Ginseng ameliorates pulmonary toxicity induced by silicon dioxide nanoparticles in rats
Raghda A. El-Sayed, Fatma M. El-Demerdash, Mohammed A. El-Magd
1Department of Environmental Studies, Institute of Graduate Studies and Research, Alexandria University, Egypt
2Department of Anatomy, Faculty of Veterinary Medicine, Kafrelsheikh University, Egypt
ABSTRACT
KEYWORDS: Silicon dioxide nanoparticles; Ginseng; Oxidative stress; Inflammation; Pulmonary toxicity
1. Introduction
Silica nanoparticles, particularly silicon dioxide nanoparticles(SiONPs), are widely used in engineering, industries, food additives, and as carriers for targeted drug and gene delivery in nanomedicine. SiONPs extensive use in nanomedicine was attributed to their appropriate biocompatibility and ultimate stability against biodegradation[1]. The permissible dose for SiONPs as a food additive (namely E551 in some food products such as noodles,soups, and coffee creamers) is 124 mg/day[2]. Wider use of these nanoparticles requires a thorough understanding of their toxic influence on animal and human health. Numerous studies reported cellular toxicity for SiONPs with hazardous health effects[2-4].Therefore, there is a rising argument regarding health and safety concerns for using SiONPs as a food additive. This cytotoxicity mainly depends on SiONPs physicochemical properties such as size, cell type, dose, and even specific coatings, ligand incorporation, or surface modifications[4]. The main determinants for this toxicity are the size and dose with the highest cytotoxic and genotoxic effects of smaller size and higher concentration[4].
A large body of research concluded that silica NPs, similar to other NPs, exert their cytotoxic effect on both cancer and normal cells mainly through induction of oxidative damage, apoptosis,and inflammation. The cytotoxic effect of silica NPs against HepG2 cells is mediated by these three mechanisms[5]. Moreover,in vitro administration of SiONPs caused notable DNA damage accompanied by activation of the MAPK/ERK1/2 and Nrf2/ARE pathways in colon cancer cells[6]. Silica NPs can also induce endoplasmic stress-dependent apoptosis in macrophages through upregulation of apoptotic markers [Bcl2-associated X protein(Bax) and caspase 3] and downregulation of anti-apoptotic marker B-cell lymphoma-2 (Bcl-2)[7]. Moreover, oral treatment of rats with synthetic amorphous silica, which contains silica NPs, resulted in upregulation of inflammatory cytokines [nuclear factor kappa B (NF-κB) and transforming growth factor-beta 1 (TGFβ1)],thereby leading to liver fibrosis[8]. Athinarayanan et al.[9] found that SiONPs induced apoptosis in human mesenchymal stem cells via triggering reactive oxygen species (ROS) release and oxidative stress damage. Furthermore, oral administration of SiONPs caused endoplasmic reticulum stress which led to ROS overproduction, and the expression of NF-κB and other inflammatory cytokines in mouse liver was upregulated[10].
The lung is one of the main targets for silica NPs accumulation and probably toxicity[11]. Administration (injection or oral) of silica NPs to animals resulted in the accumulation of these NPs in the lung, liver, spleen, and kidney[8,12]. Hassankhani et al.[3]reported histopathological alterations (pneumonia, congestion,and mononuclear cells infiltration) in rat lung following oral administration of silica NPs with a size of 20 nm. Furthermore,mice injected with silica NPs (at a size of 50 nm) showed lung thrombosis[13]. Silica particles-induced pulmonary toxicity was mediated by ROS release and increased expression of the inflammatory cytokines [tumor necrosis factor-alpha (TNF-α),interleukin 1 beta (IL-1β), NF-κB, and cyclooxygenase 2 (COX2)],and the apoptotic genes (Bax, caspase 3 and FasL)[14]. Recently,Li et al.[11] reported that silica NPs induced oxidative stress-based cytotoxicity only on the normal bronchial epithelial 16HBE cells rather than on lung cancer A5490 cells.
