Understanding autism spectrum disorders with animal models: applications, insights, and perspectives
Zhu Li, Yuan-Xiang Zhu, Li-Jun Gu, Ying Cheng,*
1 Institute of Biomedical Research, Yunnan University, Kunming, Yunnan 650500, China
ABSTRACT
Autism spectrum disorder (ASD) is typically characterized by common deficits in social skills and repetitive/stereotyped behaviors.It is widely accepted that genetic and environmental factors solely or in combination cause ASD.However, the underlying pathogenic mechanism is unclear due to its highly heterogeneous nature.To better understand the pathogenesis of ASD, various animal models have been generated, which can be generally divided into genetic, environment-induced,and idiopathic animal models.In this review, we summarize the common animals used for ASD study and then discuss the applications, clinical insights,as well as challenges and prospects of current ASD animal models.
Keywords: Autism spectrum disorders; Animal models; Neurodevelopment; Genetics;Environment
INTRODUCTION
Autism spectrum disorder (ASD) comprises a clinically heterogeneous group of disorders characterized by social deficits, narrow interests, stereotyped behaviors, and verbal and non-verbal communication difficulties (Sandin et al.,2017).The worldwide incidence of ASD has dramatically increased in the past several decades, increasing from 1/150(0.67%) in 2000 to 1/54 (1.85%) in 2016 in American children,with a 4.3 times higher incidence in boys than in girls(Maenner et al., 2020).In China, the most extensive population-based study (~120 000 children) indicates that the prevalence of ASD in Chinese children aged 6 to 12 is~0.70%.According to China’s 2016 national census data, this estimate translates to approximately 700 000 Chinese children(6 to 12 years) with ASD (Zhou et al., 2020).As early as 1943,American pediatric psychiatrists reported on clinical autistic symptoms in 11 children, which became an important basis for the diagnosis of ASD (Kanner, 1968).However, there is still a lack of practical methods for the diagnosis and treatment of ASD, mainly due to high disease heterogeneity.ASD is not only a medical problem for patients but also an urgent social problem, imposing a heavy mental and financial burden on families and society (Manoli & State, 2021).Therefore,systematic investigations on the pathogenic regulators of ASD are critical to provide theoretical and experimental support for the development of new clinical diagnosis, treatment, and intervention measures.
As indicated in twin and family studies, development of ASD is predominantly attributed to genetic factors (Sandin et al.,2017; Tick et al., 2016).Dozens of rare Mendelian disorders,including fragile X syndrome, neurofibromatosis, Rett syndrome, tuberous sclerosis complex, and structural chromosomal variations, are also considered high risk factors for the development of ASD (Bourgeron, 2015; Geschwind &State, 2015).Genome-wide studies have identified hundreds of risk loci for ASD, with a considerable number also related to other neurodevelopmental diseases such as schizophrenia and intellectual disability (ID) (Geschwind & State, 2015).Copy number variants (CNVs) are another major factor responsible for ASD etiology (Chung et al., 2014).Recent studies have also revealed the contribution of raredenovosingle nucleotide mutations, which together contribute significantly to the pathogenesis of ASD (Bourgeron, 2015;Gaugler et al., 2014; Geschwind & Flint, 2015; Geschwind &State, 2015).Dysregulated immune response during early embryonic development due to environmental factors, such as chemical exposure, infection, inflammation, and emotional health of pregnant mothers, can also increase ASD risk(Ornoy et al., 2015).
Given the difficulties in obtaining samples from ASD patients, animal models that restore the clinical features of ASD are the best choice for exploring the pathogenic regulators of ASD (Wintler et al., 2020).The establishment and selection of a stable and reliable animal model of ASD is critical for elucidating the relationship between the central nervous system (CNS) and ASD pathogenesis and for investigating pathophysiological processes, behavioral changes, diagnosis, and treatment.To date, many ASD animal models have been developed, and each animal species and modeling method has its own unique advantages and disadvantages.In this review, we first summarize the standard animals used to study ASD, and then discuss the applications and clinical insights of ASD animal models based on genetic and environmental factors.Finally, we discuss the challenges and prospects of current ASD animal models.This review should provide a better understanding of ASD research and treatment.
COMMON ANIMALS USED IN ASD STUDY
Impaired social relationships and repetitive/stereotypic behaviors distinguish ASD from other developmental disorders(Rapin & Tuchman, 2008; Yenkoyan et al., 2017).Therefore,advances in the pathophysiology of ASD symptoms are classified into two categories: social communication/interaction and patterns of behavior (Mukherjee, 2017; Peretti et al., 2019) (Figure 1).Social communication and interaction deficits include poor eye contact, lack of facial expressions,flat affect, delayed (or no) speech, difficulty in understanding questions and directions, aggressive or disruptive, repeated phrases or words, and resistance to cuddling and holding.Patterns of behavior are highlighted as repetitive movements like handshaking, spinning, or rocking; hyperactivity; difficulty in body movement coordination; sensitivity to light, sound, or touch; self-harming activities such as head-banging; and specific food preferences or food patterns.
Non-human primates (NHPs), domestic animals, rodents,birds, fish, and invertebrates have been used as animal models to mimic the clinical features of ASD (Figure 1;Table 1).In this section, we summarize those animals used in ASD study and how their research advantages benefit our understanding of the pathogenesis of ASD.
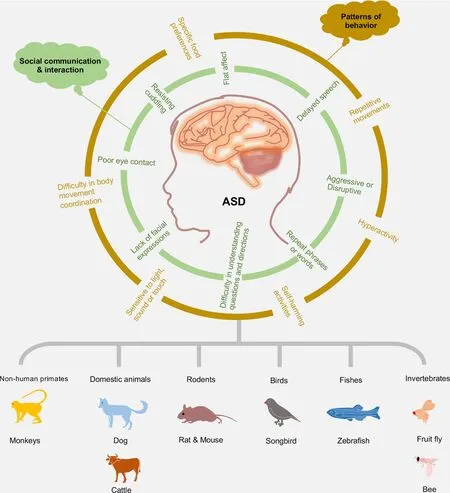
Figure 1 Diverse animal models of ASD
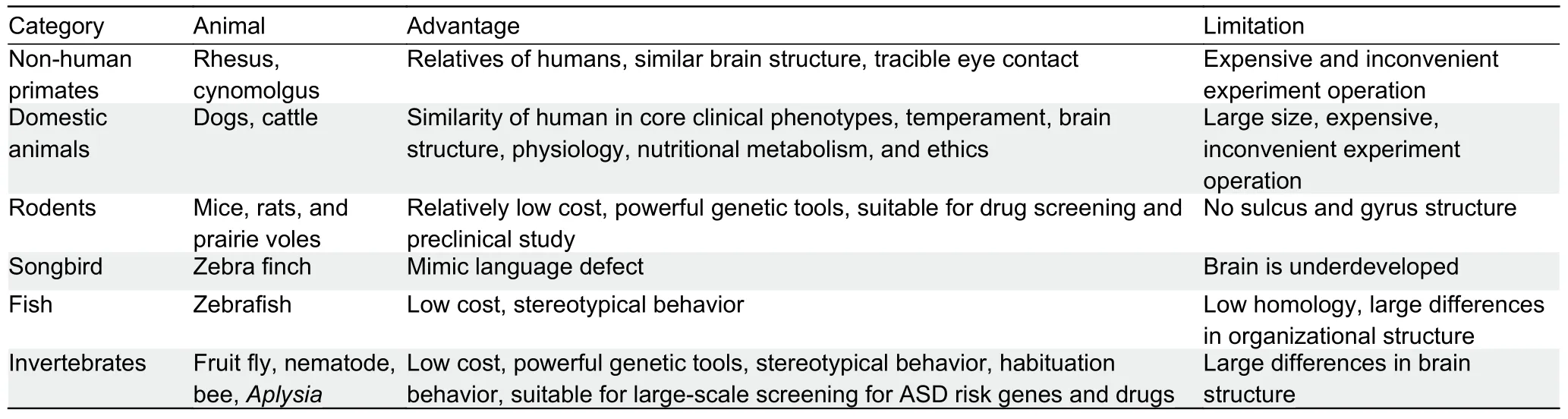
Table 1 Advantages and limitations of different animals in ASD modeling
NHPs
As animal models, monkeys, including rhesus (Macaca mulatta) and crab-eating macaques (M.fascicularis), best simulate human social behavior.Compared with rodents that have evolved for more than 70 million years, macaques separated from human evolution nearly 25 million years ago,and thus show greater similarity to humans in terms of genetics, neurobiology, and behavior (Kumar & Hedges, 1998;Rat Genome Sequencing Project Consortium, 2004).
Macaques mature about four times faster than humans.However, the early social development of macaque infants is similar to that of human infants in many aspects (Weinstein &Capitanio, 2012; Weinstein et al., 2014), including complex social groupings, communication through facial expressions,body language, and sound (Testard et al., 2021), and behavioral defects (Ghazanfar & Santos, 2004).In addition,several key brain regions associated with social behavior in macaques are similar to those in humans (Bauman &Schumann, 2018).For example, the amygdala has a very similar nuclear structure, neurochemical distribution,connectivity, and functional characteristics as humans and other NHPs (Rutishauser et al., 2015), whereas nuclear distribution differs significantly in rodent brains (Chareyron et al., 2011; Pitk?nen & Kemppainen, 2002).
The Social Responsiveness Scale (SRS) provides a quantitative measure of behavioral variability to identify individuals who do not meet the diagnostic criteria for ASD but still exhibit atypical social behaviors compared to the general population (Constantino et al., 2006).It is adaptive to NHPs.For instance, well-trained caregivers using the monkey SRS can identify macaques that exhibit atypical social response patterns related to social avoidance, social anxiety/rigidity,lack of social self-confidence, and social embarrassment(Feczko et al., 2016).Moreover, chromosome-scale genome assemblies of NHPs not only allow us to better understand these animal models but also provide an important basis for human biomedical research (Jayakumar et al., 2021).However, although NHP models can bridge rodent models of human diseases, increased costs and ethical considerations limit their use in research.
Domestic animals
Several studies have proposed that domestic animal models are complementary to traditional rodent models.For example,the dog is an innovative and unique model for many human neuropsychiatric diseases, including ASD (Bunford et al.,2017; Sándor et al., 2021).The advantages of dog models include: (a) significant inter-individual differences in social cognitive performance in dogs; (b) greater phenotypic similarity between dogs and humans than between rodents and humans; (c) symptoms that are functionally similar to the human condition; and (d) similar causes in dogs than in rodents (Topál et al., 2019).Cattle have also been utilized to study the molecular mechanism underlying ASD.A recent study tested enrichment of polymorphisms associated with cattle temperament in genes involved in four characteristics of human psychosis and personality disorder, including schizophrenia, ASD, neuroticism, and developmental delay disorder (Costilla et al., 2020).As relatively advanced mammals, domestic animals have a similar brain structure,physiology, and nutritional metabolism to humans.However,due to their large size, their maintenance and experimental operation can be inconvenient and expensive.
