticles can be tuned into faceted polyhedra exposing high percentage of{001} facets (Fig. 4d)49. The added ethanol probably contributes to facilitating the selective surface fluorination, accordingly,reducing the surface energy and increasing the percentage of exposed {001} facets.
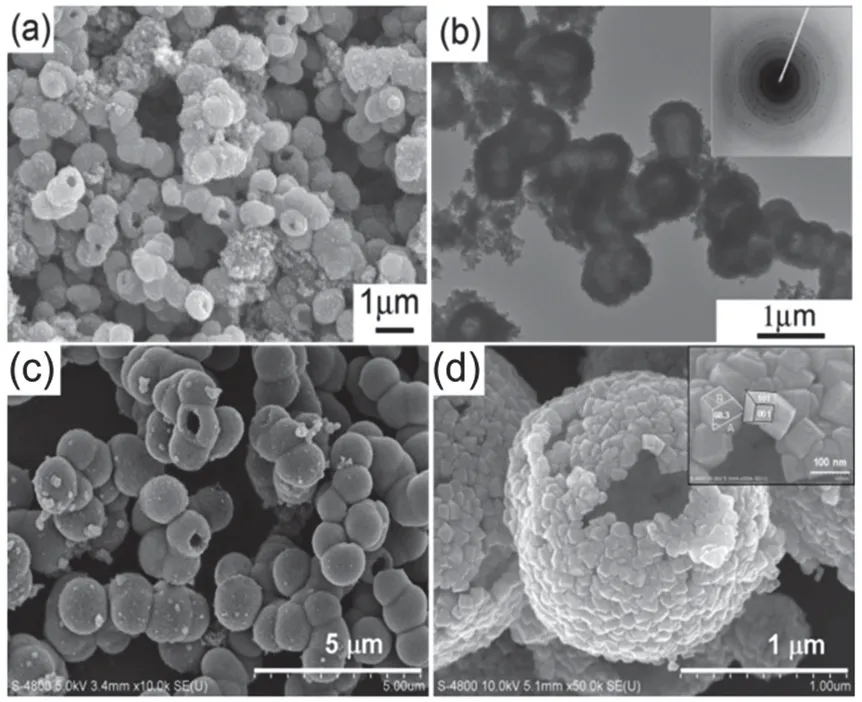
Fig. 4 (a) SEM and (b) TEM images of fluorinated TiO2 porous hollow microspheres based on FMST mechanism. Inset in (b)shows the corresponding SAED pattern, Adapted from J. Catal.,Elsevier publisher 17. (c) Urea-modified synthesis with consequence of less randomly dispersed nanoparticles, Adapted from Nanotechnology,IOP Science publisher 35. (d) Ethanol-modified synthesis with consequence of shell-building nanoparticles evolving into faceted polyhedra. Inset in (d) shows enlarged polyhedral nanobuilding units exposing high percentage of {001} facets, Adapted from J. Am. Chem. Soc., ACS publisher 49.
