Sustained-release drug delivery systems for the treatment of glaucoma
Natasha P. Kesav, Cara E. Capitena Young, Monica K. Ertel, Leonard K. Seibold, Malik Y. Kahook
1Department of Ophthalmology, University Hospitals Cleveland Medical Center, Cleveland, OH 44106, USA
2Department of Ophthalmology, University of Colorado School of Medicine, CO 80045, USA
Abstract
INTRODUCTION
Glaucoma is the second most common cause of blindness and the leading cause of irreversible blindness in the world, projected to aあect 111.1 million individuals by 2040[1].
The disease classically presents as an optic neuropathy with corresponding progressive, irreversible visual field loss. Many patients with glaucoma have elevated intraocular pressure (IOP), however a significant proportion of diagnoses occur even when IOP is within the “normal range”[2]. Although there is no cure, the aim of currently available glaucoma treatments is to prevent disease progression and vision loss by lowering IOP, which often requires lifelong therapy and strict patient compliance[3]. Available treatment options include medical therapy (both topical drops and oral medications), laser therapy, and incisional surgery.
Medical therapy is often utilized as first line treatment, however poor adherence to topical drop therapy continues to be a major clinical challenge and is a widely recognized issue[2], reported to range in incidence from 5% to 80% across 34 studies[4]. Independent risk factors for noncompliance include lack of education regarding necessity of treatment adherence, timing, and duration as well as misperceptions about the irreversible nature of vision loss. Furthermore, complex dosing regimens with one or more medications place high demands on patients’ daily routines[5]. Self-reported questionnaires and interviews cite multiple other reasons for poor adherence to medical therapy, including memory impairment, forgetfulness, and mental health issues of particular concern as the prevalence and severity of glaucoma increases with age. Yochimet al[6]explored the prevalence of cognitive impairment, depression, and anxiety in a sample of 41 adults with glaucoma above the age of 50 and found that 44% of this sample was impaired on one or more measures of cognition. Furthermore, studies indicate that a high prevalence of non-compliance is compounded by patients’ inability to adequately instill a drop into the eye. Ability tests measuring grip strength revealed that a significant number of patients, particularly those with arthritis, could not generate enough force to expel drops from the bottle, which are small in size and contain viscous liquid[7].
Another major driver of inadequate adherence is the financial burden of necessary life-long treatment. A survey of glaucoma patients by Sleathet al[8]found that 41% had difficulty paying for their prescribed ocular hypotensive medications. Additionally, some patients have to battle formulary restrictions, prior authorizations, and step therapies imposed by insurance companies. In a study by Happeet al[9]on the most common clinical and economic factors related to medication adherence, 68% of negative medication adherence outcomes were associated with such formulary restrictions. All of these factors may contribute to patients’ struggle with adherence to their treatment regimen by impeding patients’ ability to take medications as prescribed, refill prescriptions, and keep physician appointments[10].
Outside of adherence limitations, there are also significant barriers to drug delivery and systemic bioavailability of both topical and oral medications that can result in suboptimal medication levels within the eye. High tear fluid turnover and nasolacrimal drainage limit drug retention time on the ocular surface. Trans-corneal permeability restricts diffusion of medications to their targeted ocular tissue which can aあect therapeutic eきcacy[11]. Furthermore, chronic use of glaucoma drops, especially those that contain preservative agents, is associated with a higher incidence of meibomian gland disease and ocular surface toxicity. Patients may experience symptoms of burning, photophobia, and irritation from daily application of topical drops[12-13]. These side effects can eventually lead to deliberate discontinuation of or intolerance to prescribed therapy.
ADDRESSING THE UNMET NEED
Several attempts have been made to improve patient adherence to medical therapy over the last several years. Behavioral methods for measuring adherence to medications have included motivational patient interviewing (MI), color coding bottle caps, self-reporting, prescription refill rate monitoring, electronic medication monitoring systems, and combinations of each of these.
Studies have demonstrated that negative attitudes towards treatment is an important determinant of nonadherence[5]. Cooket al[14]evaluated the ability of motivational interviewing to improve patient-enacted behavioral changes in an outpatient clinic. Glaucoma educators were successfully trained to deliver MI-consistent interventions; however, study participants showed no significant improvement in readiness for change. It is unclear if these changes had implications on medication adherence[14].
Bottle cap color and bottle characteristics have also been used by patients and providers for correct identification and differentiation of drops[15]. However, this heterogenous color-coding system is subject to communication errors, especially in patients with hereditary or acquired color deficiencies. Furthermore, physicians frequently misinterpret medication classes on the basis of color cap alone leading to miscommunication between providers and patients[15-16]. Other approaches to identify barriers to patient adherence have utilized electronic monitoring, real-time IOP measurement devices, and dosing aids[17-19].
Identification of IOP spikes, both natural deviations and those related to medication noncompliance, can be informative to both the patient and provider[2]. The advent of home tonometers and pressure sensing contact lenses may assist in the detection of such IOP spikes, however these are often not readily available to patients or providers outside of research eあorts.