Rodents
Rodents (namely mice and rats) have similar neuroanatomy,biochemistry, electrophysiology, and genetics to humans.As classic animal models, they are widely used in basic scientific studies and preclinical trials due to the advantages of low price, short pregnancy, and many offspring (Simmons et al.,2021).Other rodents, such as prairie voles, have a stable spousal relationship and parental behavior, which is beneficial for studying social cognitive defects and ASD (Donovan et al.,2020).Most current ASD animal models are generated in mice and rats and will be discussed in detail in the following sections.
Birds
Little is known about the neural and genetic basis of human language development and related neurodevelopmental disorders (including ASD), in which language deficits are considered a comorbidity.Although no animal model can fully capture the behavior and genetic complexity of ASD, as an experimental language learning animal model, songbirds can supplement the shortcomings of rodent genetic models and provide essential insights into communication deficiencies.For instance, zebra finches communicate through learned vocalizations and can be used to simulate language communication disorders (Ahmadiantehrani & London, 2017;Hacohen-Kleiman et al., 2020; Panaitof, 2012).Moreover,recent genomic research has identified potential genetic substrates for the evolution and regulation of vocal communication (Warren et al., 2010).Furthermore, expression of the ASD geneFOXP1is reported to cause severe impairment in speech/language learning and affect the cultural transmission of bird songs between adult and juvenile zebra finches (Garcia-Oscos et al., 2021).
Fish
Zebrafish serve as a model system due to their precocious development, small transparent larval morphology, sensitive pharmacology, and genetic and physiological similarities to humans (de Abreu et al., 2020; James et al., 2021).For neurological research, zebrafish have been widely used in studies on brain development, synaptic growth, and other functions that regulate the CNS, making it a powerful tool for studying ASD (de Abreu et al., 2021; Gawel et al., 2020; Rea& Van Raay, 2020).With homologous preference and community aggregation, zebrafish can be used to study social behaviors (Tang et al., 2020).The phenotypic characteristics of zebrafish can be reliably evaluated by automatically reconstructing three-dimensional swimming trajectories(Stewart et al., 2015).
Invertebrates
The fruit fly (Drosophilamelanogaster) is a unique and powerful genetic model organism with a high degree of genetic conservatism and easy genome manipulation, and thus can be used to study a wide range of biological issues(Cheng & Chen, 2018).Drosophilacan help identify genes associated with ASD (Hope et al., 2019).For instance,Drosophilafed different doses of bisphenol propane show more repetitive behavior (grooming behavior) and abnormal social interactions (shorter distance from individual flies)compared with the controls (Kaur et al., 2015).Recent research on 286 gene orthologs implicated in ID with or without comorbid ASD, specifically inDrosophilaneurons, with light-off jump habituation testing, identified nearly 100 ID genes that regulate habituation learning, with some“habituation-deficient” genes implicated in ASD (Cheng & Jin,2019; Fenckova et al., 2019).
In addition toDrosophila, other invertebrates such as bees(Kocher et al., 2018),Caenorhabditiselegans(Calahorro &Ruiz-Rubio, 2011), andAplysia(Choi et al., 2011) have been used for ASD study.Although there are significant differences in brain structure between invertebrates and humans, these animals can be utilized as models to study the underlying mechanism of ASD.
DIVERSE ANIMAL MODELS EXPLORING KEY FEATURES OF ASD
Based on different construction methods, animal models of ASD mainly include genetic models, environment-induced models, and idiopathic models (Figure 2).
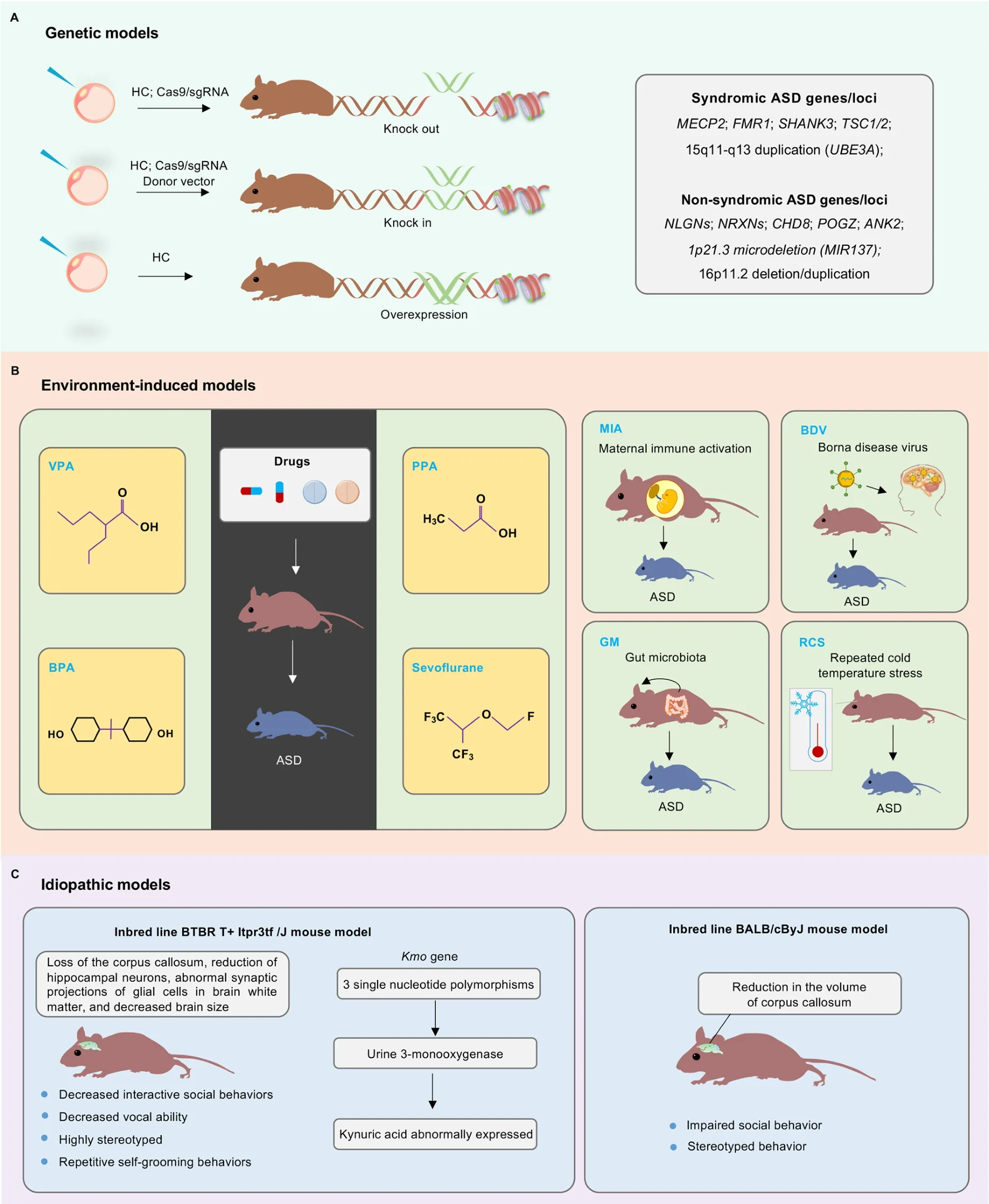
Figure 2 Schematic of major animal models of ASD
Genetic models
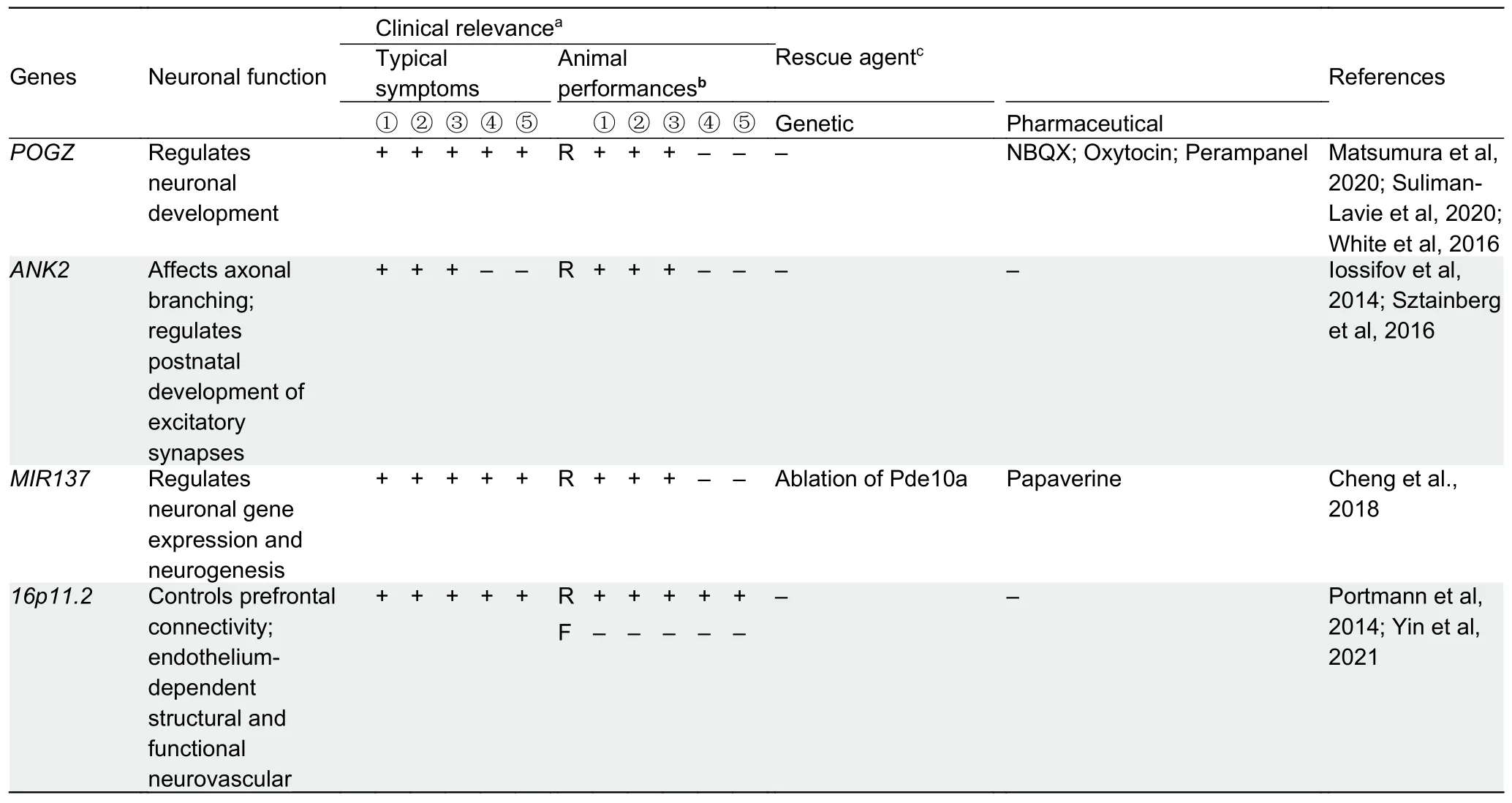
The genetic risks of ASD are conferred by hundreds of genes(Satterstrom et al., 2020), broadly affecting various biological processes, such as synaptic function and neuronal activity,postsynaptic density protein metabolism, neuronal cell adhesion, WNT signaling, and chromatin remodeling in the process of neurogenesis (Abrahams & Geschwind, 2008;Miles, 2011).Advanced sequencing and gene-editing technologies, such as homologous recombination and CRISPR/Cas9, have together resulted in the rapid development of genetic animal models of ASD (Figure 2A).To date, more than 200 ASD risk genes/loci are targeted to generate corresponding animal models (https://gene.sfari.org/database/animal-models/genetic-animal-models/).In this section, we discuss the animal models of several typical syndromic and non-syndromic ASD genes/loci.Many of these genetic models have been used to examine the rescue effectiveness of pharmaceutical agents, as summarized in Table 2.