One may note that the present FMST mechanism for F-TiO2PHM is somewhat similar to the “Ostwald ripening” mechanism proposed by Zenget al.36,47,48. Nonetheless, two undervalued aspects shall be at least highlighted for the hollowing process:first, the existence of amorphous stage; second, the critical role of fluoride. The ‘Ostwald ripening’ mechanism assumed that there are inhomogeneous size and distribution of crystallites within the starting aggregates36,47,48. During hollowing processes, large crystallites are essentially immobile while the smaller ones are undergoing mass relocation through dissolving and re-growing. Nonetheless, up to now, there is lacking evidence regarding such a radial size and distribution inhomogeneity, which is somewhat hard to be directly recorded by available experimental tools. A further cross section analysis by TEM after cutting of the starting aggregates shall be helpful.Besides such a plausible localized Ostwald ripening, we note that the phase transition from amorphous to anatase TiO2provides additional driving force for the matter redistribution and hollowing process of the starting aggregates17. In fact, this existence of amorphous intermediate and phase transition process is common and crucial for the hollowing process of many inorganic hollow structures33,50–53. However, in the absence of fluoride added, amorphous solid TiO2microspheres only evolved into the crystalline counterpart without a hollowing process17. In contrast, hollowing processes are readily proceeded after adding fluoride in the synthetic system,highlighting the crucial roles of fluoride in the hollowing processes. Moreover, the hollowing rate was related toRF, with higherRFresulting in a greater hollowing rate17. As for the specific role of fluoride added on the hollowing processes,however, there are two viewpoints regarding the underlying mechanism. One viewpoint highlights the etching effect of HF on the solid starting aggregates. In this regard, a strong acid environment is typically involved to promote HF etching.Alternatively, another viewpoint highlights the promoting effects of F?ions on the dissolution and re-crystallization process of metastable TiO2intermediates (Step 4, selftransformation, Fig. 3a). Two reactions may occur34,35:
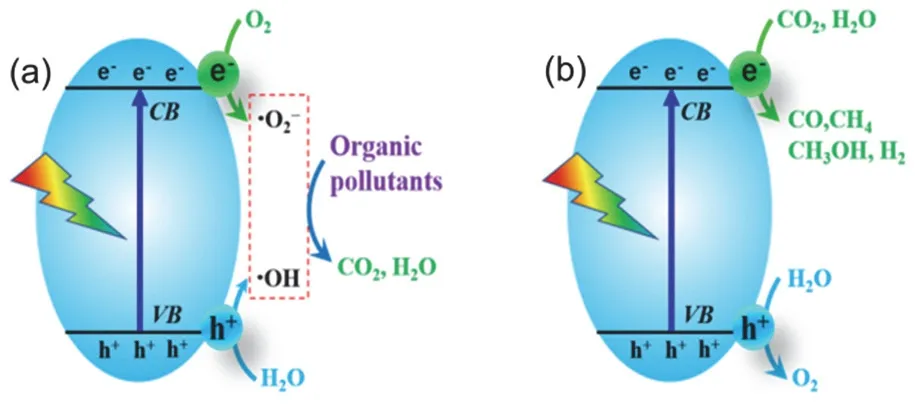
Fig. 5 Typical photocatalytic applications of fluorinated TiO2 porous hollow microspheres: (a) environmental photocatalysis,(b) energy photocatalysis.

The strong complexing ability of active F ions facilitates the dissolution of metastable TiO2(Eq. 3). Such a complexingmediated dissolution process avoids the dependence on the strong acid environment, which is supported by urea-modified synthetic protocols35. Also, the re-crystallization process is promoted by fluorination (Eq. 4), affecting the reaction dynamics and linking modes during hydrolysis and condensation reactions. In fact, this promoting effect of fluoride on the crystallization process is readily confirmed by comparing the degree of crystallinity and crystallite size of anatase crystallites obtained in the presence relative to absence of fluoride (XRD results)17.
3 Photocatalytic applications
Environmental photocatalytic technology is intensively developed as one of the most promising advanced oxidation processes (AOPs) for diverse environmental remediation, which possesses a series of advantages in comparison with conventional AOP techniques. Significantly, the oxidants involved in environmental photocatalytic reactions/processes can be simply the widely available water or oxygen molecules,which would be activated by photogenerated charge carriers to generate the highly active reactive oxidative species (ROSs),such as hydroxyl radicals (·OH) and superoxide radicals (·O2?)(Fig. 5a). Thesein situgenerated ROSs are highly reactive,which are able to completely decompose most organic pollutants into CO2and H2O, avoiding secondary pollution. Up to now, the environmental photocatalytic technologies have been widely exploited for complete removal of volatile organic compounds(VOCs) in air and for deep decomposition of diverse organic pollutants (such as dye molecules) in water54–58. Nevertheless,the produced CO2itself is the major greenhouse gas in the atmosphere, and increasing emission of the CO2overloads the self-purification capacity of the environment based on natural carbon cycling. It is ideal that the CO2produced from the treatment of organic pollutants can be further converted into valuable organic chemicals (such as solar fuels). In this regard,the photocatalytic reduction of CO2with H2O towards valueadded solar fuels (Fig. 5b), named, artificial photosynthesis, is emerged as an important process of energy photocatalytic technology59. Artificial photosynthesis can not only reduce the content of CO2in the atmosphere, inhibit greenhouse effect and relieve the environmental pressure, but also produce renewable resources and convert the hard-to-store solar energy into chemical energy which is easy to store and use. Evidently.advanced photocatalysts with much higher photocatalytic efficiency is crucial for developing practical photocatalytic techniques. Unfortunately, up to now, the apparent photocatalytic efficiency of most available photocatalyst systems is still quite low, which cannot meet the demands for large scale treatments of waste water/gas in high flux and/or high concentration.