Because patient compliance with daily therapy and home monitoring is limited for a number of reasons, patientindependent drug-delivery systems are attractive alternative options. The optimal system for treating open angle glaucoma (OAG) or ocular hypertension (OHT) would be one with a sustained release zero-order kinetic and a multi-drug delivery platform that relies minimally on patient action[20]. These devices also allow for a controlled delivery system to maintain therapeutic concentration in the eye, while increasing drug permeation and bioavailability in ocular tissues. Recently, this need has driven the development of depots and devices such as punctal plugs, external ocular inserts, and injectable reservoirs to address these issues.
As novel, minimally invasive drug delivery systems are being developed, their eきcacy, duration and safety must be carefully balanced so that physicians will trust and recommend them to patients who will adopt them. In order to achieve this, it is critical to examine existing patient attitudes and preferences. Patient-based surveys reveal that despite enthusiasm for alternative therapies in the pipeline, there is still hesitancy around alternative methods, especially those that are more invasive[21-22]. In a study of patients with glaucoma, 55% indicated they would prefer to use drops instead of undergoing other treatments including punctal plug depots, periocular or intraocular injections, or invasive surgery[21]. In contrast, there is more enthusiasm from ophthalmologists in pursuing new technologies, with surveys indicating that 88.9% of providers would prefer a sustained release contact lens as a treatment modality for their patients[22].
A variety of technologies are being studied to develop more durable, patient friendly, and cost-effective ocular delivery systems with the goal of increased compliance and better IOP control. This requires optimization of formulation, release kinetics, and duration of action with minimal side eあects[23-24]. Although previous research has illustrated a broad shift in miniature platforms that show potential to meet such needs, approval and/or adoption into clinical practice has not yet occurred for many. To date, there is only one US Food and Drug Administration (FDA)-approved sustained delivery device for treatment of OAG or OHT, but several are in the pipeline. This review aims to provide an update on the novel sustained release drug delivery platforms currently available and those in development for the treatment of OAG and OHT, with an emphasis on the benefits and challenges of each.
METHODS OF LITERATURE SEARCH
PubMed was searched from inception through March 2020. The search was limited to only English language and both human and animal studies were included. MeSH controlled vocabulary terms and related keywords were combined using appropriate Boolean operators to find literature discussing sustained-release drug systems used to treat glaucoma. The reference lists of all primary studies, review papers, and gray literature were searched for additional references as well as the registers of current and ongoing trials, including public clinical trial registries on clinicaltrials.gov (NCT02250651, NCT01180062, NCT02247804, NCT02129673, NCT02371746, NCT01845038, NCT02914509, NCT02312544, NCT04285580, NCT02636946, NCT01481077, NCT02384772, NCT02862938, NCT00705770, NCT03318146, NCT02358369).
EXTERNAL OCULAR INSERTS
Extraocular inserts are sterile preparations that are specially sized and shaped to fit into the conjunctival cul-de-sac[13,25]. By increasing pre-corneal residence times at predictable rates compared to conventional drug delivery, they eliminate the need for reliable patient administration of a drop. There are insoluble, soluble, and biodegradable subtypes in development[13,25].
The Ocusert (Alza Corporation, Mountain View, CA, USA) pilocarpine ocular therapeutic system was the earliest ratecontrolled, time independent drug delivery system FDA approved in the United States in the early 1970’s. This was a pilocarpine-eluting reservoir within a thin ethylene-vinyl acetate microporous membrane supported by a white titanium dioxide ring[26]. It was placed in the inferior fornix for one week throughout which timed pilocarpine was releasedviaa diffusional release mechanism at a constant rate of 20 to 40 μg/h[12]. Although reports showed positive outcomes[27-29], Ocusert’s therapeutic value was limited by side eあects such as dislodgement of the unit and “dose dumping” which resulted in a burst effect at unintended times of use[29-30]. Although Ocusert’s pilocarpine system was discontinued and taken off the market, the design has been adopted for the treatment of posterior segment diseases such as noninfectious uveitis and cytomegalovirus retinitis[31-32].
The Bimatoprost Ocular Ring (Allergan plc, Dublin, Ireland) is composed of an inner polypropylene ring within a preservativefree silicone matrix that is impregnated with 13 mg of bimatoprost. The insert, available in diameters of 24-29 mm, is placed into the upper and lower fornices and is designed to be worn for 180d. The design has the advantage of a large surface area which may allow for delivery of a combination of ocular hypotensive agents and can therefore address the inconvenience of multidrop regimens[33]. A multicenter, phase 2, noninferiority trial was conducted to evaluate the bimatoprost insert in 130 patients with primary OAG and OHT. While the study showed consistent IOP lowering of 20%, at the end of the 6-month trial, the bimatoprost insert was somewhat less effective than twice daily timolol eye drops. There was a mean reduction in IOP of 3.2-6.4 mm Hg in the implant group compared to 4.2-6.4 mm Hg in the timolol group with no patients needing rescue therapy. In this study, however, a total of 28 dislodgements were reported in 15 patients and the bimatoprost insert group had a higher percentage of treatment related adverse events compared to the topical timolol group (45.3%vs34.8% respectively)[33]. From this study, 75 patients continued on through 13mo in an open label, single arm, insert safety study which demonstrated the insert continued to provide IOP reduction for 13mo (mean IOP reduction 4 mm Hg) with 97.3% retention. Rescue therapy with topical bimatoprost was needed in 13 of 63 patients[34]. A clinical trial of a fixed combination bimatoprost and timolol ring was completed in 2018 but there are currently no publicly available results (NCT02742649).