Syndromic ASD genes:
MECP2:Rett syndrome (RTT) is a progressive neurodevelopmental disorder that causes mental retardation,with an incidence of 1/10 000–15 000 (Amir et al., 1999).It is frequently classified within ASD given their shared clinical symptoms of repetitive movements, impaired motor coordination, and social withdrawal.The primary cause of RTT is mutation of the methyl CpG binding protein 2 (MECP2)gene, a typical monogenic cause of ASD.MECP2is located on the X chromosome, andMECP2mutation in males is usually lethal; thus, RTT affects females almost exclusively(Amir et al., 1999).Mouse models ofMECP2mimic the symptoms of RTT, including impaired social behavior (Amir et al., 1999; Li et al., 2020a; Orefice et al., 2019; Pizzamiglio et al., 2021).
At present, classicMecp2-mutant mice include deletion of exon 3 (Chen et al., 2001), deletion of exons 3 and 4 (Guy et al., 2001), and 308X point mutations (Shahbazian et al.,2002).AlthoughMecp2-knockout mice show most of the developmental and behavioral defects of patients with RTT,identifying ASD-like behaviors inMecp2-overexpressing mouse models can be challenging (Collins et al., 2004).Mecp2-knockout mice have also been used to explore the regulatory mechanisms of other ASD risk genes, such asMir137, whose expression is controlled by Mecp2 in a promoter binding-dependent manner (Szulwach et al., 2010).In rat models, loss ofMecp2results in growth retardation,malocclusion, lack of exercise, weak forelimb grip, and significant social communication defects (Wu et al., 2016).
Transgenic cynomolgus monkeys (Macacafascicularis)overexpressing humanMECP2show ASD-like behaviors and stable inheritance of transgenic germlines (Liu et al., 2016).Moreover, aMECP2mutant cynomolgus monkey model generated using transcriptional activator-like effector nuclease(TALEN) technology to edit theMECP2gene (Chen et al.,2017) has been applied to explore related phenotypes using unique eye-tracking tests and magnetic resonance imaging analysis, showing similar physiological, behavioral, and brain structural abnormalities to those in ASD patients (Zhang et al.,2019).Recently, the circuit abnormalities related toMECP2and autism-like traits in monkeys have been mapped to homogenous ASD subgroups, thus providing a new strategy to deconstruct the clinical heterogeneity of ASD (Cai et al.,2020).These studies suggest that NHP ASD models are feasible and reliable and can be applied to study ASD-related neural mechanisms and potential therapeutic interventions.

Table 2 Genetic animal models targeting classical ASD risk genes
Continued
FMR1:Fragile X syndrome (FXS) is mainly caused by the excessive expansion of the CGG trinucleotide in the 5'UTR of the fragile X mental retardation 1 (FMR1) gene, with a small portion caused by point mutations of theFMR1gene (Richter& Zhao, 2021).Boys tend to exhibit more severe clinical symptoms than girls, including ID, speech delay, anxiety,attention deficit disorder, hyperactivity, seizures, and physical deformities (Richter & Zhao, 2021).Similar toMECP2,FMR1is considered as another monogenic cause of ASD.
Loss ofFmr1in male mice is not lethal.The dendritic spines of cerebral cortex neurons inFmr1knockout mice are longer,thinner, and more curved than those in wild-type mice, and the dendritic spines of the apical dendrites are denser (Comery et al., 1997).Invivotwo-photon calcium imaging found that the adaptability of neurons inFmr1-knockout mice to repeated whisker stimulation is inadequate, suggesting that adaptive impairment of the cortical sensory circuit may be a potential cause of ASD tactile defense (He et al., 2017).In addition toFmr1-knockout mouse models, several clinically relevant mouse models have been generated.For example, the recurrent missense mutations inFMR1encoding protein FMRP (FMRP-R138Q) are reportedly associated with FXS(Collins et al., 2010).In a knock-in mouse model expressing the FMRP-R138Q protein, neuronal density increasing in the hippocampus is related to synaptic ultrastructural defects and increased surface expression of AMPA receptors (Prieto et al.,2021).Based on biochemical analysis, high-resolution imaging, electrophysiological recording, and behavioral testing, the R138Q mutation can lead to impaired long-term hippocampal enhancement and social cognitive deficits in mice (Prieto et al., 2021).Social dominance and hierarchy changes inFmr1-knockout rats have been evaluated through a penetrating experiment, providing new insight for understanding complex social dynamics in an Fmr1-dependent manner (Saxena et al., 2018).
SHANK3:SH3 and multiple ankyrin repeat domains protein 3(SHANK3) is a postsynaptic density protein that interacts with a variety of ionotropic and metabotropic glutamate receptors and is associated with the actin cytoskeleton (Duffney et al.,2013).SHANK3mutation is associated with ASD and Phelan-McDermid syndrome, with the latter characterized by global brain retardation, mental disability, speech delay or loss, and mild deformities (Tatavarty et al., 2020; Wang et al., 2020).InShank3-knockout mice, the cortex-striatum-thalamic loop in mutant mice is overactive, leading to impaired social behaviors (Wang et al., 2016).Early recovery of Shank3 expression inShank3-knockout mice can prevent the ASD-like behavior phenotype (Jaramillo et al., 2020).Loss of Shank3 in male rats does not lead to the enhanced social approach behavior that typically occurs after playback of pro-social ultrasonic vocalizations (Berg et al., 2018).
In addition to rodent models, the first heritableShank3bmutant zebrafish model also show ASD-like behavior and changes in synaptic protein homer1 and synaptophysin levels(Liu et al., 2018).Moreover,SHANK3-knockout cynomolgus monkeys have been generated (Tu et al., 2019; Zhou et al.,2019b), which show motor deficits, repetitive behaviors, social and learning disorders, and sleep disorders.
TSC1/2:Tuberous sclerosis complex (TSC) is an autosomal dominant disease characterized by tuber-like benign tumors in multiple organs (including the brain and kidney).It can develop into malignant tumors, often accompanied by ASD(Lam et al., 2018).TSC1orTSC2mutation can lead to a high incidence of complications in ASD, and the protein product dimerizes and negatively regulates mTOR signaling transduction in mammalian targets.
The cerebellum primarily controls motor activity, while its non-motor functions are also associated with psychiatric disorders, such as ASD (Wolf et al., 2009).For example,compared with TSC patients without ASD, TSC patients with ASD show hypermetabolism in the deep structure of the cerebellum based on functional imaging.Furthermore,compared with the control group, human induced pluripotent stem cell (hiPSC)-derived Purkinje cells (major cerebellum neurons) from patients with pathogenicTSC2mutations exhibit excessive mTORC1 pathway activation, neuronal differentiation deficiency, RNA regulation deficiency,decreased excitability, and lower synaptic activity (Sundberg et al., 2018).However, upon loss of TSC2, the expression levels of FMRP, glutamate receptor δ2 (GRID2), and pre- and post-synaptic markers (such as synaptophysin and PSD95)decrease in hiPSC-derived Purkinje cells (Sundberg et al.,2018).In mouse models, following specific deletion ofTsc1orTsc2in Purkinje cells, mice exhibit a battery of ASD behaviors, including impaired memory, repetitive behaviors,and altered vocalizations (Reith et al., 2013; Tsai et al., 2012).Since the absence of Purkinje cells is one of the most common anatomical abnormalities in ASD patients, this model provides experimental support for related pathophysiological studies.
15q11–q13 duplication (UBE3A):Among the known causes of ASD, duplication of human chromosome 15q11–q13, which contains large repeat sequences, is the most frequently associated cytogenetic abnormality.Disruption of this region causes different disorders, including autism, Angelman syndrome, and Prader-Willi syndrome (Pinto et al., 2010).Duplications of chromosome 15q11–q13 account for up to 3% of ASD, where increased levels of UBE3A, an E3 ubiquitin ligase, are usually observed (Baron et al., 2006; Smith et al.,2011).In patients with ASD, a mutation that impairs UBE3A phosphorylation (p.T485A) can cause elevated UBE3A activity (Iossifov et al., 2014; Yi et al., 2015).Increased expression of UBE3A negatively regulates the function of cerebellin 1 precursor (Cbln1) in the ventral tegmental area,resulting in impaired synaptic transmission and sociability(Krishnan et al., 2017).In mice, ASD-like behaviors can be induced when expressing excessive UBE3A, resulting in impaired retinoic acid (RA)-mediated neuronal homeostatic synaptic plasticity (Xu et al., 2018).UBE3A can regulate ALDH1A2, the rate-limiting enzyme of RA synthesis, and administration of an ALDH1A antagonist can rescue impaired social behaviors associated with ASD (Xu et al., 2018).Other genes involved in 15q11–q13 and their corresponding animal models have been reviewed extensively (see Takumi, 2011).