The whole photocatalytic processes for either environmental or energy-related applications are complex. Generally speaking,four basic interconnected processes are involved for various photocatalytic redox reactions (including photocatalytic organic pollutant decomposition and CO2reduction) over TiO2or other photocatalysts. First (light absorption, charge generation): light absorption and excitation produces photogenerated charge carriers (holes and electrons). Second (charge separation and transport), those charges are separated against recombination,and transporting from bulk toward the catalyst surface. Third(reactant adsorption and activation), reactants are adsorbed and activated by interfacial charge transfer from catalyst surface to targeted reactants. Fourth (surface reactions), the surface redox reactions occur with targeted pathway and kinetics. Accordingly,a photocatalytic material system is generally consisting of four cooperative functional modules, that is, light-active centers,charge transportation channel, adsorption centers and the catalysis-active centers, which are closely interconnected and working cooperatively in dominating the whole photocatalytic processes. Essentially, modifying photocatalytic processes could be primarily achieved by engineering the multilevel structures of photocatalytic material system, including electronic,crystallographic, surface, and textural structures. However,simply modifying a specific structural parameter, modulating a single functional module, and tuning a single photocatalytic step will usually be hard to significantly improve the overall photocatalytic efficiency, not to mention that the associated possible negative effects on other correlated processes. For example, because of introduced impurities and defects associated localized electronic states, doping would usually enhance visible light absorption but impair the charge transportation. All the photocatalytic modules shall be modified as an integrated organic whole to cooperatively modulate the correlated photocatalytic steps. The hierarchical super-structured photocatalysts, with abundant multileveled structural parameters, possessed multi-aspect structural advantages for synergetic tuning of the four functional modules, which would enable a comprehensive optimization of the overall interconnected complex photocatalytic processes and overall photocatalytic efficiency18. Especially, the constructed porous TiO2microspheres is an excellent model example of hierarchical super-structured photocatalysts, in which the integrated engineering of multilevel microstructures would be readily achieved for the synergetic tuning of the four functional modules and thus the great enhancement in photocatalytic performances.
3.1 Hollowing effects
In addition to the universal merits of primary photocatalytic nanomaterials, the unique superstructure of porous hollow photocatalysts brings special advantages in improving light harvesting and mass transfer. Firstly, it is generally proposed that the hollow photocatalysts allow multi-reflections of irradiated light within their interior cavities, endowing them with enhanced light-harvesting (Fig. 6a)58. Indeed, the presentF-TiO2PHMs usually show a stronger absorption in the UV-visible region(310–700 nm) than P2559.