The Topical Ophthalmic Drug Delivery Device, or TODDD (Amorphex Therapeutics, Andover, MA, USA) is a continuous release soft polymer drug depot that delivers medication while floating atop the sclera beneath the upper eye lid. Animal safety and efficacy studies tested the platform with 3 mg of timolol and 600 μg of latanoprost in normotensive rabbits[35]and beagle dogs[36], respectively, resulting in approximately a 37% IOP reduction from baseline in both studies. An open label study evaluating safety and tolerability in 14 adult humans showed a 70% retention rate of the prototype after four weeks of continuous wear[37].
Other controlled release device platforms have incorporated a number of natural polymers into drug delivery vehicles to enhance bioavailability and increase retention time on the ocular surface. The adaptation of the collagen shield was developed by Agbanet al[38]and was tested in animal studies for the delivery of pilocarpine hydrochloride over 14d. Pilocarpine was loaded into the collagen matrix shield consisting of nanoparticles optimized to be the most ideal cross-linking agents, consisting of polyvinylpyrrolidone (PVP) capped zinc oxide (ZnO/PVP). Other vehicles, such as the New Ophthalmic Delivery System (NODS), have been manufactured using a water-soluble polyvinyl alcohol (PVA) film placed through the lower conjunctival sac with a radiolabeled flag loaded with pilocarpine nitrate. When compared to a 2% pilocarpine nitrate solution in a Phase 1 clinical trial, the pilocarpine NODS showed an eightfold increase in precorneal bioavailability and higher compliance amongst test subjects[39].
PUNCTAL PLUGS
Punctal plugs have a successful track record for the treatment of dry eye syndrome and intracanalicular drug delivery systems are currently used to treat post-operative inflammation which makes them an enticing option for glaucoma drug delivery as well. Punctal plugs normally function by blocking the punctum and canaliculus to reduce tear drainage and increase the amount of tears on the ocular surface. They can also be impregnated with various medications which are slowly released over a period of time. A hydrogel intracanalicular plug containing 0.4 mg of dexamethasone (Dextenza?; Ocular Therapeutix, Inc., Bedford, MA, USA) is currently commercially available for the treatment of post-operative inflammation and pain[40]and has been shown to be highly favorable and preferred over topical therapy in patient interviews[41].
Similarly, punctal plugs are in the clinical pipeline as a sustained release drug delivery platform for the treatment of glaucoma. Mati Therapeutics (Austin, TX, USA) is developing the Evolute novel Punctal Plug Delivery System (PPDS) for the treatment of multiple ocular conditions including OAG and OHT. This platform uses an L-shaped plug with a nonbiodegradable latanoprost core which can be inserted at the slit lamp. A safety and efficacy study[42](NCT01229982) demonstrated a clinically significant, prolonged reduction in mean IOP from baseline by 5.7 mm Hg or 22.3% at 4wk (95%CI -6.5, -4.9). In two additional phase 2 multicenter trials, a total of 134 subjects underwent simultaneous placement of Evolute PPDS in both the upper and lower puncta. In both studies, mean IOP reductions from baseline were statistically significant at all time points. Retention rate of plugs through week 12 was 92%-96% and 48%-58% in the lower and upper puncta, respectively[43]. Retention of punctal plugs over time remains one of the biggest concerns and must be further investigated in future studies and in larger groups of patients. The L-PPDS is reported to be well tolerated with most adverse events similar to those reported for commercial punctual plug designs, such as mild tearing and discomfort[44]. These trials show promise for a sustained release ophthalmic drug deliveryviaPPDS.
Travoprost, another prostaglandin analogue, has also been incorporated into a punctal plug delivery device. The travoprost punctal plug, OTX-TP (Ocular Therapeutix, Inc., Bedford, MA, USA), is a resolvable hydrogel rod which swells to fill the canalicular space and gradually releases travoprost from within an interior poly(lactic acid) microsphere matrix over a 90d period. A phase 2 double dummy study was conducted in which 73 patients were randomly assigned to receive OTX-TP with twice daily artificial tears or timolol 0.5% twice daily with a drug-free punctal plug[44]. The study found a clinically significant IOP reduction from baseline in both groups, although this reduction was greater in the timolol group (4.5-5.7 mm Hg compared with 6.5-7.6 mm Hg). This eあect was attributed to the increased drug contact time in the group with placebo punctal plugs. Retention rates of the plug were consistent with expected dissolution of the plug (91% at day 60 and 48% at day 90). A single-arm feasibility study of OTX-TP found sustained IOP lowering eあects in 26 eyes of 17 Asian participants over 30d. Peak eあect (24% IOP reduction) was observed at day 10 with 100% retention. Plug retention declined to 42% by day 30 with a mean 15.6% IOP reduction. Only one subject was intolerant of the plug due to epiphora and required removal. This study was limited however by sample size and short duration[45]. A subsequent placebocontrolled phase 3 trial evaluated OTX-TP for 75d versus a comparator arm using a nondrug-eluting punctal plug. This trial was conducted across more than 50 sites with 554 subjects enrolled. Ultimately, the trial did not demonstrate significant superiority in the mean reduction of IOP at 2, 6, and 12-week follow-ups. The main adverse event was dacryocanaliculitis (7% in OTX-TP groupvs3% in placebo)[46]. The OTX-TP system is no longer being developed according to personal communication with the manufacturer.