Non-syndromic ASD genes:
NLGNs:Neuroligins (NLGNs) are cell adhesion molecules on the postsynaptic membrane and consist of excitatory and inhibitory synapses.NLGNs interact with neurexins (NRXNs)to promote the formation of presynaptic and posterior membranes (Vieira et al., 2021).The fiveNLGNgenes expressed in humans (NLGN1,NLGN2,NLGN3,NLGN4X,andNLGN4Y) are all associated with ASD, withNLGN3andNLGN4most closely related to ASD symptoms (Heshmati et al., 2018).Indeed, animal models suggest thatNLGN-gene knockout can cause synaptic changes and abnormalities in neurotransmitters of the brain, leading to the emergence of ASD symptoms (Südhof, 2017).
BothNlgn1-knockout and Nlgn1-P89L-knock-in mice show spatial memory impairment, but onlyNlgn1-knockout mice show vigorous combing and stereotyped behavioral phenotypes (Blundell et al., 2010; Nakanishi et al., 2017).Nlgn2-knockout mice also exhibit ASD-like behaviors, normal social behaviors, increased anxiety-like behaviors, decreased pain sensitivity, and poor motor coordination (W?hr et al.,2013).
Mice withNlgn3gene deletion show impaired ultrasound vocalization and social deficits (Modi et al., 2019; Radyushkin et al., 2009).Mutation inNlgn3can lead to impaired oxytocin signaling in dopaminergic neurons and changes in the behavioral responses of mice to social novelty tests (H?rnberg et al., 2020).Additionally, Nlgn3-R451C-knock-in mice exhibit ASD-related behavioral phenotypes, including deficiency in social novelty preference, hyperactivity, and repetitive behavior, indicating thatNlgn3mutations damage striatal circuits, leading to repetitive behavior (Rothwell et al., 2014).Other studies suggest that abnormal gamma oscillations in the prefrontal cortex may be a leading cause of social behavior disorders in Nlgn3-R451C-knock-in mice.Social defects can be effectively reversed by manipulating the inhibitory effect of parvalbumin intermediate neurons in the prefrontal cortex(Cao et al., 2018).
NLGN4gene mutations are found in many patients with ASD and other neurodevelopmental disorders.For example,single amino acid substitution (R101Q) mutations in NLGN4 can cause synaptic dysfunction in autistic patients (Cast et al.,2021).InNlgn4-knockout mice, PSD-95 in the cerebral cortex and GABAA receptor and porphyrin immunoreactive synapse density in the hippocampal CA3 region are significantly decreased (Hammer et al., 2015).
NRXNs:Neurotoxins (NRXNs) serve as the presynaptic binding ligand of NLGN and play important roles in synaptic adhesion, differentiation, and maturation (Missler et al., 2003).At present, most models come from mice in which all three genes (NRXN-1,NRXN-2, andNRXN-3) are knocked out or from NRXN-1α/2α double knockout mice.Compared with wildtype mice, triple- and double-knockout mice show fewer inhibitory synapses in the brainstem and neocortex,respectively (Missler et al., 2003).
ASD patients often show high comorbidity with attention deficit hyperactivity disorder (ADHD).In rats, loss ofNrxn-1αresults in significant non-social cognitive deficits and hyperactivity, similar to ADHD (Esclassan et al., 2015).CNTNAP2, which encodes contactin-associated protein-like 2 protein, is another member of the neurexin family.TheCNTNAP2gene is indispensable in promoting dendritic axons,maintaining synaptic stability, and transporting transmitters(Gdalyahu et al., 2015; Varea et al., 2015).Mice withCntnap2mutations exhibit behavioral defects, hyperactivity, and seizures (Pe?agarikano et al., 2011).Other studies have revealed that loss ofCntnap2in rats can lead to changes in social interaction, stereotyped behavior, and sensory behavior(Scott et al., 2020).Interestingly, recent studies on songbirds have shown that, across taxonomic classes,Cntnap2plays an important role in neural connectivity critical for vocal learning(Condro & White, 2014).
CHD8:Chromodomain helicase DNA-binding protein 8(CHD8) controls epigenetic and transcriptomic regulation and acts as a master regulator of many ASD risk genes (Cotney et al., 2015).Mutation of CHD8 is a highly penetrant risk factor of ASD (De Rubeis et al., 2014; Talkowski et al., 2012; Werling et al., 2018).
As a recently identified ASD gene, the functions of CDH8 have been investigated in several animal models.For example, CHD8 ortholog knockdown in mice increases brain weight and volume and in zebrafish results in macrocephaly,consistent with the macrocephaly of ASD patients with CHD8 mutations (Bernier et al., 2014; Katayama et al., 2016; O'Roak et al., 2012; Platt et al., 2017).CHD8 haploinsufficiency in mice causes ASD-like phenotypes associated with a delay in neuronal development and small but global changes in the expression of many genes in the brain during development(Katayama et al., 2016).In germline heterozygous frameshift Chd8 mutation mice,Chd8+/del5mice show standard core features of ASD, including social interactions and repetitive behaviors, but also exhibit cognitive impairment correlated with increased regional brain volume (Gompers et al., 2017).
There is a male bias (84%) in autistic subjects with CHD8 mutations (n=24) (Stessman et al., 2017).In mice carrying heterozygous mutation ofChd8(Chd8+/N2373K), which mimics human CHD8 (Asn2373LysfsX2) mutation, male mutant mice display a range of abnormal ASD-like behaviors during development from pup to adult, but their female counterparts do not (Jung et al., 2018).The differential patterns in male and femaleChd8+/N2373Kmice are determined by sexually dimorphic changes in neuronal activity, synaptic transmission,and transcriptomic profiles, validating that human CHD8 mutation indeed results in sexually dimorphic changes in mice(Jung et al., 2018).
Moreover, recent study has also shown that cerebellar granule neuron progenitor (GNP)-specific deletion of CHD8 in mice impairs cellular proliferation and differentiation, and leads to cerebellar hypoplasia and motor coordination defects, but not to ASD-like behavioral abnormalities (Kawamura et al.,2021).
POGZ:Pogo transposable element derived with ZNF domain(POGZ) is widely expressed in human tissues, including the brain.Expression analysis has shown that POGZ is expressed in the mouse cortex and hippocampus in the early developmental stages but decreases in the nucleus of both cortical and hippocampal neurons at postnatal day 30 (P30)(Ibaraki et al., 2019).In the developing cerebellum, POGZ is mainly detected in the nucleus of Purkinje cells, while at P15 and P30, POGZ expression is observed in the granular and molecular layers (Ibaraki et al., 2019).
Recent studies provide compelling evidence that loss-offunction mutations in POGZ are associated with abnormal development and behavior (Stessman et al., 2016; White et al., 2016; Ye et al., 2015).POGZ is one of the chromatin regulators of several genes implicated in ASD (De Rubeis et al., 2014; Suliman-Lavie et al., 2020).Many individuals with POGZ mutations, known as White-Sutton syndrome, manifest ID, ASD, specific facial features, and other phenotypic spectra(Fukai et al., 2015).A recent study suggested that ASD-relateddenovomutations in Pogz impair neuronal development in the developing mouse brain and induce pluripotent cell lines from an ASD patient (Matsumura et al.,2020).In addition, mice with heterozygousdenovoPogz mutation exhibit ASD-like abnormalities and reduced anxietyrelated avoidance (Cunniff et al., 2020; Matsumura et al.,2020).Nervous system-specific deletion of Pogz in mice leads to microcephaly, impaired growth, and ASD-like behaviors that mimic several human symptoms, suggesting that Pogzdependent heterochromatin dysregulation can lead to cerebellar circuit dysfunction and behavioral abnormalities in ASD (Suliman-Lavie et al., 2020).
ANK2:ANK2 is a member of the ankyrin gene family, which encodes two primary ankyrin B (ankB) polypeptides, including a 440 kDa polypeptide (giant ankB) expressed only in the nervous system (Chan et al., 1993; Kunimoto, 1995), and a 220 kDa polypeptide expressed in various tissues.Clinical history shows that defect/mutation of giant ankB alone is sufficient to cause non-syndromic ASD.ANK2is not directly related to gene regulation or synaptic function, and most ASD patients diagnosed withANK2mutations are non-syndromic and show average intelligence (Iossifov et al., 2014;Sztainberg & Zoghbi, 2016).
Mice lacking the two ankB peptides die during the neonatal period, and long axon bundles are lost, including pyramidal tracts and corpus callosum (Lorenzo et al., 2014; Scotland et al., 1998).For the neuronal-specific giant ankB, giant ankB-mutant mice show increased axonal branching in cultured neurons and a transient increase in excitatory synapses during postnatal development, providing evidence that normal structural connections require a considerable amount of ankB in the CNS (Yang et al., 2019).Dysfunction of giant ankB leads to inheritable impaired communicative and social behaviors, as well as superior executive function, suggesting giant ankB-deficiency/mutation is a potential cause for the abnormal structural connectivity and penetrant behaviors in mice and humans carrying ASD-related ANK2 mutations(Yang et al., 2019).
1p21.3 microdeletion (MIR137):Among chromosome 1p21.3 microdeletion carriers, most have been shown to have ASD(11 out of 12 patients) and all have ID (Carter et al., 2011;D'Angelo et al., 2015; Tucci et al., 2016; Willemsen et al.,2011).Interestingly, although each patient exhibits different genomic deletions on chromosome 1p21.3, the minimal overlapping regions only include theMIR137gene.Genetic studies have also identified that theMIR137gene is highly associated with ASD, schizophrenia, and bipolar disorder(Duan et al., 2014; Pinto et al., 2014; Schizophrenia Psychiatric Genome-Wide Association Study (GWAS)Consortium, 2011).Thus, miR-137 appears to be a critical pathogenic regulator mediating the core clinical features of ASD in these patients.
To study the role of miR-137, we generatedMir137germline-knockout (gKO) and nervous system-knockout (cKO)mice and found that complete loss of miR-137 in gKO and cKO mice leads to postnatal lethality (Cheng et al., 2018).In contrast, heterozygous gKO and cKO mice are viable,indicating that all chromosome 1p21.3 microdeletion carriers are hemizygous.The partial loss of miR-137 in heterozygous gKO and cKO mice can cause synaptic overgrowth and impaired synaptic plasticity related to ASD, as well as impaired repetitive behaviors, learning, and social behaviors(Cheng et al., 2018).Multi-omics studies have revealed that one of the miR-137 mRNA targets, phosphodiesterase 10a(Pde10a), is elevated inMir137-knockout mice, and the application of the Pde10a inhibitor papaverine or lentivirusinduced Pde10a knockdown significantly ameliorates the deficits observed in heterozygousMir137-knockout mice.Given the known genetic link betweenMIR137and neuropsychiatric diseases, our study provides direct evidence that the dysregulation of miR-137 is involved in the molecular pathogenesis of ASD.