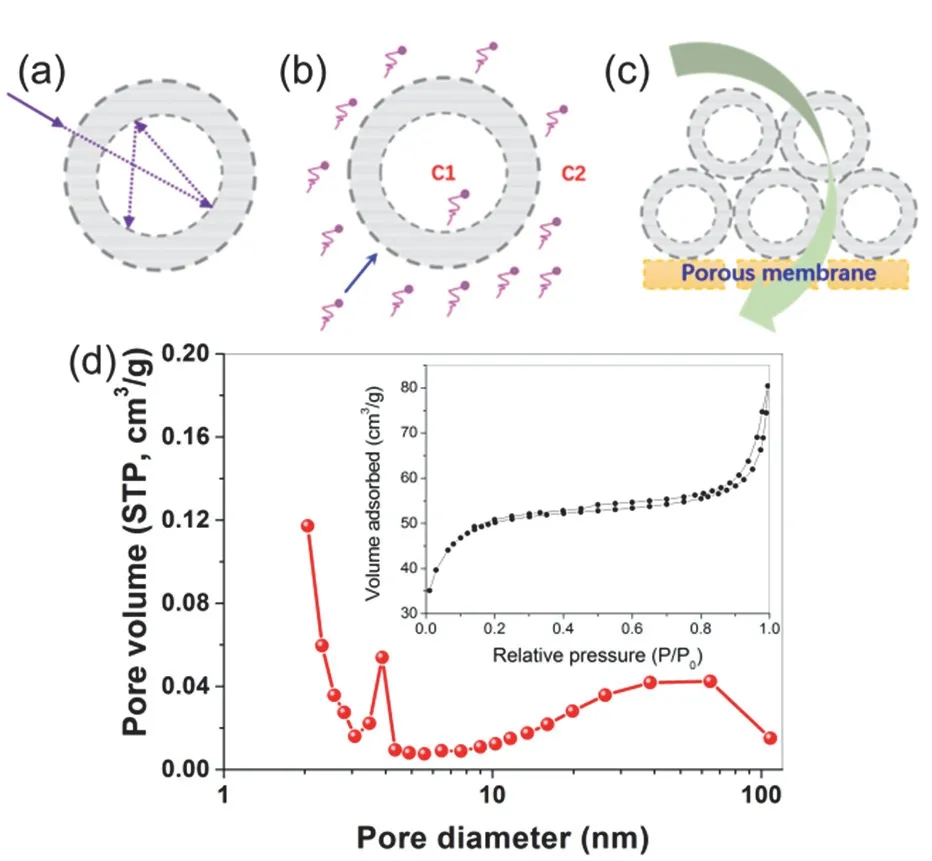
Fig. 6 The unique superstructure of TiO2-based porous hollow photocatalysts brings special advantages in improving (a) light harvesting, (b) mass transfer and (c) membrane antifouling, Adapted from J. Am. Chem. Soc., ACS publisher 58. (d) Nitrogen sorption isotherm (inset) and corresponding pore size distribution of fluorinated TiO2 porous hollow microspheres via two-step templating method:keeping a 0.02 mol·L?1 TiF4 aqueous solution containing preformed microspherical SiO2 template at 60 °C for 12 h, Adapted from Cryst.Growth Des., ACS publisher 38.
Moreover, the mass transfer and reactant capture is largely promoted in hollow photocatalysts (Fig. 6b). The formation of F-TiO2PHMs is accompanied by hierarchical organization of primary nanoparticles. As a result, those F-TiO2PHMs are composed of at least three levels of hierarchically porous structures covering micropores, mesopores and macropores38:(i) fine intra-aggregated pores (less than several nanometers) due to the aggregation of primary particles, (ii) large interaggregated pores (several to several tens nanometers) due to mesoscale packing of secondary aggregation particles in the shell wall, and (iii) assembly of a self-supporting, continuous outer shell enclosing an internal cavity space. It should be noted that both the pore size and their contributions can be adjusted to some extent by the experimental variation. The typical hierarchical porosity is clearly reflected in Fig. 8, a typical pore size profile forF-TiO2PHMs analyzed from N2sorption measurement38. Notably, two separate hysteresis loops are presented (inset in Fig. 6d), and the corresponding pore size profile showed a bimodal distribution across the mesoporous region (Fig. 6d). Smaller mesopores (2–20 nm) are usually related to primary intra-agglomeration driven by Gibbs free energy, while the larger ones (20–50 nm) are associated with secondary inter-aggregation involving condensation between primary agglomerates35. The high specific surface area and hierarchically porous framework is beneficial for improving mass transfer and adsorption of reactant molecules. Meanwhile,the initial concentration gradient inside and outside the porous hollow chamber give rise to localized driving force promoting the directional diffusion of reactant molecules from bulk solution towards the surface of hollow photocatalyst (Fig. 6b). Owing to the collective properties of hollow photocatalysts in promoting light harvesting and mass transfer, it is well demonstrated that these prepared F-TiO2PHMsexhibit superior photocatalytic activities towards decomposing VOCs in air as well as textile dyes in aqueous solution17,34–39.