As of 2020, there have been over 100 issued patents for punctal plug systems for use in a variety of ocular conditions. Although it has the appeal of a flexible drug delivery profile and noninvasive approach, this approach is not without limitations. These include foreign body sensation, localized pain and retention issues. The IOP lowering effect is also limited by inconsistent delivery from the punctum to the tear film. Possible infection of the lacrimal drainage system, expulsion of plug, or ocular irritation are other complications that have been reported but can readily be identified with regular follow-up visits.
CONTACT LENS
Contact lenses have emerged as an alternative to topical drop administration because they take advantage of selective site targeting. Additionally, their use is already accepted amongst many patients for vision correction. In a survey evaluating acceptance of sustained release devices in 150 patients, the majority of subjects (56%) indicated that they would accept contact lenses[47]. The latest advancements in contact lens technology has aided in both real-time monitoring of IOP and improved sustained drug release. Drug-eluting contact lenses were first tested more than 50 years ago but their utility as a drug delivery platform has historically been limited by rate of drug delivery[48]. To extend the duration of the drug particulate system release, nanoparticles, drug-polymer films, vitamin E barriers, and liposomes have been incorporated into contact lenses, each with variable results.
Ciolino and colleagues reported the results of a latanoprosteluting contact lens placed in New Zealand white rabbits for one-month. The goal was to deliver the same amount of medication in one day as one drop of topical latanoprost. While the results showed feasibility, investigators were unable to translate the pharmacokinetics in the context of human circadian IOP variations[49]. In follow up, Ciolinoet al[49]conducted a preclinical efficacy crossover-design study in four glaucomatous monkeys evaluating a latanoprost eluting methafilcon contact lens. A central aperture was cut from the film so as to retain visual acuity. Dose variations included low dose (CLLO) and high dose (CLHI) formulations and were compared to topical latanoprost therapy. The lenses were placed for one week and were compared to topical latanoprost administered for 5d with a three-week washout period between consecutive treatments. The authors found that both formulations were at least as effective as topical latanoprost (IOP reduction of 2.9-6.6 mm Hg in topical latanoprost, 4.0-7.8 mm Hg in CLLO, and 6.0-10.2 mm Hg in CLHI). Further, the topical latanoprost solution resulted in more variation in IOP reduction during diurnal measurements as compared to each contact lens, which in turn demonstrated a favorable consistent reduction in IOP[49].
Previous trials of a contact lens platform for drug delivery reported issues such as high burst release kinetics and low drug loading[50-51]. Several techniques have been employed to combat these issues. Yanet al[52]applied molecular imprinting technology to improve drug uptake and achieve controlled release of bimatoprost from contact lenses.In vivorabbit tear fluid data showed a low burst release and increase in bimatoprost retention time in this novel molecular imprinted contact lens compared to the conventional soak and release method[52]. There was concern however that these kinetic advancements affected the elasticity and swelling properties of the contact lens, therefore further studies are warranted to establish eきcacy and safety profiles in humans.
Extended drug release contact lenses have also been modified with vitamin E diあusion barriers which increase release time while still retaining critical lens properties[51-54]. Vitamin E increases the diffusion pathway in the lens matrix ultimately reducing the drug transport rate and increasing the loading concentration from 10% to 40%[53]. It has also been shown to increase the release duration of both drugs resulting in a platform that can provide extended drug delivery for about 2d[51]. Hsuet al[51]reported on the safety and efficacy profile of these vitamin E loaded contact lenses forin vitroandin vivostudies in beagle dog models of glaucoma for four days. They also reported on the feasibility of combination delivery of timolol maleate and dorzolamide hydrochloride in these contact lenses. The release durations of both medications with 20% vitamin E incorporation increased by 35 and 14-fold for timolol and dorzolamide, respectively. They found that when the two medications were co-loaded and released simultaneously, the release durations increased around 1.7 and 1.2-fold compared to individual loading. Furthermore, the IOP reduction was maintained for about a week after removal of the contact lens, potentially due to slow accumulation of the medications inside corneal epithelial cells or binding of the drug to high affinity targets such as the iris or ciliary body[54]. These studies support the utility of vitamin E-loaded contact lenses for enhancing IOP reduction with improved bioavailability and compliance.