16p11.2 microdeletion and duplication:Microdeletion and microduplication at 16p11.2 (containing 29 genes) are strongly associated with autism, accounting for about 1% of cases(Weiss et al., 2008).To explore which of the 29 genes in this genomic region are critical for the corresponding phenotypes of 16p11.2 microdeletion and microduplication, Golzio et al.(2012a) conducted functional screening in a zebrafish model and identified KCTD13 as a key gene responsible for the mirrored neuroanatomical phenotypes in humans.
Given that 16p11.2 microduplication is also reported in schizophrenia (McCarthy et al., 2009), animal models used for ASD often focus on loss-of-function of 16p11.2.For example,16p11.2-deficient mice exhibit diminished prefrontal connectivity, thalamo-prefrontal miswiring, and reduced longrange functional synchronization (Bertero et al., 2018).Compared to wild-type male littermates, male mice with 16p11.2 heterozygous deletion (16p11.2+/–) produce strikingly fewer vocalizations during first exposure to an unfamiliar estrous female (Yang et al., 2015) and a complete lack of habituation similar to that observed in some autistic individuals(Portmann et al., 2014).In addition, partial loss of 16p11.2 can lead to endothelium-dependent structural and functional neurovascular abnormalities in16p11.2+/–male mice and in male mice with endothelium-specific 16p11.2 deletion,suggesting a potential role for endothelial impairment in ASD(Ouellette et al., 2020).Moreover, based oninvivotwo-photon imaging analysis in awake mice, layer 2/3 excitatory neurons in the motor cortex of adult male16p11.2+/–mice are dysregulated, showing overactivation during the initial phase of learning, with prolonged learning-induced spine reorganization (Yin et al., 2021).Local infusion or intraperitoneal injection of clozapine N-oxide (CNO) is sufficient to ameliorate these impaired cellular and behavioral phenotypes, suggesting a novel role of noradrenergic neuromodulation in improving delayed motor learning in16p11.2+/–male mice (Yin et al., 2021).
Environment-induced models
Environmental factors can also act as important pathogenic regulators in the etiology of ASD (Cheroni et al., 2020; Hertz-Picciotto et al., 2018).Children with ASD often show oxidative stress and methylation damage, which may be related to environmental pollution, exposure to chemical or toxic substances, and viral infection (B?lte et al., 2019; Varghese et al., 2017).In addition, when the mother is affected by certain antibodies and neurotoxins, her offspring can exhibit ASD-like symptoms.Animal models of ASD induced by drugs or other substances (~45 models) have the advantages of simple, fast,and low-cost operation.However, each animal model only reflects a few aspects of the possible pathogenesis of ASD(https://gene.sfari.org/database/animal-models/inducedanimal-models).Nevertheless, these environment-induced animal models can be used to study nervous system function,screen new drugs, and explore treatment protocols for ASD(Figure 2B; Table 3).
Drug-induced models:
Valproic acid (VPA):VPA was first used as an anti-epileptic drug and later as a mood stabilizer in epilepsy, bipolar disorder, migraine, and schizophrenia (Evatt et al., 2009).In animal experiments, researchers have simulated typical ASD models of newborn mice by exposing female mice to VPA during pregnancy.Prenatal and postnatal VPA exposure in mice can induce autism-like behaviors, and inflammatory cytokines and oxidative/nitrosative stress markers in the prefrontal cortex and hippocampal homogenate are increased in VPA-treated offspring (Elnahas et al., 2021).The degree of neuropathological changes depends on the dose and duration of VPA exposure (Bringas et al., 2013).Combined with histopathology and immunohistochemistry, the prenatal VPA model is better than the postnatal VPA model at inducing behavioral and neuropathological changes, thus highlighting the superiority of prenatal VPA exposure as a translation model for understanding the pathophysiology of ASD and developing new targets for treatment.In rat models where mothers are exposed to VPA, their offspring show changes in serotonin levels in the prefrontal cortex, hippocampus,cerebellum, and peripheral blood, similar to the results of current human clinical studies, suggesting that VPA application may be a good candidate for generating animal models of ASD (Sacco et al., 2010).
Propionic acid (PPA):PPA is a short-chain fatty acid, the final product of intestinal bacterial metabolism, and a common food preservative.Many studies have demonstrated that PPA can cause ASD-like behaviors and neuroinflammatory responses in rats.For example, rats treated with PPA show less interest in specific objects and weaker social behavior, as well as reactive astrocyte proliferation and microglia activation in brain tissue, indicating that PPA application can lead to a congenital neuroinflammatory response (MacFabe et al.,2011).The impacts of PPA on social behavior, anxiety behavior, and ultrastructure of central amygdaloid nucleus have also been explored in rats, with acute administration of a relatively low dose of PPA (175 mg/kg) found to significantly affect social behavior (Lobzhanidze et al., 2019).Although these results provide an experimental basis for using PPA to establish rodent ASD models, the direct correlation betweenPPA models of ASD and human ASD requires further verification.
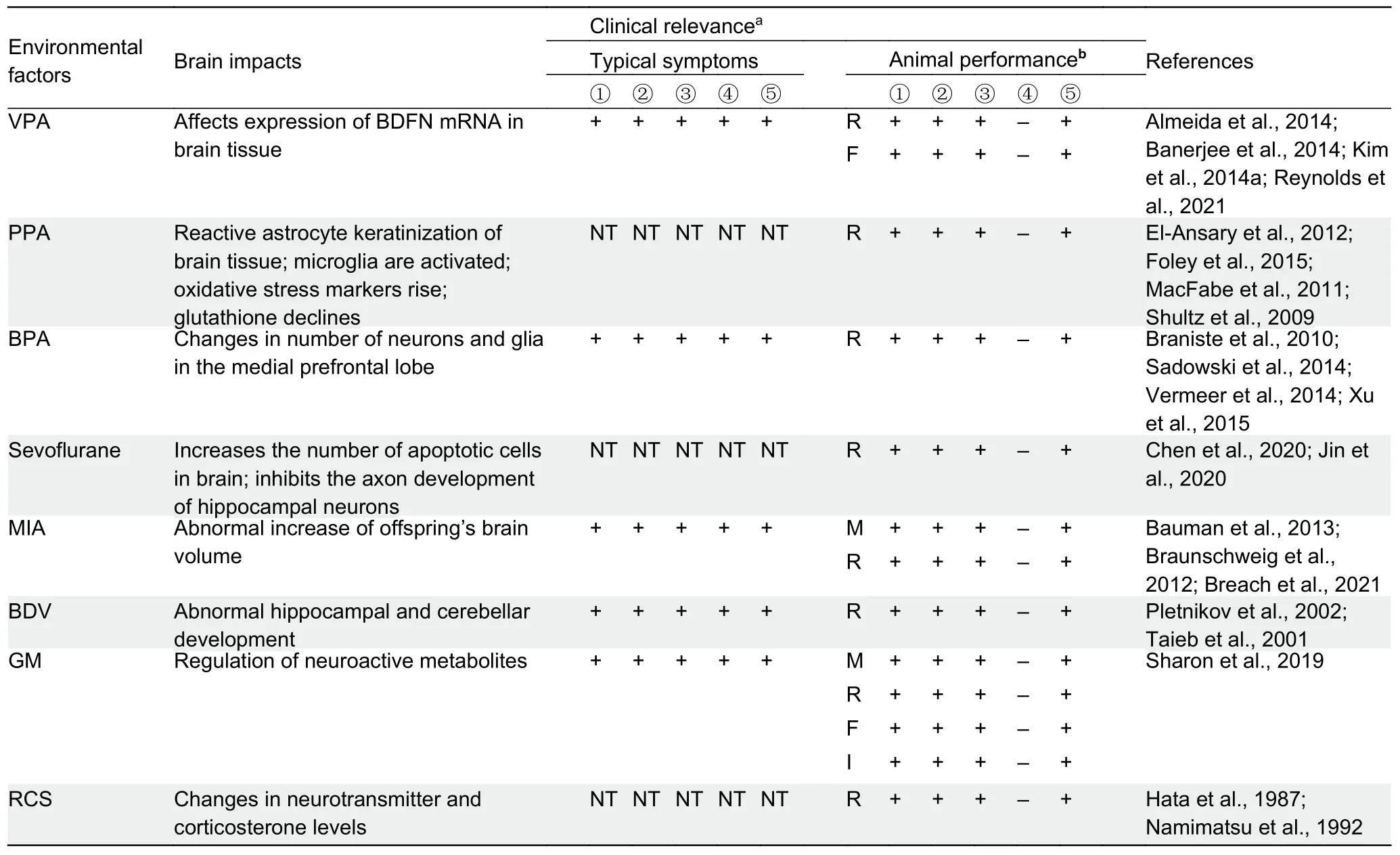
Table 3 Environmental-induced ASD animal models
Bisphenol propane (BPA):BPA is an important organic chemical material and a significant derivative of phenol and acetone and is widely used in the packaging of canned food and beverages.Various studies have provided evidence on neural correlates of human exposure to BPA.For instance,prenatal maternal exposure to BPA can lead to changes in the microstructure of white matter in preschool-aged children and plays an intermediary role in the relationship between BPA exposure in early life and internalization (Grohs et al., 2019).In addition, as an endocrine disruptor, exposure to BPA during fetal and perinatal periods can lead to abnormal neurodevelopment and behavioral changes in children(Elsworth et al., 2013; Nakamura et al., 2012).Studies exploring the relationship between endocrine interferon and ASD/ADHD have revealed that BPA is a low-dose developmental neurotoxic drug (Mustieles & Fernández,2020).
Social cognition tests on three consecutive generations of mice after BPA exposure during pregnancy show that these mice exhibit more exploratory behaviors compared with the controls (Wolstenholme et al., 2013).However, thirdgeneration mice cannot recognize new stimuli (strange mice),indicating long-term disruptive social memory in offspring.These results suggest that BPA may cause neurodevelopmental diseases in a cross-generational way(Wolstenholme et al., 2019).A recent study of 46 autistic children and 52 normal children found that those with ASD show a direct association with BPA exposure (Stein et al.,2015).Metabolomics analysis has also shown a correlation between ASD and the metabolic pathways of essential amino acids, i.e., precursors of neurotransmitters, such as tryptophan and serotonin.Furthermore, compared with age-matched nonautistic children, there are two fundamentally different types of serotonin synthesis abnormalities in autistic children, i.e.,overall brain capabilities and focal abnormalities (Sarrouilhe &Dejean, 2017).