For practical water treatments, theseF-TiO2PHMs,characteristic of assembled micro-sized superstructures, also have unique merits with respect to better recyclability and lesser membrane fouling (Fig. 6c), relative to normal nanosized photocatalyst59. Moreover, it is commonly observed that a significant fraction of F-TiO2PHMs tends to fuse together in form of dimers, trimers, or chain-like assemblies35. This dimerization process should be induced by titania condensation as two separate F-TiO2PHMs consisting of Ti-OH group on the surface contact each other. The recyclability and membrane antifouling features would be further intensified by their spontaneously forming chain-like assemblies. The better membrane antifouling performance ofF-TiO2PHMs have been simply demonstrated in crossflow microfiltration process, as compared with P25 nanoparticles59. After 2 h of filtration, the membrane permeate flux decreased by 31.5% for P25, whereas only a decrease of 14.9% occurred for F-TiO2PHMs60.
3.2 Surface fluorination effects
Note that fluoride is involved in all the above-mentioned synthetic systems. Taking account of the strong complexing ability of fluorine toward titanium, there is typically surface fluorination forF-TiO2PHMs. A typical high-resolution X-ray photoelectron spectroscopy spectrum of F 1sregion for the preparedF-TiO2PHMs indeed present a peak centered atca.684.44 eV, which was related to surface adsorbed F ions (i.e.,formation of ≡Ti―F surface species)54–56. Otherwise, the typical F 1speak corresponding to doped F?(i.e., lattice Fdoping, typically located at 688–689 eV12) is not detected. This is understandable considering the low-temperature hydrothermal conditions for preparingF-TiO2PHMs relative to the higher calcinations temperature normally for effective lattice F-doping.
The surface fluorination exhibits great impacts on the photocatalytic performance. The surface fluorination effects can be simply summarized as follows.
(1) The surface fluorination tunes the reactant adsorption capacity and mode, depending on the specific physiochemical properties of reactant molecules (Fig. 7)61,62. Normally, the surface fluorination will reduce surface charge, and thus favoring the adsorption of the positively charged organic pollutants, but inhibiting the adsorption of negatively charged organic pollutant. Usually, surface fluorination results in higher photocatalytic activity just because of a higher adsorption capacity. Furthermore, surface fluorination would modulate the photocatalytic selectivity of TiO2photocatalyst by tuning the selective adsorption. As an example, Liuet al.49reported that the surface-clean hollow TiO2spheres (surface fluoride removed by annealing at 600 °C) preferentially decomposes methylene blue (MB) in comparison to methyl orange (MO). On the contrary, the fluorinated hollow TiO2spheres exhibit preferential decomposition of MO over MB, since the selective adsorption of MB is inhibited.
(2) Surface fluorination significantly tunes the interfacial charge transfer. For hole transfer, after surface fluorination, the direct hole transfer is retarded, but the indirect hole transfer is enhanced by producing mobile free OH radicals. It isproposed that, for hole mediated activation of adsorbed H2O (H2Oads),mobile free OH radicals (·OHfree, Eq. 5, Fig. 7) are facilitated over the surface fluorinated TiO2(Ti-F), but surface adsorbed OH radicals (·OHads, Eq. 6) are dominated on surface-clean TiO2(Ti-OH)34:
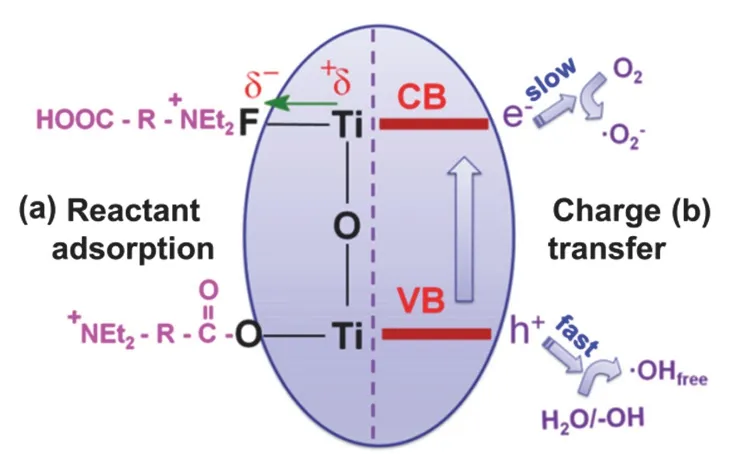
Fig. 7 Surface fluorination effects: (a) modifying reactant adsorption capacity and mode; (b) tuning charge transfer kinetic and mechanism.