Panax ginseng Meyer, as a member of Araliaceae family, has multiple beneficial properties including anti-oxidant, anti-cancer,anti-inflammatory, and anti-stress[15]. Ginsenosides, the main derivatives of ginseng, have improved effects on health through induction of immune response against diseases, improving mental performance, and reducing stress and anxiety[16]. Ginseng and ginsenosides also have potent anti-cancer effects in several experimental models both in vitro (against a large variety of cancer cells including liver, breast, pancreas, and colon) and in vivo[17].These therapeutic and preventive effects are mainly mediated through the ability of ginseng and its active derivatives to trigger DNA damage, and apoptosis in cancer cells[17]. Moreover, ginseng has a strong anti-inflammatory effect through suppressing IL-1β,IL2, IL4, IL6, COX2, and TNF-α expression[18,19].
Therefore, this study aimed to evaluate the protective and therapeutic effect of ginseng against SiONPs toxicity in rat lung.
2. Materials and methods
2.1. Materials
SiONPs (98.7% purity, 20 nm size, 160 m/g specific surface area,2.4 g/cc true density, non-porous, white powder) was purchased from SkySpring Nanomaterials, Inc., USA (product # 6809NM, Lot #6809-60112). Ginseng dried plant (in form of powder) was obtained from Pharco Pharmaceutical Company, Egypt. All chemicals used in the present study were of analytical grade.
2.2. Experimental design
Sixty white male albino rats (12-16 weeks age and 160-180 g) were obtained from the Production Center for Natural Toxins and Raw Plasma at the Helwan Farm of the Egyptian Company for Vaccines and Medicines. Two weeks before starting the experiment, animals were adapted to the laboratory conditions. Animals were fed a basal diet ad libitum and housed in cages supplied with a light/dark system of 12 h/12 h and at 21 ℃.
Rats were randomly assigned into five groups. Group 1 was orally administered with saline for 5 weeks and served as a control.Group 2 was given ginseng (75 mg/kg, 1/10 of LD) daily for 5 weeks[20]. Group 3 received SiONPs at a dose of 200 mg/kg which is equivalent to 1/10 of LD[8] daily for 5 weeks. Group 4 (protective group) was pretreated with ginseng for 1 week before SiONPs and continued for further 4 weeks. Group 5 (therapeutic group) was administered with SiONPs 1 week before ginseng administration and continued for further 4 weeks. In all groups, the duration of treatment for either ginseng or SiONPs was equal (5 weeks).SiONPs and ginseng were dissolved in saline, freshly prepared, and orally administered by gavage.
None of the rats treated with SiONPs showed notable signs of morbidity and only one died in group 3 during the whole experimental time. At the end of the experiment, rats were euthanized by decapitation, and the lungs were immediately removed. Lung was divided into four parts: the first part was fixed in 10% formalin (for histopathology), the second part was stored at -20 ℃ (for biochemical assays), the third part was kept at -80 ℃ (for RNA extraction), and the fourth part was freshly utilized (for comet assay and flow cytometry).
2.3. Biochemical assays
Lung specimens were homogenized, centrifuged (7 500 rpm for 15 min), and the obtained supernatant was collected as previously described[21]. The levels of lipid peroxidation marker malondialdehyde (MDA), hydrogen peroxide, reduced glutathione(GSH), superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), and glutathione peroxidase (GPx) were determined in lung homogenates using commercially available kits(Biodiagnostic, Egypt) following manufacturer’s instructions and as previously described[22].
2.4. Determination of DNA damage by comet assay
DNA damage parameters (tail length, percentage of degraded DNA,and tail moment) of lung cells were determined by alkaline singlecell gel electrophoresis (comet assay) as previously described[23]. In this assay, fluorescent microscopy, GelRed stain (red fluorescence),and Komet 5 image analysis software (Kinetic Imaging, Liverpool,UK) were used. One hundred cells were examined per sample.
2.5. Molecular analysis by real-time PCR
Relative expression of inflammation-related genes (IL-1β
, TNF-α
,NF-κ
B, COX2, TGFβ
1), and apoptotic (Bax and caspase 3) and antiapoptotic Bcl-2 genes in the lung was detected using real-time PCR(qRT-PCR). Total RNA was isolated using a commercial kit (Gene JET RNA Purification Kit) following the manufacturer’s protocol(Thermo Scientific, # K0731, USA). The cDNA was synthesized by reverse transcription using a commercial kit (RevertAid H Minus Reverse Transcriptase) as described in the manufacturer’s instruction (Thermo Scientific, # EP0451, USA). The qPCR mixture included cDNA, Syber green master mix (2× Maxima, Thermo Scientific, # K0221, USA), and primers. Theβ
-actin gene was used as a reference (internal control). The primers were designed by the Primer 3 web-based tool based on rat sequences retrieved from GenBank databases (Table 1). The thermal cycling and melting curves conditions were done as previously described[21]. The relative gene expression presented as fold change was calculated using the 2method.