In mouse models, during neural development,intergenerational exposure to BPA can disrupt social interactions in mice and the normal expression of the PSD gene (Wolstenholme et al., 2019).InDrosophila, the application of different doses of BPA (0, 0.1, 0.5, 1 mmol/L)can lead to differences in autonomous movement (Kaur et al.,2015).Drosophilatreated with BPA (especially 0.5 mmol/L BPA) also exhibit more ASD-related behaviors than the controls, including repetitive behaviors (grooming behavior)and abnormal social interactions (shortened distance from surrounding flies) (Kaur et al., 2015).
Sevoflurane:During the rapid and sensitive period of CNS development, harmful stimuli can cause serious consequences.The effect of anesthetic exposure during pregnancy on CNS development in offspring is still unclear but has aroused widespread concern.For instance, long-term exposure to sevoflurane, a commonly used general anesthetic in pediatrics, can lead to social defects in mice (Zhou et al.,2019a).Moreover, in addition to neurons, astrocytes may be important targets of sevoflurane toxicity, suggesting that the morphological integrity of astrocytes is essential for synaptic formation and neurobehavior (Zhou et al., 2019a).
In mouse models, narcotic sevoflurane can cause neurotoxicity in the developing brain, as well as adverse neurobehavioral outcomes, including inattention, insufficient social interaction, and learning and memory disabilities (Jin et al., 2020).Newborn mice inhaling sevoflurane exhibit dysfunction in learning ability (Satomoto et al., 2009).The number of apoptotic cells in the brain tissue of neonatal mice exposed to sevoflurane increase significantly after anesthesia,leading to the continuous loss of learning behavior in adulthood (Satomoto et al., 2009).Furthermore, mice exposed to sevoflurane exhibit impaired social communication, which is not caused by loss of interest or smell as they tend to show normal exploratory behavior and olfaction (Satomoto et al.,2009).These results indicate that exposure to sevoflurane during the neonatal period can lead to abnormal learning and social behaviors, resulting in ASD-like characteristics.
In pregnant mice exposed to sevoflurane when germ cells are epigenetically reprogrammed during embryonic development, >38% of F1 mice exhibited anxiety and social interaction disorders and 44%–47% of F2 and F3 mice without direct sevoflurane contact showed the same behavioral problems (Wang et al., 2021).In addition, in the sperm of F1 mice, more than 1 200 differentially accessible loci were identified, 69 of which were also found in the sperm of F2 mice, most of which were found in the regulatory regions of genes closely related to ASD, includingArid1b,Ntrk2, andStmn2(Wang et al., 2021).These results suggest a longlasting impact of sevoflurane in the pathogenesis of ASD,transmitting through male germlines between and across generations.
In rat models, compared with other brain regions of offspring, the hippocampus, a vital component of the brain involved in learning and memory, is more susceptible to repeated sevoflurane exposure (Chen et al., 2020).In addition to affecting motor sense and emotional and social behavior in offspring mice, repeated exposure to sevoflurane can cause memory deficits, probably by inhibiting the axonal development of hippocampal neurons (Chen et al., 2020).
Maternal immune activation (MIA) models:Many studies have shown that MIA in pregnant women can cause significant damage to fetal development.During pregnancy, maternal immunoglobin G (IgG) can provide passive immunity to the fetus through the placenta; however, protective and pathogenic antibodies will be transmitted to the fetus in the same way (Zimmerman et al., 2007).In the presence of autoimmune diseases or antigens, autoantibodies are activated during or before pregnancy, and damage caused by maternal autoantibodies to the fetus can cause congenital developmental disorders (Haddad et al., 2020; Zimmerman et al., 2007).
There is a known association between maternal IgG antibodies that are reactive to proteins in the fetal brain and autistic childhood outcome.Animal experiments have shown that maternal anti-fetal brain protein antibodies are closely related to offspring ASD-like symptoms (G?adysz et al., 2018).After injecting IgGinutero, offspring mice respond more positively to new things during adolescence but show anxietylike behaviors at the adult stage and react strongly to external stimuli (Ariza et al., 2017; Singer et al., 2009).In addition, in mice injected intravenously with brain-reactive IgG antibodies from the “mothers of autistic children” and “mothers of normal children”, offspring exposed to IgG antibodies from the“mothers of autistic children” show impaired sensory and increased anxiety, suggesting that maternal IgG in autistic children can cause long-term behavioral abnormalities(Braunschweig et al., 2012).Similarly, in a maternal autoantibody model of rhesus monkeys, offspring brain volume is abnormally increased (Bauman et al., 2013).
Maternal infections or fever during pregnancy are also associated with autism or developmental delay (Zerbo et al.,2013).For example, the pro-inflammatory cytokine interleukin-6 (IL-6) has been identified as the cause of the ASD-like phenotype associated with prenatal exposure to MIA (Kumari et al., 2020).Moreover, tumor necrosis factor α (TNF-α), IL-2,IL-6, and IL-17A are also important in the pathogenesis of ASD (Eftekharian et al., 2018).For example, recent evidence suggests that the underlying inflammatory pathway links MIA-related ASD with the activity of T helper 17 (Th17)lymphocytes and its effector IL-17A.Furthermore, antibody blockade of IL-17A signaling can prevent ASD-like behavior in offspring exposed to MIA (Wong & Hoeffer, 2018).
Borna disease virus (BDV) models:BDV is a widely distributed, non-segmental, non-cytolytic neurotrophic, singlestranded RNA virus.It can infect vertebrates and lead to multiple immune-mediated CNS diseases, depending on various host and viral factors (Taieb et al., 2001).In animal infections, BDV can persist in the CNS and lead to changes in brain cell function, neurodevelopmental abnormalities, and behavioral disorders (Taieb et al., 2001).Although BDV infection has been observed in both humans and animals, the epidemiology of BDV in humans, especially children, is unclear.Based on immunofluorescence, western blotting, and radioligand analysis, Honda et al.(2018) reported on the prevalence of BDV antibodies (7.4%) in Japanese ASD children, thus providing valuable baseline data on the epidemiology of BDV in children for future research.Therefore, as a unique teratogenic factor, BDV could be used to study the pathophysiological mechanism underlying the interaction between heredity and the environment and to help carry out preclinical drug treatment trials.Additionally, in BDV-induced ASD rat models, infected young rats exhibit various behavioral dysfunctions, including impaired sensory, motor,social, emotional, and cognitive functions (Pletnikov et al.,2002).
Gut microbiota (GM) models:Traditionally, the core symptoms of many neurological diseases are considered to involve genetic variations that affect brain development and function.However, as a new research hotspot, the gut microbiome is now recognized as another important source of variation that can also affect specific behaviors of ASD (Wu et al., 2021).For example, recent studies have shown that GM can affect mouse behavior by regulating neuroactive metabolites, indicating that the gut-brain axis may be closely related to the pathogenesis and pathophysiological processes of ASD (Hsiao et al., 2013).Transplant experiments have confirmed that the GM in ASD patients is sufficient to induce obvious ASD-like behaviors (Sharon et al., 2019).InCntnap2-knockout mice, it has been shown that the microbiome and host genes unexpectedly regulate impaired behaviors in an interdependent manner (Buffington et al., 2021).Furthermore,the hyperactive phenotype ofCntnap2-knockout mice is caused by host inheritance, while the gut microbiome mediates the social behavior phenotype.Interestingly, specific microbial interventions can selectively save social deficits inCntnap2-knockout mice by up-regulating metabolites in the tetrahydrobiopterin synthesis pathway.These results indicate that behavioral abnormalities may have different origins (host genetics and microbes), changing the way we think about neurological diseases and their treatment (Buffington et al.,2021).
Repeated cold temperature stress (RCS) models:Other environment-induced models, such as RCS, can lead to changes in neurotransmitter and corticosterone levels in the rat brain (Hata et al., 1987; Namimatsu et al., 1992).Rats exhibit impaired locomotor activities and anxiety after RCS(Hata et al., 1988).Moreover, offspring of rats treated with RSC (during pregnant days 9–12.5) show autism-like behavioral abnormalities (Tazumi et al., 2005).Given its operational reproducibility, the RCS model has become a commonly used autism model, but the underlying mechanism is currently unclear; thus, its reliability needs to be further demonstrated.
Idiopathic models:The etiology of ASD is complex, and various factors together lead to the pathogenesis of ASD.Genetic and environment-induced models cannot simulate all pathological features of ASD.Therefore, strains of mice and rats that better mimic the core symptoms of ASD can be generated using idiopathic models, which are helpful for identifying novel ASD risk genes.At present, eight inbred lines have been used as idiopathic models, most of which are based on BTBR T+Itpr3tf/J and BALB/cByJ strains(https://gene.sfari.org/database/animal-models/inbred-animalmodels/; Figure 2C).
Inbred line BTBR T+Itpr3tf/J mouse model:The BTBR T+Itpr3tf/J (BTBR) mouse strain is a widely used animal model of ASD (Meyza & Blanchard, 2017; Queen et al., 2020).BTBR mice show behavior consistent with most core clinical features of ASD and exhibit stable progeny replication (Endo et al., 2019; Meyza & Blanchard, 2017).In behavioral experiments, BTBR mice show decreased interactive social behaviors, decreased vocal ability, and highly stereotyped and repetitive self-grooming behaviors (Dodero et al., 2013;Ellegood et al., 2013).The abnormal behaviors of BTBR mice are mainly caused by three single nucleotide polymorphisms in theKmogene, which encodes urine 3-monooxygenase(McFarlane et al., 2008).Urine 3-monooxygenase can regulate the synthesis of kynurenic acid, one of the metabolites of tryptophan, which is abnormally expressed in other mental diseases such as schizophrenia (McFarlane et al., 2008).
Clinically, ASD patients often show corpus callosum hypoplasia or corpus callosum volume reduction, leading to speech disorders and social communication disorder symptoms (Frazier & Hardan, 2009).Based on imaging studies, the most significant neuroanatomical features of BTBR mice include loss of the corpus callosum, extreme reduction of hippocampal neurons, abnormal synaptic projections of glial cells in brain white matter, and decreased brain size (Stark et al., 2008).At the same time, gray matter volume in BTBR mice is reduced in the ventral tegmental area, cingulate gyrus, lateral thalamus, posterior thalamus,occipital and parietal cortices, and subcortex, but increased in the olfactory bulb, medial prefrontal and insular cortices,amygdala, and dorsal hippocampus (Pagani et al., 2016).These findings are consistent with the continuous decrease of gray matter volume in the brains of clinical ASD patients over time.Thus, abnormalities in other brain structures of BTBR mice may be helpful in clinical diagnosis in future studies.