Though sustained drug delivery may be achieved using contact lenses, critical patient needs still must be considered. While retention studies in the context of sustained release therapies have not yet been done, one prospective study by Sulleyet al[55]revealed that the first-year retention rate for neophyte contact lens wearers was only 77.6%. The most commonly cited reasons for discontinuation in the remaining 22.4% of patients included problems with vision (41%), discomfort (36%) and handling (35%)[55]. Contact lens wear is also a primary risk factor for developing microbial keratitis since 50.3% of such infections are associated with contact lens use[56]. Furthermore, specific groups of patients, such as the elderly, may exhibit manipulation difficulties which may increase the risk of contamination. This platform may be a more attractive option for younger glaucoma patients who were contact lens wearers prior to their diagnosis of glaucoma.
Subconjunctival InjectionsSubconjunctival injections are widely used to deliver medications for conditions such as uveitis and are an attractive option for sustained release platforms considering the volume available within the subconjunctival space[23,57]. Amongst these novel platforms is the VS-101, also known as the Eye-D latanoprost insert. This is an extended-dose subconjunctival insert developed by Biolight Life Sciences (Tel Aviv, Israel) for the treatment of glaucoma. The 77-patient Phase 1/2a study (NCT02129673) demonstrated the ability to lower diurnal IOP by an average of 24% for a sustained 12-week period from a single placement of the insert in one of the three tested doses. The proof of concept trials achieved safety and efficacy endpoints and provided insert size, structure and location data to improve retention[58].Graybug Vision (Redwood City, CA, USA) presented preclinical study results for GB-401, a subconjunctival injection for use up to 6mo[59]. GB-401 is an encapsulated microparticle formulation of a proprietary beta-adrenergic antagonist prodrug that hydrolyzes into an active agent to lower IOP and act as a neuroprotective agent. A significant reduction in IOP of approximately 20% was recorded within the first week following injection of the formulation in pigmented rabbits bothin vitroandin vivo[60]. Patients with OAG and OHT are being recruited for the upcoming first-inhuman phase 1/2a study.
Nanoparticle technology is also under investigation for use within this space. Published studies have reported the development of biodegradable polylactic-co-glycolic acid (PLGA) nanoparticles and liposomes for drug delivery. Commercially, PLGA is co-eluted with dexamethasone in the Ozurdex intravitreal implant (Allergan plc, Dublin, Ireland) to achieve 6mo of drug delivery for the treatment of uveitis. Subconjunctival administration of PLGA nanoparticles are known to facilitate a prolonged release rate with minimal toxicity to ocular tissues due to the slow biodegradation of nanoparticles[24]. A brinzolamide (BRN) formulation incorporating two types of PLGA nanoparticles was injected into the subconjunctival space of normotensive Albino rabbits. The reduction of IOP in both BRN-loaded PLGA nanoparticle preparations was greater than topical BRN suspension alone for up to 10d[24]. Other subconjunctival nanoparticle delivery platforms have also been investigated. Nget al[61]synthesized a blend of poly(lactose-co-caprolactone) (PLC)- and poly(ethylene glycol) (PEG) biodegradable microfilms loaded with timolol maleate for subconjunctival injection in nonhuman primates with OHT. IOP was reduced by 50.1%±8.5% from baseline which lasted for 140d[61]. Alkoxylphenacyl-based polycarbonate (AP-PLC) is another polymer matrix that was investigated in a model containing brimonidine tartrate (BRT). The data demonstrated that these microfilms were eあective in sustaining BRT release for >90d[62]. These studies indicate that PLGA, PLC-PEG, and AP-PLC nanoparticles may serve as an eきcient carrier for prolonged, controlled ocular delivery due to their biodegradability and biocompatibility characteristics. Liposomes are neutrally charged vesicular lipid systems that form encapsulated complexes with lipophilic drugs. These complexes can be injected subconjunctivally and enhance permeability and increase residence time of the drug[23,63]. A unique dipalmitoyl phosphatidylcholine (DPPC) formulation of liposomal latanoprost was testedin vivoin 23 normotensive New Zealand white rabbits and produced IOP lowering of 2-3 mm Hg which was sustained for 50d[63]. Combined formulations have also been tested in liposomal drug carriers. Fahmyet al[57]tested an encapsulated liposome in glaucomatous rabbit eyes designed to deliver latanoprost and thymoquinone, an herbal anti-inflammatory chemical that lowers IOP. This combination was able to sustain a significant IOP reduction when compared to non-treated glaucoma rabbits for 84h[57].
Although substantial IOP reduction with these systems has been demonstrated in animal models, robust human clinical studies to date are lacking. The first-in-human evaluation of liposomal latanoprost was conducted by Wonget al[64]to evaluate safety and clinical eきcacy. The study enrolled adults with OAG or OHT who each received 100 μL of liposomal latanoprost subconjunctival injection. They found a reduction of 13 mm Hg or 47% from baseline, which is at least as effective as previous reports of topical latanoprost solution. Only one subject was intolerant to the injection due to preexisting ocular surface disease[64](NCT01987323). While the results of this human trial are exciting, further larger human clinical studies are needed to investigate clinical efficacy, safety and tolerability before these can come to market.