Table 1. Forward and reverse primers used in the qRT-PCR.
2.6. Cell cycle analysis by flow cytometry
Flow cytometry was applied to detect the effect of different treatments on the number of cells in each phase of the cell cycle and was performed as previously described[24] with some modifications.Briefly, freshly dissected lung tissues were lysed by collagenase enzyme (0.1%, Invitrogen, USA), then mesh filtered (0.7 mm nylon)and centrifuged (4 000 rpm/5 min). After fixation, the cells were stained with propidium iodide and analyzed by the Attune flow cytometer (Applied Bio-system, USA). The number of cells was counted in each cell cycle phase and was presented as a percentage of the total number of cells.
2.7. Histopathological investigation and lung damage score
After fixation in 10% formalin, lung specimens were dehydrated through alcohols, cleared in xylene, and then embedded in paraffin wax. Sections (5 μm) were stained with hematoxylin and eosin. Four slides from each lung specimen were analyzed by a light microscope(Olympus BX 41, Japan). The lung damage score was blindly determined in 7 randomly chosen fields per each slide at ×400 magnification. This score included the following histopathological alterations: inflammation, mononuclear cells infiltration, size and number of granuloma-like structure (GLS), pathological emphysema, and hemorrhage. The score grades were: - (no lesion),+ (mild damage), ++ (moderate damage), +++ (high damage), and++++ (severe damage).
2.8. Statistical analysis
In all experiments, the obtained data were presented as mean± standard error of mean (SEM) and significance was set at P <0.05. One-way Analysis of Variance (ANOVA) and the post hoc test Tukey’s Honestly Significant Difference (Tukey’s HSD) were used to compare means via Graph Pad Prism 5 software (San Diego, CA,USA).
2.9. Ethical statement
All experiments were conducted in accordance with US National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and followed Helsinki declaration of animal ethics. The study protocol was reviewed and approved by the Research Ethics Committee of Kafrelsheikh University (KFS 125/10).
3. Results
3.1. Ginseng ameliorated SiO2NPs-induced oxidative stress
The levels of MDA and the content of HOwere significantly (P <0.05) higher in SiONPs-treated rats than the control group (Table 2).However, rats treated with ginseng showed significantly (P < 0.05)lower levels of MDA and HOcompared with those of SiONPstreated rats. On the other hand, SiONPs-administered rats exhibited markedly lower levels of GSH, SOD, CAT, GPx, and GST than the control group (P < 0.05) (Table 2). These antioxidant markers were significantly (P < 0.05) increased in SiONP rats pre-treated or posttreated with ginseng. Besides, normal rats treated with ginseng showed significantly (P < 0.05) higher levels of antioxidant markers than the control group (Table 2).

Table 2. Effect of ginseng on lipid peroxidation and antioxidant enzymes in rat lung.
3.2. Ginseng relieved DNA damage initiated by SiO2NPs
DNA damage in the lung was assessed by the comet assay (Figure 1 and Table 3). SiONPs-administered rats had significantly (P <0.05) higher tail length, tail DNA%, and tail moment, indicating higher DNA damage in this group than the control group. The DNA damage was significantly (P < 0.05) decreased in SiONPs rats pretreated or post-treated with ginseng, with the lowest damage in the post-treated rats. The ginseng control group showed non-significant(P > 0.05) differences in DNA damage compared with the control group.
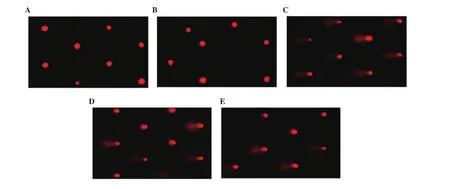
Figure 1. Effect of SiO2NPs and ginseng on lung DNA damage detected by the comet assay. A: control; B: ginseng; C: SiO2NPs; D: ginseng pre-treated; and E:ginseng post-treated group.