Inbred line BALB/cByJ mouse model:Compared with highly social inbred mice, such as C57BL/6J and FVB/NJ, the inbred BALB/cByJ mouse strain shows significant social disorder and stereotyped behavior.Imaging studies have demonstrated that BALB/cByJ mice have a reduced corpus callosum volume.However, BALB/cByJ mice require a specific control group and do not fully present all typical clinical symptoms of ASD (Fairless et al., 2008, 2012).
CLINICAL INSIGHTS OF ASD ANIMAL MODELS
There is currently no effective treatment for ASD, resulting in a heavy burden on individuals, families, and society.Many drug therapies only treat peripheral symptoms, such as aggression,anxiety, and depression, rather than improving the core symptoms of ASD and lack effectiveness and safety.Therefore, systematic research and evaluation of innovative treatment methods to ameliorate the core social defects of ASD are needed (Anagnostou & Hansen, 2011; Doyle &McDougle, 2012a, 2012b).
Based on ASD animal models, the effects of hundreds of genetic and pharmaceutical rescue agents have been examined, indicating the great value of these models.For example, it has been shown that mTOR signaling is significantly associated with the neurological and behavioral phenotypes of certain ASD animal models, such asTsc1/2-mutant mice, and impaired social behaviors can be corrected using the mTOR inhibitor rapamycin (Haji et al., 2020; Sato et al., 2012).Complete loss of eukaryotic initiation factor 4E(eIF4E)-binding protein 2 gene (Eif4ebp2) can lead to an imbalance in the excitatory/inhibitory ratio and ASD-like behaviors, while application of metabotropic glutamate receptor 1 (mGluR1) antagonists (JNJ16259685) or mGluR5 antagonists (fenobam) can reverse these defects (Aguilar-Valles et al., 2015).Similarly, in VPA mouse models of autism,mGluR5-antagonist (2-methyl-6-phenylethyl-pyrididine, MPEP)can ameliorate stereotyped repetitive behaviors (Mehta et al.,2011).Moreover, by inhibiting ionotropic glutamate receptors(NMDARs), d-cycloserine (DCS) can rescue ASD-associated social defects and repetitive behaviors in multiple animal models, includingNLGN-1?/?,Shank2?/?, and BTBR inbred mice (Budreck et al., 2013; Burket et al., 2013; Won et al.,2012).
In the section below, we will briefly discuss several recent hotspots of ASD treatment that have benefited from animal model studies (Figure 3).
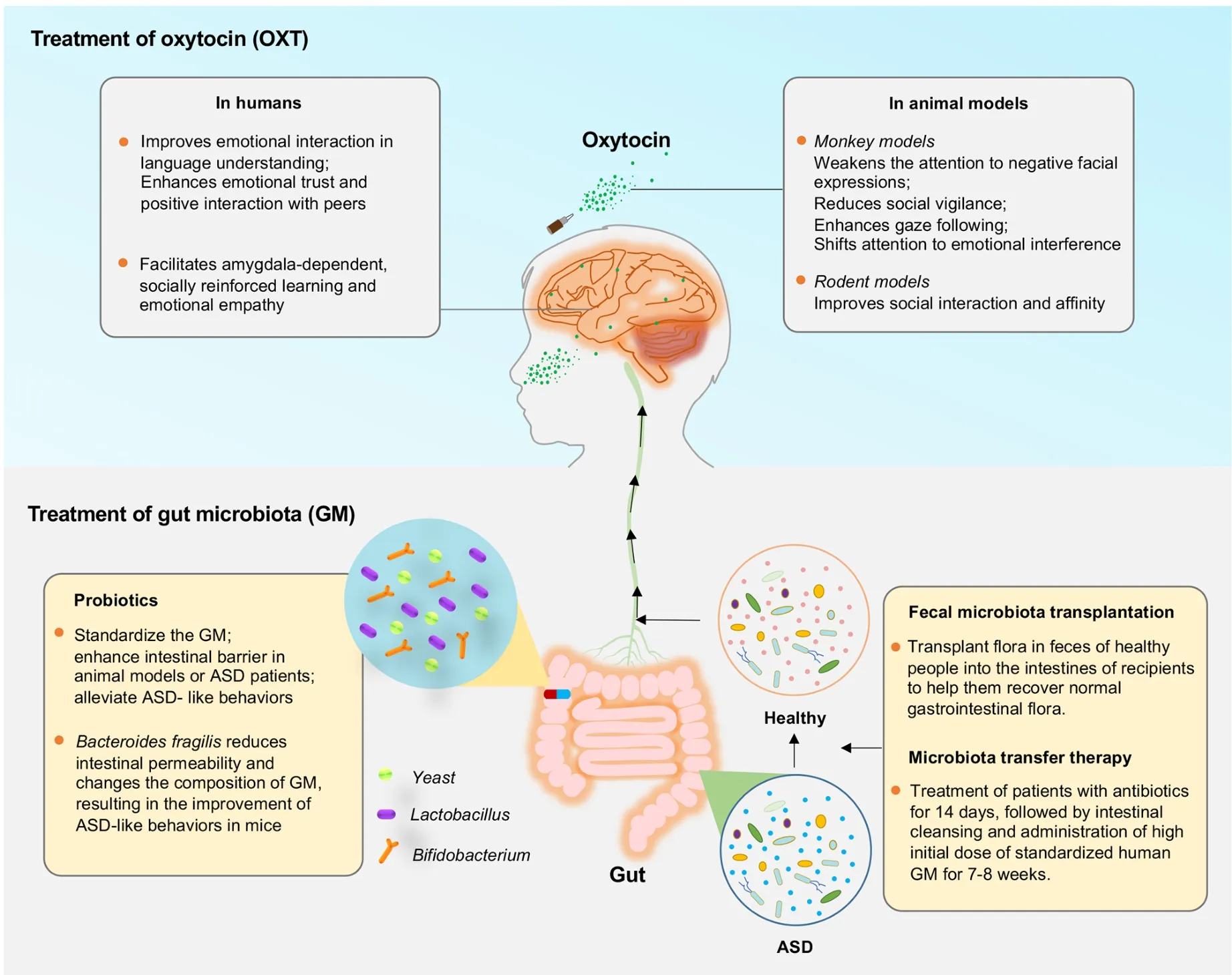
Figure 3 Clinical insights of ASD animal models
Oxytocin (OXT) treatment
As a brain neuropeptide, OXT is related to human social interactions and trust, and its application may improve social function in some autistic patients (Guastella & Hickie, 2016).Intranasal application of OXT is practical for autism, especially for enhancing language comprehension, emotional trust, and positive interactions with peers (Kosaka et al., 2012).Moreover, OXT can facilitate amygdala-dependent, socially reinforced learning and emotional empathy in men(Hurlemann et al., 2010).
In monkey models, OXT application can selectively weaken the attention of rhesus monkeys to negative facial expressions(Parr et al., 2013), reduce social vigilance (Ebitz et al., 2013),enhance gaze following response (Putnam et al., 2016), and shift attention to emotional interference factors (Landman et al., 2014).Application of OXT can also reduce fMRI response to fear and aggressive faces in macaques and selectively reduce functional coupling between the amygdala and occipital and infratemporal cortical regions (Liu et al., 2015).Moreover, in rodent models, OXT is reported to be closely related to social interaction and affinity (Silverman & Crawley,2014).At present, there are about 10 genetic models targeting OXT and its receptor (OXTR) (https://gene.sfari.org/database/animal-models/rescue-animal-models).These animal models have confirmed the rescue effects of several pharmaceutical agents, including OXT, cocaine, and arginine vasopressin,strongly suggesting the clinical value of drug treatments targeting OXT and OXTR.
Encouraged by successful OXT treatment in animal models,a randomized placebo-controlled study of 38 male autistic patients aged 7–16 years was conducted to evaluate the effects of OXT (nasal spray); however, the study found no significant improvement in emotional cognition, social skills, or problem behavior, highlighting that intervention methods for ASD patients should be considered carefully (Yatawara et al.,2016).However, in a recent long-term follow-up treatment study, multi-dose intranasal OXT treatment effectively induced long-lasting adaptations in core social brain regions (posterior superior temporal sulcus and amygdala) from the four weeks of intranasal OXT administration until four weeks (even up to one year) post-treatment (Bernaerts et al., 2020).Therefore,OXT may be a promising molecule to promote social behavior(Gauthier et al., 2016).OXTR gene expression is low in POGZWT/Q1038R mice, and intranasal OXT administration can effectively restore impaired social behavior in these mice(Kitagawa et al., 2021).Up to 20 clinical trials are currently testing compounds for ASD treatment, including vasopressin in OXT and OXTR animal models.
Treatment of GM
Potential probiotic therapies for specific behavioral symptoms of gastrointestinal and human neurodevelopmental disorders have been identified using ASD mouse models (Hsiao et al.,2013).Indeed, changes in the GM can regulate gastrointestinal physiology, immune function, and even behavior, suggesting a certain correlation between specific bacteria in the GM and ASD-related phenotypes (Coretti et al.,2017; Wu, 2017).Thus, probiotics, fecal microbial transplantation (FMT), and microbial transfer therapy (MTT)have been applied for treating ASD.
Many studies have shown that probiotics, such asBifidobacterium, yeast,Lactococcus, andLactobacillus, can prevent and treat animal models and human diseases, such as obesity, depression, colorectal cancer, and Crohn’s disease (Tomova et al., 2015).Indeed, in rodent models of ASD, application ofBacteroidesfragiliscan reduce intestinal permeability and change intestinal microbiota composition,resulting in improved ASD-like behaviors (Hsiao et al., 2013).In a recent study of 131 autistic children and adolescents(male:female=122:19; age: 86.1±41.1 months), probiotics were shown to ameliorate several symptoms of ASD (Mensi et al., 2021), suggesting that probiotics can standardize intestinal microbiota, enhance the intestinal barrier in animal models or ASD patients, and alleviate ASD-like behaviors.However,whether probiotics play a positive role in humans remains controversial, and more clinical evidence is needed.
The transplantation (i.e., FMT) of fecal flora from healthy people into the intestines of recipients to help them recover normal gastrointestinal flora has been used to treat gastrointestinal and other diseases (Antushevich, 2020).FMT from EphB6-deficient mice results in ASD-like behavior in antibiotic-treated C57BL/6J mice, whereas FMT from wild-type mice ameliorates autism-like behavior in EphB6-deficient mice(Li et al., 2020b).Although it is workable in animal models,there are many adverse reactions caused by FMT, such as diarrhea, abdominal colic, and transient low fever.Nevertheless, FMT can normalize the intestinal microbiota of ASD patients and improve their constipation symptoms(100%) (Rossen et al., 2015).