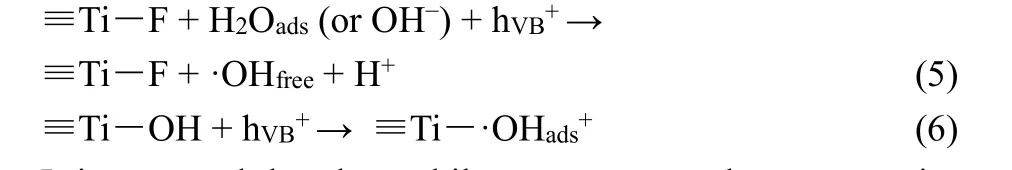
It is expected that the mobile ·OHfreeare much more reactive than the surface-bound ·OHads, and thus the photocatalytic decomposition rate of most organic pollutant in aqueous solution can be enhanced upon surface fluorination.
(3) Surface fluorination enhances surface electron storage,leading to retarded electron transfer but inhibited charge recombination. Because of the strong electronegativity of fluorine, the surface ≡Ti―F seems to act as an electron-trapping site and to reduce interfacial electron transfer rates by tightly holding trapped electrons34. The enhanced electron storage in the surface ≡Ti―F moiety retards electron transfer (to O2), but inhibits charge recombination, which in turn significantly facilitates the interfacial indirect hole transfer and generation of more free OH radicals (Fig. 7).
All these three aspects shall be comprehensively considered in understanding the unique photocatalytic redox properties of surface fluorinated photocatalysts (Fig. 7). Notably, the overall photocatalytic performance of F-TiO2could be further enhanced if electron transfer is improved. Typically, co-catalysts and adspecies can be introduced to promote electron transfer. As a case in point, Choi’s team reported that the simultaneous modification of TiO2with platinum nanoparticles and surface fluoride was beneficial for both hydrogen production and organic pollutant degradation63.
3.3 Synergetic effects
Compared to the primary nanostructures, the porous hollow superstructures exhibit much better compatibility and tolerance to guest modifications, enriching the abundance of compositional/structural parameters that synergistically tune the physicochemical properties and photocatalytic processes. In this regard, besides the above-mentioned collective properties of hollow superstructures and surface fluorination effects of F-TiO2PHMs, the photocatalytic performance of F-TiO2PHMscan be further enhanced by synergetic host and guest modifications,such as ion doping, group functionalization and nanoparticle loading. The synergetic host and guest modifications can be achieved by either post-treatments or one-potin situmodification based on CIST mechanism. Upon synergetic host and guest modifications, the light-harvesting range and intensity can be enhanced, the charge recombination can be reduced, the mass transfer and adsorption can be promoted, and the surface reactivity can be tuned also by introducing special surface functionality or nanoparticular cocatalyst, consequently, the whole photocatalytic processes can be systematically modulated to optimize overall photocatalytic performances. For example,by controlling the surface chemistry and the surface atomic structure of the shell-building primary nanoparticles of theFTiO2PHMs, we have achieved selective adsorption and photocatalytic decomposition of azo dye molecules49, providing an example concerning the design of advanced TiO2photocatalysts with desirable catalytic selectivity beyond reactivity and stability. By synergetic cooping, the environmental photocatalytic activity of thoseF-TiO2PHMs can be further enhanced for the decomposition of VOCs (acetone and toluene) in air and organic pollutants (Rhodamine B, Methyl orange) in aqueous solution54–56. The lattice F-doping are significantly facilitated by special synergetic codoping55,56. For example, the concomitant participation of Zr4+or Sn4+promotes lattice substitution of O2?ions by F?, as evidenced by the presence of an additional F 1speak atca. 688.30 (Zr/Fcodoping) or 688.80 eV (Sn/F-codoping)55,56. It is proposed that electron transfer-mediated charge compensation between the Zr4+(or Sn4+) and F?impurities reduces the diffusion barrier and thus facilitates the lattice doping. The synergetic codoping influences both the surface states and textural characteristics of resulting hollow TiO2photocatalysts.