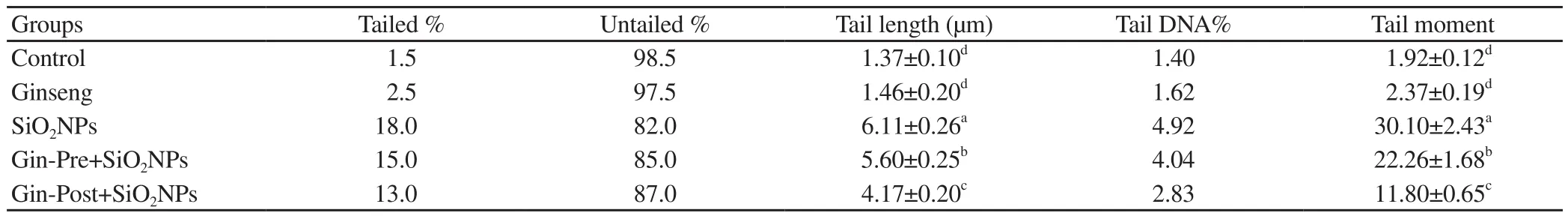
Table 3. DNA damage parameters by comet assay.
3.3. Ginseng reduced apoptosis triggered by SiO2NPs
The qRT-PCR was used to detect the changes in Bax, Bcl-2, and caspase 3 mRNA levels in the lung following different treatments.Untreated SiONPs rats significantly increased the mRNA levels of Bax and caspase 3 while decreasing Bcl-2 mRNA levels compared with the control group (P < 0.05) (Figure 2). Rats pre- and posttreated with ginseng reversed SiONPs-induced change (P < 0.05),with the best improvement found in the SiONPs group pre-treated with ginseng (Figure 2). On the other hand, the ginseng control group showed significantly higher Bcl-2 mRNA levels than other groups (P < 0.05).

Figure 2. Effect of SiO2NPs and ginseng on the expression of lung apoptosis-related genes (A: Bax, B: Bcl-2, and C: caspase 3) by qRT-PCR. Data are presented as fold change (mean) ± SEM (n = 7/group). Different letters show significant difference at P<0.05.
3.4. Ginseng restored cell cycle phase arrested by SiO2NPs
Administration of SiONPs arrested the cells in the G/Gphase as revealed by a significant (P < 0.05) increase in the number of cells in this phase compared to the control group (Figure 3). However,SiONPs administration significantly decreased the number of cells in the S phase (P < 0.05). Pre-treatment and post-treatment with ginseng (as prevention and treatment) restored the number of cells in each cell cycle to a level comparable to that of the control group,with the best effect in the group pretreated with ginseng.
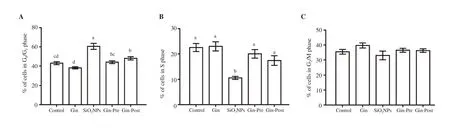
Figure 3. Effect of SiO2NPs and ginseng on the percent of lung cells measured by flow cytometry. Cell percentage in the three cell cycle phases (A: G0/G1, B: S,and C: G2/M) is shown in bar graphs. Data are expressed as mean ± SEM. Columns carrying different letters are significantly different at P<0.05 (n = 7/group).
3.5. Ginseng decreased inflammation induced by SiO2NPs
The obtained qRT-PCR results revealed significant (P < 0.05)increases in mRNA levels of pro-inflammatory cytokines IL-1β
,TNF-α
, NF-κ
B, COX2, and TGFβ
1 in lung tissue of rats treated with SiONPs as compared to the control group (Figure 4). Administration of ginseng either before or after SiONPs significantly (P < 0.05)decreased the levels of all these mRNAs, with the lowest level in the pre-treated group, compared with the untreated SiONPs group.The ginseng control group exhibited a significantly lower IL-1β
expression than other groups.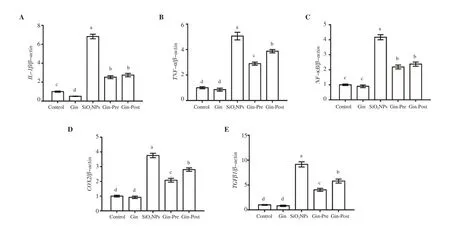
Figure 4. Effect of SiO2NPs and ginseng on the expression of lung inflammation-related genes as detected by qRT-PCR (A: IL-1β, B: TNF-α, C: NF-κB, D:COX2, E: TGFβ1). Data are presented as fold change (mean) ± SEM (n = 7/group). Different letters show significant difference at P<0.05.