Intracameral ImplantsIntracameral implants have also been developed to address treatment adherence issues. Bimatoprost SR (BimSR; Allergan plc, Dublin, Ireland), an intracameral pellet implant within a biodegradable NOVADUR solid copolymer PLGA matrix platform is the first sustained drug delivery implant approved by the US FDA. This solid, rodshaped implant is designed to slowly release bimatoprost and lower IOP for 4-6mo. Once the implant is administered into the anterior chamber, the matrix is hydrolyzed to release bimatoprost in a non-pulsatile, zero-order kinetic fashion[67-73]. The BimSR implant was created to target drug delivery directly to the iris and ciliary body, thereby altering matrix metalloprotein production and reducing extracellular matrix in outflow tissues[65-66]. By upregulating this pathway, drug distribution is limited to the relevant tissue targets thus lowering the incidence of adverse eあects.
BimSR has demonstrated targeted drug delivery and eきcacy in nonclinical studies. A drug distribution study using normotensive beagle dogs showed a selective, sustained dilation of aqueous outflow vessels and a steady IOP reduction maintained through day 66 in treated eyes[68]. Leeet al[69]also evaluated dose strengths up to 120 μg in beagle dogs and found that the IOP lowering response provided by BimSR has no plateau in eあect like topical bimatoprost does. Another distribution study was conducted in which twenty-four beagle dogs received either bilateral intracameral 15 μg BimSR or daily administration of bimatoprost 0.03% ophthalmic solution. Pharmacokinetic data showed that 80.5% of the bimatoprost load was released by day 51 and 99.8% by day 80. The peak concentration in the iris-ciliary body was 4 log units higher after a single BimSR administration than after 7d of once-daily topical dosing with bimatoprost 0.03%. Further, there was low or undetectable drug levels in off-target extraocular tissue, suggesting the advantage of the implant in enhancing drug delivery while preventing common adverse events seen with topical PGA treatment[70]such as orbital fat atrophy.
In human clinical trials, an interim 6-month analysis of phase 1/2 trials of BimSR showed IOP lowering in a rapid and sustained manner in OAG patients at four tested dose strengths (6, 10, 15, and 20 μg)[71]. Continuation of the safety and efficacy study was completed after a 2-year trial (NCT01157364) and demonstrated that a single administration of BimSR controlled IOP in 40% of patients for up to 12mo and in 28% of patients for up to 24mo[72]. There was a reduced incidence of adverse events in BimSR treated eyes, which overall had favorable long-term safety and tolerability profiles. Furthermore, after month 24, 82.9% of patients had a positive treatment experience and reported that they were extremely or very likely to have another implant procedure if given the choice[67,72]. Two 20-month phase 3 trials of BimSR were conducted involving 528 patients with OAG or OHT. The treatment groups were randomized to either BimSR 10 or 15 μg or topical timolol 0.5% twice daily. BimSR treatment groups demonstrated ability to decrease IOP by 30% from baseline in the 12-week primary efficacy period, which achieved non-inferiority versus topical timolol. The safety was most favorable for the 10 μg dose strength[67]. The most commonly reported ocular adverse reaction was conjunctival hyperemia (27%; Allergan media). Corneal endothelial loss was not uncommon in the FDA approval studies and this remains an area that requires further investigation to quantify the incidence and severity in real world settings. In 2020, the FDA approved DURYSTA (Allergan plc, Dublin, Ireland), a 10 μg bimatoprost implant for intracameral administration to treat OAG or OHT[73]with the caveat that it can only be used once in each patient to avoid the potential risk of corneal endothelial loss that was seen with multiple injections over time. Allergan currently has five ongoing Phase 3 studies with Durysta.
Travoprost XR (Aerie Pharmaceuticals, Durham, NC, USA) is another biodegradable intracameral implant. This device uses novel sterile nanoparticle replication engineering technology to provide continuous travoprost elution. A preclinical study of six normotensive Beagle dogs who underwent intracameral implantation of Travoprost XR demonstrated a 35.5% IOP reduction over eight months (-6.4±0.6 mm Hg from a baseline of 18.6±0.2 mm Hg)[75]. In the phase 2a study, ENV515 was administered to 21 glaucoma patients and compared to oncedaily topical Travatan Z administered to the non-study eye. The study achieved its primary eきcacy endpoint of a change from baseline in diurnal IOP by day 25 (-6.7 mm Hg in the implant group and -6.6 mm Hg in topical treatment eyes,P<0.001). A 12-month study was then undertaken in which ENV515 was administered to eyes of patients with OAG including those who were treated previously with prostaglandins. Topical timolol 0.5% ophthalmic solution once daily was administered to the fellow eye. The implant demonstrated 25% IOP reduction and non-inferiority to timolol for 11mo with a mean reduction in IOP of 6.7±3.7 mm Hg. Similar to studies of BimSR, the most common adverse event was dose-related transient hyperemia[76].