Owing to the fascinating Schottky junction effects, surface plasmon resonance (SPR) effects as well as the cocatalyst effects of noble metal (such as Au and Ag) nanoparticles, they are intensively employed to modify TiO2photocatalysts.Incorporating plasmonic Ag or Au nanoparticles into the matrix of theF-TiO2PHMs has been also demonstrated to significantly enhance the visible-light response and the whole-spectrum photoactivity57,59. By FMST pathway, the F-TiO2PHMs are firstly fabricated, which are composed of primary polyhedral anatase nanocrystals with abundant {001} facets and substantial surface defects. After following photochemical deposition, those surface tailored F-TiO2PHMs are successfully decorated uniformly with tiny Au nanoparticles of average size of 14 nm(Fig. 8a–g)59. The resulting Au modified TiO2hollow microspheres (Au1/THMs) exhibit remarkably improved photocatalytic CO2reduction performance under full spectrum,visible-light and AM 1.5 G irradiations (Fig. 8h)59, relative to both P25 and F-TiO2PHMs without Au decoration (Au0/THMs).In short, Au decoration enhances both full spectrum and visiblelight photocatalytic CO2reduction activity. In addition, Au decoration largely tunes the photocatalytic CO2reduction selectivity59. Upon Au decoration, photocatalytic reduction of CO2to CH4is remarkably improved under both full spectrum and visible-light irradiation (Fig. 8h). The strong CO affinity to surface decorated Au nanoparticles limits the release of CO molecules from the surface of hybrid photocatalyst, promoting their further hydrogenation to CH4.
Because of the synergetic effects of the porous and hollow frameworks, the abundant favourable microscopic surface features (surface fluorination, the high-energy {001} facets, and surface Ti3+/Vodefects), and the surface Au modification, the light harvesting, charge dynamics, CO2adsorption and activation over the hybrid Au1/THMs photocatalyst system are systematically modulated to promote the photocatalytic CO2reduction activity and selectivity. The multiple synergetic effects are illustrated in Fig. 959.
Fig. 8 (a, b) TEM images, (c) HRTEM image, (d) HAADF-STEM image and (e–g) the corresponding EDS elemental mappings of the typical sample Au/THMs. The inset in panel (a) is the corresponding SAED pattern, and the inset in panel (b) is the enlarged image of the outermost shell wall, (h) comparison of the CH4 and CO generation rates for various samples obtained under full spectrum light irradiation,visible-light and AM 1.5 G irradiation.
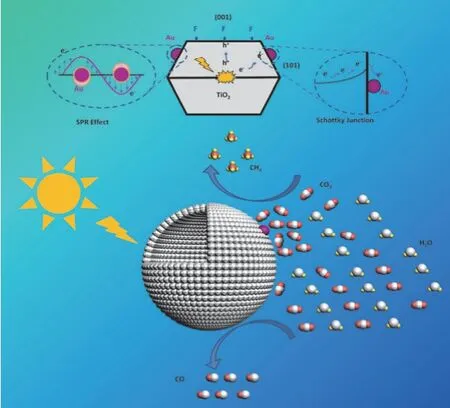
Fig. 9 The synergetic mechanism for enhanced photocatalytic CO2 reduction with H2O to CH4 and CO over hybrid photocatalyst of TiO2 hollow microspheres decorated with Au nanoparticles (Au1/THMs).