3.6. Ginseng improved histology of the lung deteriorated by SiO2NPs
Control and ginseng-treated rats exhibited normal lung architecture.Alveoli had a thin wall containing both pneumocyte typeⅠand pneumocyte type Ⅱ with some normally emphysematous alveoli(Figure 5A and B). In contrast, lung tissues of untreated SiONPs rats (Figure 5C) showed clear signs of inflammation in form of notable infiltration of mononuclear cells forming GLS. These GLS contained condensed cells with large basophilic nuclei and scanty cytoplasm. The alveoli adjacent to this granuloma were highly emphysematous and had a very thin alveolar wall with hemorrhage.SiONPs rats pre-treated with ginseng showed improved lung histology indicated by a significant decrease in inflammatory signs,and small scanty GLS and emphysema without hemorrhage (Figure 5D). SiONPs rats pos-treated with ginseng exhibited a slight degree of improvement in lung histology with moderate inflammatory signs.Some GLS showed large basophilic nuclei, and emphysema which were smaller than those of the SiONPs group but larger than the pre-treated group with moderate hemorrhage (Figure 5E).
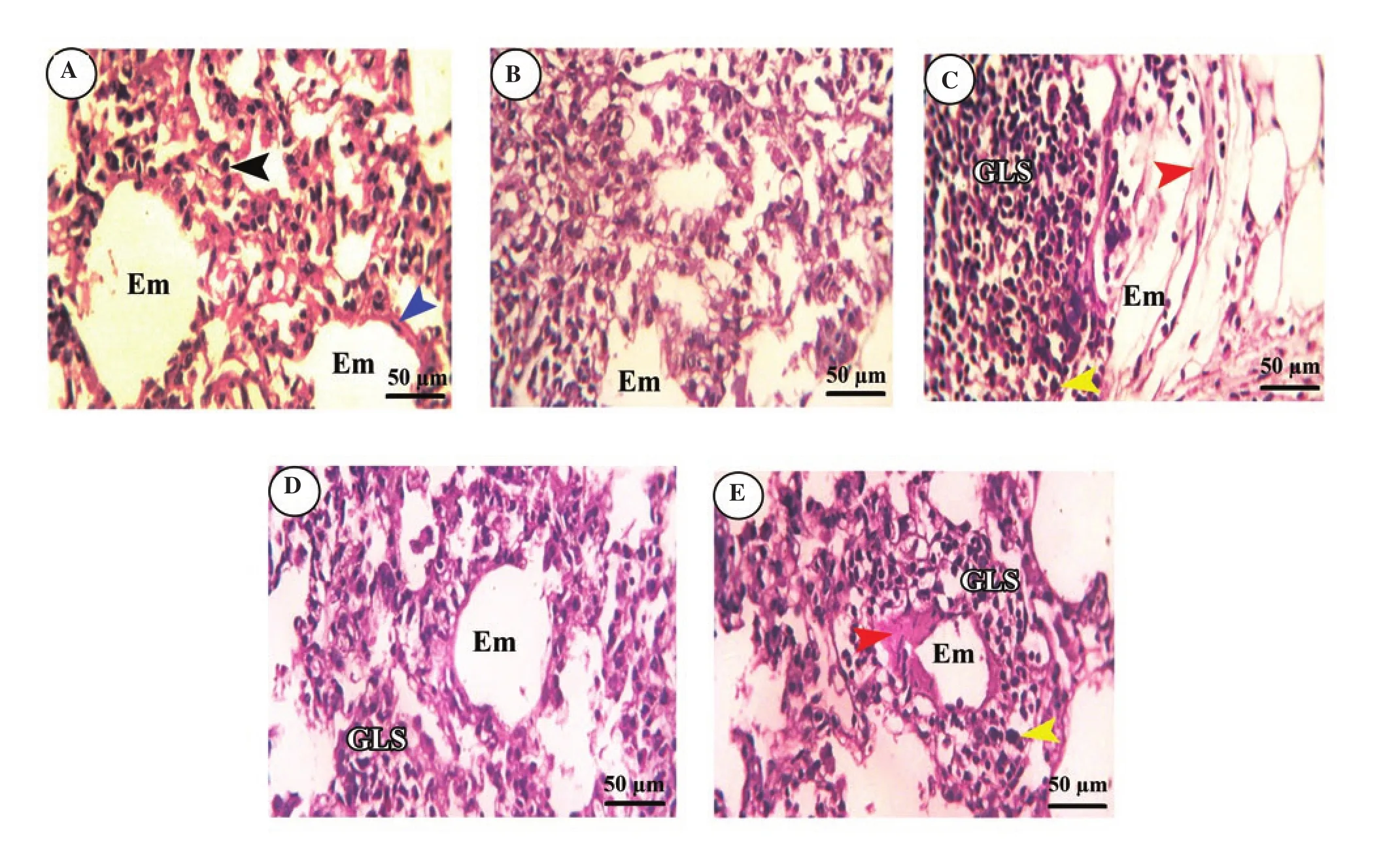
Figure 5. Effect of SiO2NPs and ginseng on lung histology by a light microscope. The lungs of the control group (A) and ginseng group (B) show pneumocyte typeⅠ(blue arrowhead) and pneumocyte typeⅡ(black arrowhead) with some normally emphysematous alveoli (Em). (C) The lung of the SiO2NPs group shows granuloma-like structure (GLS) containing condensed cells (yellow arrowhead). The adjacent alveoli were highly emphysematous and had a very thin alveolar wall with hemorrhage (red arrowhead). The lungs of of the ginseng pre-treated (D) and post-treated groups (E) show few GLS with large basophilic nuclei (yellow arrowhead), and emphysema (Em) with moderate hemorrhage (red arrowhead). All images were captured at ×400, scale bar = 50 μm.