Similarly, MTT, in which patients are treated with antibiotics for 14 days, followed by intestinal cleansing, and administration of a high dose of standardized human GM for 7–8 weeks, may be another way to treat ASD (Hamilton et al.,2012).MTT not only improves gastrointestinal symptoms(such as constipation, diarrhea, dyspepsia, abdominal pain)and ASD-related symptoms, but also normalizes the microbiota of ASD patients, with improvement for up to two years post-MTT (Kang et al., 2017, 2019).
CHALLENGES AND PROSPECTS
ASD is a complex neurodevelopmental disorder caused by a variety of pathogenic regulators.Although susceptible factors have been clarified through epidemiological data and clinical observations, our understanding of this disease remains relatively poor.At present, animal models remain the best choice for studies on the causes and treatment of ASD.Moreover, continuous technological improvements and breakthroughs, such as multiphotoninvivoimaging and single-cell technology, will deepen our understanding of the pathogenesis of ASD and provide necessary support for the development of new diagnostic methods and treatment(Figure 4).
Assessment of current animal models
The more effective a model is, the better it can reflect human diseases.Validation is essential for evaluating the reliability of animal models and cannot be replaced when assessing the efficacy of drug treatments (Figure 4).Three types of validation are widely used to assess the closeness of a model to human disease (Chadman, 2017).Firstly, structural validity requires that the model conform to a certain theoretical hypothesis, and pathophysiological changes must be consistent with the hypothesis or theory.From this point of view, models more consistent with structural validity include ASD animal models with single-gene variation and confirmed toxic drugs.Secondly, surface validity requires that the model simulate typical characteristics of the disease in many aspects, such as behavior.From this point of view, almost all current ASD animal models conform to this feature to different degrees, given that typical clinical symptoms (such as stereotyped and impaired social behavior) are present.However, except for songbirds, no animal models are suitable for studying linguistic defects in ASD patients.Thirdly,predictive effectiveness requires that the pharmacological and non-pharmacological responses of the model are consistent with clinical treatment performance and can provide predictability for long-term treatment and pathogenesis research.From this point of view, although there is no ideal drug to treat impaired social communication and repetitive stereotyped behavior in human ASD, current ASD animal models are capable of predictive effectiveness to a certain extent.Importantly, many drugs approved for other diseases can effectively alleviate ASD symptoms without knowing the underlying mechanism, and thus basic research using ASD animal models is necessary.Of note, given its high heterogeneity, there is no current agreement on which animal model is best to investigate the pathogenesis of ASD, and finding shared causes from independent models is an excellent way to identify novel pathogenic regulators of ASD.
Developing new animal models for ASD
In addition to current ASD animal models, developing new models with novel animals that are evolutionarily close to humans, such as tree shrews and pigs, is important (Figure 4).
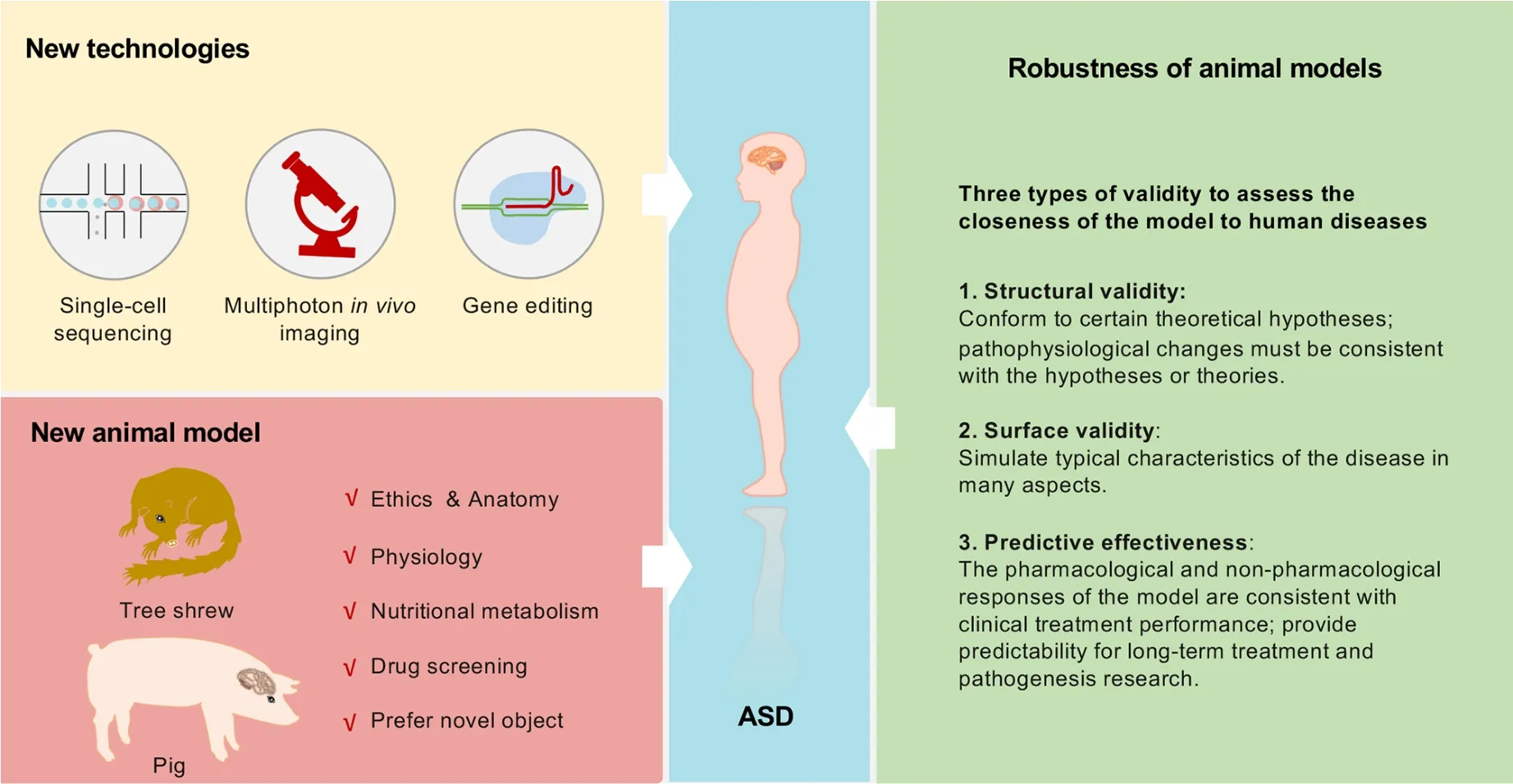
Figure 4 Development of robust and novel animal models for ASD
The tree shrew (order Scandentia) is a small animal closely related to primates and is considered an intermediary between rodents and primates (Savier et al., 2021).It has a small body size, low maintenance cost, and a relatively short reproductive cycle, making it an ideal model for studying various human diseases (Chen et al., 2020; Xu et al., 2020; Zhang et al.,2020).Extensive characterization of critical factors and signaling pathways in the immune and nervous systems shows that tree shrews have conservative and unique characteristics compared with primates (Fan et al., 2019; Yao,2017; Ye et al., 2021).Tree shrews have a more developed nervous system and a stress system similar to humans,suggesting they may be a good choice for models of mental illness behavior.Indeed, tree shrews exhibit strong novelty preferences similar to those in rodents and primates (Khani &Rainer, 2012).A recent study on social avoidance behavior toward unfamiliar conspecifics showed notably differences between tree shrews and mice, suggesting that tree shrews may be an ideal animal model for exploring social avoidance and prosocial behaviors (Ni et al., 2020).Tree shrews have also been used to establish social frustration, learned helplessness, and chronic mild stress models (Meng et al.,2016).Based on drug antidepressant experiments,clomipramine has been shown to reverse social withdrawal behavior in depressed animals, while fluoxetine has a reversal effect on learned helplessness.In contrast, carbetocin has been shown to have a significant therapeutic impact on decreased interest, social withdrawal, and learned helplessness (Meng et al., 2016).Moreover, visuospatial cognitive task experiments have demonstrated that trees shrews have much higher cognitive abilities than rodents,including reverse learning, reward, and punishment expectations (Ohl et al., 1998).Thus, using tree shrews to explore the pathogenesis of ASD is likely to become a hotspot in ASD research.
Pigs are another potential candidate animal.Compared with rodents, pigs have advantages in anatomy, physiology, and nutritional metabolism, and are more suitable for animal models of human diseases.Various human disease model pigs have been generated by gene-editing and somatic cellcloning technology.In recent years, pig models have been used in studies on diabetes, cardiovascular diseases, genetic diseases, tumors, and neurodegenerative diseases.Studies have shown that mini pigs explore novel objects significantly more than familiar objects, indicating that pigs exhibit measurable social novelty behavior (Moustgaard et al., 2002;S?ndergaard et al., 2012).Previous studies have identified promising tasks in pig cognitive research, e.g., universal space that allows simultaneous measurement of multiple behavioral domains.The use of appropriate tasks can facilitate the collection of reliable data on pig cognition (Gieling et al., 2011).Recent study investigated the effects of intrauterine growth retardation level (IUGR, score 0?3; i.e.,“normal” to “severe”) in pigs at birth, and found that some low birth-weight piglets can perform spatial tasks and object recognition tests, but performance is modulated by IUGR levels (Schmitt et al., 2019).Moreover, their relatively large littermate size makes pigs an ideal model for drug screening.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Z.L.and Y.C.conceived the review.Y.X.Z., L.J.G., and Y.C.prepared the illustrations.Z.L.and Y.C.wrote the manuscript.All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Dr.Da-Hua Chen for critical discussion.
- Zoological Research的其它文章
- Novel rhino-like SHJHhr mice with thyroid dysfunction
- A new species of Micryletta (Amphibia: Anura:Microhylidae) from the Langbian Plateau in southern Vietnam
- The high diversity of SARS-CoV-2-related coronaviruses in pangolins alerts potential ecological risks
- Site-specific and seasonal variation in habitat use of Eurasian otters (Lutra lutra) in western China:implications for conservation
- High-quality chromosome-level genome assembly of redlip mullet (Planiliza haematocheila)
- Challenging Wallacean and Linnean shortfalls:Ectatosticta spiders (Araneae, Hypochilidae)from China