The iDose Travoprost implant (Glaukos, San Clemente, CA, USA) is a titanium travoprost-eluting intracameral delivery system placed through a small corneal incision and anchored to the trabecular meshwork. The implant is encapsulated in a membrane that controls the release of travoprost for continuous elution into the anterior chamber. Once depleted, the implant can be removed and replaced for continued treatment. Two different rates of elution were compared in a multi-center, randomized double masked Phase 2 clinical safety and eきcacy study. At the 12-week primary endpoint, mean IOP lowering was 8.5 mm Hg (33% reduction), 8.0 mm Hg (32%), and 7.6 mm Hg (30%) in intervention arms of iDose fast-elution, iDose slowelution, and timolol 0.5%, respectively (NCT02754596). Corneal endothelial cell loss will be an important endpoint to examine once iDose studies are publicly available since the device resides in the angle and near the corneal endothelium. A similarly designed Phase 3 trial is pending with a primary completion date of June 2021. Travoprost has also been incorporated into another intracameral resorbable implant, OTX-TIC (Ocular Therapeutix, Bedford, MA, USA). This implant contains micronized travoprost released over a period of 4-6mo. The first cohort of five patients showed a greater average IOP reduction in the implant treated eye compared to the fellow eye treated with once-daily topical travoprost. Evidence of biodegradation was noted at 4mo[77]. A phase 1, prospective, multi-center, open label, dose escalation clinical trial evaluated the safety, efficacy, durability, and tolerability for up to 18mo in patients with a single insertion of OTX-TIC. Data from the first two cohorts of 9 subjects showed decreased mean IOP values that remained reduced from baseline throughout the study period and beyond. There were no serious adverse events reported[78]. Third and fourth cohort studies are underway to power long-term statistical evaluation.
Topical prostaglandin analogs (PGAs), such as bimatoprost and travoprost, have been associated with adverse eあects such as conjunctival hyperemia, skin and iris pigmentation, and eyelash growth. Despite this, their once daily dosing and high eあectivity make them a popular option for first line treatment in many patients[69]. In a study of BimSR, there was limited distribution of the medication to the eyelid, conjunctival, and periorbital tissues thus theoretically limiting the potential side effects seen at these sites with topical PGAs[68]. Similarly, the iDose travoprost implant had no reported cases of hyperemia[79-80]though distribution of the medication was not directly assessed and other intracameral PGA-impregnated implants have not directly investigated these eあects.While early data of these sustained delivery platforms promise enhanced adherence and an overall reassuring safety profile, there are several contraindications and disadvantages of implants compared to topical therapy (Table 1). These implants require injections or placement intraoperatively. Due to the invasive nature of administration, complications include risk of implant migration, hypersensitivity reactions to components of the product, and endophthalmitis. Furthermore, other current and previously used implants and/or shunt devices placed in the anterior chamber have demonstrated an increased risk of endothelial cell loss with time[81]. In a five-year follow-up study of the supraciliary Micro-Stent (CyPass; Alcon Laboratories, Inc, Ft. Worth, TX, USA) device, significant endothelial cell count loss (>30%) was seen in a higher proportion of implanted subjects than control subjects (27.2%vs10%)[82]. Because of this, Alcon removed the device from the market in 2018. While the intracameral drug delivery implants are often much smaller than many of these other intraocular implants, the risk to the corneal endothelium must be thoroughly evaluated. Overall, the costs and benefits of these implants must be weighed in each patient in order to minimize adverse reactions and optimize patient safety.