The result of histopathological score revealed severe histological changes in untreated SiONPs rats and a variable degree of improvement in ginseng pre-treated and post-treated rats,(Supplementary Table 1).
4. Discussion
Induction of oxidative damage, apoptosis, and inflammation are the main mechanisms by which nanoparticles can induce cytotoxicity[25-27].These three mechanisms can damage the cell membrane, mitochondria,nucleus, and macromolecules, thereby causing structural and functional disruption for cellular components[5,6,11,28]. This study aimed to evaluate the ameliorative effect of ginseng on the pulmonary toxicity induced by SiONPs by assessing these three mechanisms.
SiONPs, similar to the majority of other NPs, induce apoptosis in both cancer and normal cells through stimulation of excessive ROS release and subsequently oxidative stress damage to essential cellular components[5,6,11]. Administration of silica NPs resulted in overproduction of free radicals as evidenced by increased lipid peroxidation biomarker MDA and decreased intracellular antioxidant level of GSH[29]. Consistent with these results, we also found that rats administered with SiONPs had significantly higher levels of oxidative stress markers (MDA and HO) and lower levels of GSH and decreased activities of antioxidant enzymes (SOD,GPx, CAT, GST). Excessive free radicals induced by SiONPs could damage various cellular components (protein, lipid, and DNA) and disrupt morphology and function of the cell membrane,mitochondria, and nucleus, thereby leading to apoptosis[22,25,30].Moreover, as ROS overproduction could interfere with important cellular signaling pathways, exposure of cells to SiONPs could, via ROS overproduction, interfere with these signaling pathways that modulate apoptosis, inflammation, and cell proliferation. In contrast,SiONPs-rats pre- or post-treated with ginseng alleviated SiONPsinduced oxidative stress. This ameliorative effect of ginseng restricted the extent of tissue damage and inhibited cytotoxicity induced by SiONPs, which could be attributed to the potent antioxidant activity of ginseng[15].