(1) Synergetic effects in enhancing wide-spectrum light absorption. First, the light trapping effects of hollow cavities enhances the UV light absorption. Second, the SPR effects of decorated Au nanoparticles enhances the visible light absorption.Together, the light harvesting capacity and intensity in both ultraviolet and visible light regions are greatly enhanced.
(2) Synergetic effects in reducing photogenerated charge recombination. First of all, two unique junctions are coexisted.One is related to facet junctions at interface of {001} and {101}facets. Another one is related to the Schottky junctions at the Au/TiO2interface. Both facet and Schottky junctions would facilitate the spatial charge separation, decreasing charge recombination efficiency. In addition, SPR-mediated local electromagnetic field effects of decorated Au nanoparticles will facilitate the interfacial charge separation. Furthermore, surface Ti-F groups and surface defects will facilitate the surface trapping of photo-induced electrons, reducing the surface charge recombination.
(3) Synergetic effects in promoting surface charge transfer and redox processes. The apparent charge transfer/utilization efficiency largely depends on reactants adsorption and activation, which is in turn determined by the surface host/guest chemistry. In particular, the CO2adsorption capacity is significantly improved by decorating with Au nanoparticles,which have a strong affinity to CO2molecules. Meanwhile, the CO2adsorption modes can be adjusted by introducing surface OH groups, surface Ti3+/Vodefects and the surface decorated Au.Significantly, the CO2molecule can be dissociated into one adsorbed CO moiety and one adsorbed O atom on Au/TiO2. Such chemically dissociated adsorption and activation of CO2,combined with the cocatalyst effect of Au nanoparticles, will accelerate the hydrogenation of surface bond CO to selectively generate CH4in the hybrid Au1/THMs photocatalyst system.
In brief, the Au decoration effects can be synergistically combined with the attractive porous hollow matrix and the fascinating surface atomic geometry, defects and chemistry of FTiO2PHMs, which together enable an integrated engineering of the light harvesting, charge separation and transfer, as well as the CO2adsorption and activation processes on TiO2photocatalysts,advancing the promising photocatalysts for effective artificial photosynthesis.
4 Outlook and conclusions
In the past few years, considerable progress has been made concerning the synthesis and applications of F-TiO2PHMs. The synthetic strategies forF-TiO2PHMs include simplified twostep templating method, as well as template-free method based on FMST mechanism. Especially, FMST based synthesis method is more convenient to scale up for large scale production,because of its relatively simple synthesis procedures, high reproducibility, and low production loss. Moreover, FMST pathway enables a flexible control in cavity size, shell thickness,porosity, as well as particle size and uniformity. The integration of interior cavity, hierarchical porosity and surface fluorination within as-prepared F-TiO2PHMs endow them with interesting physiochemical properties. TheF-TiO2PHMsphotocatalysts exhibit superior photoactivity, desirable recyclability and tunable selectivity. Further exploiting the potential applications of these F-TiO2PHMsis anticipated on the basis of compatible and synergetic host and guest modifications.
- 物理化學(xué)學(xué)報(bào)的其它文章
- Recent Progress of Perovskite Oxide in Emerging Photocatalysis Landscape: Water Splitting, CO2 Reduction, and N2 Fixation
- Review of Z-Scheme Heterojunctions for Photocatalytic Energy Conversion
- TiO2-Supported Single-Atom Catalysts for Photocatalytic Reactions
- 微波輔助快速制備2D/1D ZnIn2S4/TiO2 S 型異質(zhì)結(jié)及其光催化制氫性能
- Controllable Synthesis of g-C3N4 Inverse Opal Photocatalysts for Superior Hydrogen Evolution
- Carboxyl-Functionalized Graphene for Highly Efficient H2-Evolution Activity of TiO2 Photocatalyst