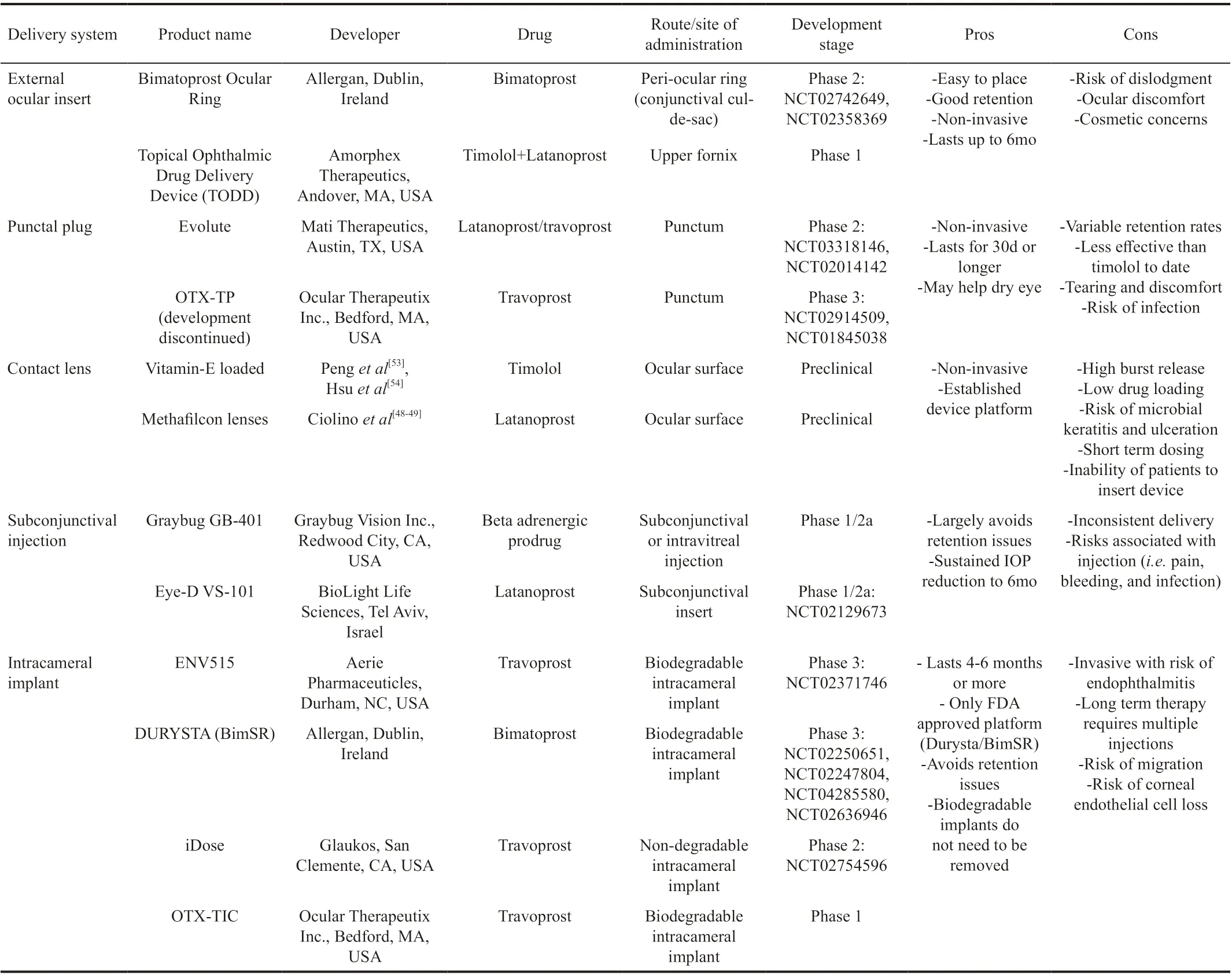
Table 1 Sustained release delivery platforms in various developmental stages, each indicated for the treatment of OAG and OHT
CONCLUSION
Adherence to prescribed therapeutic regimens remains a critical issue for patients and practicing eye care professionals alike. The innovative approaches of emerging platforms that are independent of patient adherence oあer promising options for drug delivery that may expand the glaucoma specialist’s armamentarium. For any therapeutic intervention to be successful, it must be trusted and accepted by both the provider and the patient. Although innovative sustained drug delivery platforms can eliminate patient adherence issues, previous survey data has demonstrated that patients themselves may still prefer the less invasive topical drop regimens. Therefore, further investigations to understand patient and provider preferences and potential barriers to acceptance and adoption of these innovative platforms is required. Further, the cost of these platforms to patients and the medical system, with a focus on reimbursement pathways, is another important factor to consider prior to implementation and widespread clinical acceptance over topical drop therapy.
External ocular inserts, intracameral depots, contact lenses, punctual plugs, and injectables represent just a few of the potential routes for sustained drug delivery with significant potential. However, they each have their own risks and limitations when compared to currently available therapies. While many of these platforms have shown therapeutic potential in preclinical and clinical studies, most of the available data is comprised of animal studies and small human trials and is therefore not yet widely generalizable. Further, data on duration of effect is also somewhat lacking. Additional large, human based comparator studies need to be performed on each of these therapeutic options in order to better understand where they fit into clinical practice prior to widespread adoption.
As sustained release delivery platforms continue to evolve at today’s rapid pace of innovation, clinicians and patients alike can look forward to additional therapeutic options that may be available to them in the future.
ACKNOWLEDGEMENTS
Authors’ contributions:Kesav NP, Young CEC, Ertel MK, Seibold LK, Kahook MY contributed to the conception and design of the research, acquisition of data and interpretation of data, and the writing of the manuscript. All authors have given final approval and consent for publication.
Conflicts of Interest:Kahook MY is a consultant for Alcon, Allergan, New World Medical, and Equinox and receives patent Royalties from Alcon, J&J Vision, New World Medical, and Aurea Medical. Seibold LK is a consultant for New World Medical.Kesav NP,None;Young CEC,None;Ertel MK,None.
 International Journal of Ophthalmology2021年1期
International Journal of Ophthalmology2021年1期
- International Journal of Ophthalmology的其它文章
- Instructions for Authors
- Comment on “Early results of circularity and centration of capsulotomy prepared by three diあerent methods”
- Comment on “Impact of ultrasound and optical biometry on refractive outcomes of cataract surgery after penetrating keratoplasty in keratoconus”
- Reviewer Acknowledgements
- Endothelial keratoplasty combined with scleral fixation intraocular lens
- A direct observation of aqueous humour flow in vivo after implantable collamer lens with a central hole implantation