DNA damage usually occurs due to oxidative damage in cells[31].Our results revealed that the administration of SiONPs induced DNA damage in the lung as detected by the comet assay. This genotoxic effect was followed by apoptosis as indicated by significant increases in the expression of the apoptotic genes (Bax and caspase 3) and a significant decrease in the expression of the anti-apoptotic marker Bcl-2 in the lung. Consistent with our results, previous studies showed a similar effect (DNA damage and apoptosis) for silica NPs on HepG2 cells[5], colon cancer cells[6],and macrophages[7]. Nemmar et al.[32] also found that administration of silica NPs at a lower dose (0.25 mg/kg) resulted in a remarkable DNA damage in the lung, heart, liver, kidney, and brain of mice.These results imply that SiONPs could cause DNA damage and apoptosis by inducing oxidative stress in both normal (healthy) and cancer cells. This dual-purpose effect could be either beneficial(anti-cancer effect) or hazardous (oxidative damage to normal cells).Taken together, using SiONPs in chemotherapeutic preparation should be applied with caution to avoid their side effects on normal healthy cells. On the other hand, we found that treatment with ginseng ameliorated genotoxic and apoptotic effects induced by SiONPs. Similarly, Jin et al.[33] reported that ginseng could reduce DNA damage and apoptosis in the lung through the activation of endogenous antioxidant status and inhibition of ROS release.However, this effect was reversed in cancer cells where ginseng and ginsenoside induced apoptosis in cancer cells as revealed by increased expression of apoptotic genes (p53, p21, and caspase 3)and decreased expression of Bcl-2[34]. This infers that the function of ginseng and ginsenoside may differ depending on the target cells, normal (healthy), or cancer cells. Subsequently, ginseng could be synergically used with SiONPs to combat cancer cells and antagonize the toxic effects of SiONPs in normal tissues.
DNA damage following exposure to air pollution particles including silica NPs could originate not only from oxidative stress but also from inflammation[35]. In the present study, the lungs of rats administrated SiONPs exhibited significantly higher mRNA levels of inflammatory cytokines IL-1β, TNF-α, NF-κB, COX2, and TGFβ1 than the control group. These findings were supported by the results obtained from the histological examination which clearly showed signs of inflammation, such as invasion with mononuclear cells and congestion of blood vessels in lung tissue. Consistent with our findings, Hu et al.[10] and van der Zande et al.[8] reported upregulation of hepatic NF-κB and other inflammatory cytokines following oral administration of SiONPs and synthetic amorphous silica, respectively. The pulmonary inflammation induced by SiONPs and other silica NPs could be secondary to oxidative stress triggered by these NPs. Excessive release of cytokines was usually accompanied by severe pulmonary tissue damage[36]. These cytokines recruited leucocytes infiltration, especially neutrophils,increased permeability of lining endothelial cells, thereby leading to edema around alveoli. This effect might be due to the immediate effect of nanoparticles on the lining endothelial cells that could activate the release of nitric oxide and other vasodilatation factors[37].We found notable signs of inflammation including strong infiltration of mononuclear cells forming GLS containing condensed cells.Similar to our findings, Yang et al.[38] also found diffuse greyish white GLS at lung edges following intratracheal instillation of silica NPs in mice. Hassankhani et al.[3] showed severe lung lesions in mice lung after oral administration of silica NPs for a few days including hemorrhage, congestion, pneumonia, and infiltration of the inflammatory cells. The interstitial hemorrhage observed in the lung could be due to reduced vascular resistance induced by SiONPs or secondary to the inflammatory response[39].
According to the results of our study and Hassankhani et al.[3],histopathological alterations were observed in the lung following oral administration of silica NPs, proving that the lung is one of the leading targets for silica NPs accumulation. In contrast, no pulmonary lesions were observed in mice lung after intravenous injection of silica NPs[40]. These controversial results may be attributed to different sizes of used silica NPs. Indeed, smaller NPs(with an average size of 20 nm) were applied in the present study and the study of Hassankhani et al.[3], while Nishimori et al.[40]used larger ones (70 nm). Previous studies denoted that the size and dose are the main determinants for NPs toxicity with the highest cytotoxic effects at a smaller size and higher concentration[4,27].Xie et al.[12] reported that silica NPs (with sizes of 20- and 80-nm) can be accumulated in mice lung following intravenous injection and remained for 30 d due to endocytosis by macrophages.These macrophages did not only engulf silica NPs but also secret inflammatory cytokines, thereby initiating and maintaining inflammatory conditions[38].
Treatment with ginseng inhibited pulmonary inflammation as revealed by the downregulation of IL-1β, TNF-α, NF-κB, COX2,and TGFβ1 inflammatory cytokines. This effect may be attributed to the anti-inflammatory components present in ginseng. In other studies, ginseng showed a strong anti-inflammatory effect through suppression of IL-1β, IL-2, IL-4, IL-6, COX2, TNF-α, and inducible nitric oxide synthase[18,19]. Similarly, ginseng can also inhibit NF-κB and its downstream target COX2 in addition to the apoptotic marker p53 in lung cancer cells and lung tumors in mice[41]. Ginseng extracts also decreased TGFβ1, TNF-α, and IL-6 serum levels in a rat model of hepatic fibrosis[42].
Our results showed that SiONPs caused lung cell cycle arrest in the G/Gphase. In agreement with the results of the present study, Zhang et al.[43] reported that exposure to silica NPs induced G/G-phase cell cycle arrest and proliferation inhibition in mice spermatogenic cells. Taken together, it is likely that SiONPs and silica NPs could trigger cell cycle arrest and hinder cell proliferation by reducing the release of meiotic regulators, while oxidative stressdependent DNA damage could cause apoptosis. In contrast, other studies showed that silica NPs (with a size of 50 nm or more) caused cell cycle arrest in S and G/M phases of normal human hepatocyte cells (HL-7702), while no significant changes at the smaller size of 25 nm[44]. This contradiction may be attributed to variation in experiment type (in vivo vs. in vitro), target organ/cells (lung vs.hepatocytes), and time of exposure to NPs (sub-chronic vs. acute).Administration of ginseng restored the number of cells arrested in G/Gby SiONPs to a level comparable to that of the control groups.The effect of ginseng on the cell cycle phases was extensively studied on cancer cells, rather than normal cells. Li et al.[45] found that ginsenoside arrested cell cycle of colorectal cancer cells at G/Gand G/M phases through modulation of Wnt/β-catenin, PI3K/Akt, and NF-κB signaling pathways. Wong et al.[41] reported an arrest only in the G/M phase following exposure of lung carcinoma(LLC-1) cells to ginseng, and this effect was mediated by MAPK and p53 signaling. Collectively, ginseng can exert a cytostatic effect only in cancer cells and this effect differs according to the type of cancer cells.
Pre-treatment with ginseng conferred better improvement against SiONPs-induced pulmonary toxicities than post-treatment,indicating the importance of using ginseng as prevention better than treatment. Although these data could provide new insights regarding the ameliorative effect of ginseng on lung toxicity induced by SiONPs, the underlying mechanism of this effect remained to be determined at different exposure times (acute and chronic).Therefore, further studies are required to unveil this underlying mechanism.
In conclusion, ginseng, with its strong antioxidant and antiinflammatory properties, can relieve SiONPs-induced pulmonary toxicity through inhibition of oxidative stress, DNA damage,apoptosis, and inflammation in the lung. Thus, ginseng may be applied as an adjuvant treatment for pulmonary toxicity induced by SiONPs. Further studies are required to practically approve whether this treatment is applicable to humans.
Conflict of interest statement
The authors declare no conflict of interest.
Authors’ contributions
FE designed the work and drafted the article. RE collected data and performed data analysis. ME designed the work, conducted the experiment, performed data interpretation, and wrote the first and final versions of the article.
 Asian Pacific Journal of Tropical Biomedicine2021年6期
Asian Pacific Journal of Tropical Biomedicine2021年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Total flavonoids from Saussurea involucrata attenuate inflammation in lipopolysaccharide-stimulated RAW264.7 macrophages via modulating p65, c-Jun,and IRF3 signaling pathways
- Immunostimulatory effect of ethanol extract of Chondracanthus tenellus in RAW 264.7 macrophages in vitro
- Caffeic acid and protocatechuic acid modulate Nrf2 and inhibit Ehrlich ascites carcinomas in mice
- Potential of polyphenols in curbing quorum sensing and biofilm formation in Gramnegative pathogens
