Longitudinal decrease in platelet counts as a surrogate marker of liver fibrosis
Neta Gotlieb, Naama Schwartz, Shira Zelber-Sagi, Gabriel Chodick, Varda Shalev, Oren Shibolet
Abstract
Key Words: Cirrhosis; Platelets; Count; Trend; Prediction; Range
INTRODUCTION
Liver cirrhosis is an important public health concern and a significant cause of morbidity and mortality worldwide. The global prevalence of cirrhosis ranges from 4.5% to 9.5% of the general population and is likely to increase due to the aging of hepatitis C virus (HCV) patients and rise in non-alcoholic fatty liver disease (NAFLD)[1,2]. In 2017, Cirrhosis caused more than 1.32 million deaths globally, compared with less than 899000 deaths in 1990. Most of the cases were secondary to decompensated liver disease[3]. Chronic liver disease (CLD) is usually indolent and asymptomatic early in its course, thus many cirrhotic patients are diagnosed late, when manifestations of portal hypertension (HTN) such as variceal bleeding, ascites or hepatocellular carcinoma (HCC) appear. Early diagnosis of cirrhosis is important in order to enroll patients into HCC surveillance programs and offer therapeutic interventions to halt or reverse disease progression. Nearly 1.5% of patients with cirrhosis remain undiagnosed throughout life, therefore, better diagnostics tools using laboratory and imaging modalities are needed[4].
Patients with advanced liver disease and cirrhosis may present changes in laboratory values such as thrombocytopenia, hypoalbuminemia, abnormal clotting function, anemia, and changes in hepatocellular and cholestatic liver enzymes. Thrombocytopenia (platelet count < 150000/μL) is one of the most common abnormalities in patients with cirrhosis, seen in up to 78% of cirrhotic patients[5]. Thrombocytopenia carries important prognostic information in terms of the presence of cirrhosis, portal hypertensive complications, hepatocellular carcinoma, post-liver resection and the post-transplant course[6]. Indeed, there is a correlation between the degree of thrombocytopenia and the stage and severity of liver disease; severe thrombocytopenia (< 50000/μL) is a poor prognostic factor associated with significant morbidity, indicating an advanced liver disease with established portal HTN[7,8]. This strong association has been corroborated by a study indicating that liver diseases is the underlying cause of thrombocytopenia in 58% of outpatients from all hospital departments[9].
The pathogenesis of thrombocytopenia in CLD and liver cirrhosis is multifactorial. Possible causes include splenic sequestration of platelets, suppression of platelet production in the bone marrow, decreased thrombopoetin production in the liver and an autoimmune mediated destruction[5]. Additionally, platelets actively participate in pathophysiologic processes in the liver, resulting in fibrosis and cirrhosis; previous studies including animal models showed that platelets have a major role in liver inflammationviainteractions with the hepatic sinusoidal endothelium and myeloid cells, inducing diverse hepatic processes ranging from liver repair and regeneration to necroinflammation and fibrosis[10,11]. Additionally, studies hypothesized that circulating platelet-neutrophil aggregates can induce neutrophil activation, thus driving end organ damage in patients with cirrhosis. Indeed, various liver diseases are associated with neutrophil recruitment; these include cholestatic liver injury, alcoholic hepatitis, drugs and chemical-induced injury[12].
While the association between thrombocytopenia and cirrhosis is well-established, little is known about the association between subtle changes in platelet counts over time and the long-term risk of cirrhosis development. Few previous studies have shown that platelet counts may start to fall earlier in the course of NAFLD and HCV induced liver diseases[13,14]. Additionally, platelet counts have been incorporated into non-invasive tools for the diagnosis of liver fibrosis and cirrhosis. Among others, these are the aspartate aminotransferase-to-platelet ratio index (APRI), fibrosis-4 (FIB-4) score and NAFLD fibrosis score which are used to assess the presence of liver fibrosis[15,16]. However, the platelet values in the aforementioned scores and studies were taken as a single value at a single time point. No study has tested the association between platelet trends within the normal range and cirrhosis incidence. Current computerized systems allow the collection of big data sets and enable the detection of subtle platelet changes, decades prior to the diagnosis of liver cirrhosis. Subtle trends in laboratory results are now being incorporated in machine learning algorithms which utilize artificial intelligence to generate predictive models more effectively than conventional methods, through detection of hidden patterns within large data sets.
In this study we aimed to explore whether platelet counts trajectories over time can advance the diagnosis of early liver disease and its predictive ability across the different etiologies of cirrhosis, in parallel to different fibrosis scores. In addition, we aimed to test the association between platelets decline and portal HTN complications (variceal bleeding, ascites, hepatic encephalopathy, HCC) among cirrhotic patients.
MATERIALS AND METHODS
Setting
A nested case-control study with diagnosed cirrhosis patients and matched controls, utilizing the Maccabi Health Services (MHS) database was performed. MHS is a 2.3-million-member state-mandated health services organization, representing 25% of the local population of Israel[17]. MHS's data are automatically collected and include information regarding all diagnoses, comorbidities, hospitalizations, emergency department visits, physician visits, outpatient specialist visits, purchase of medications, laboratory tests and radiologic imaging results. MHS’s database was established and collects data from 1998, with a 99% members’ retention rate which enables a unique opportunity to assess the long-term trends in laboratory results. All biochemical assessments are performed by a single laboratory that maintains a quality management system, as required and using the same standard laboratory methods[18]. The data are automatically and continuously updated, and are not dependent on active reporting by physicians.
Study population
Cases included all cirrhotic patients aged 18 to 80 years diagnosed between 2001 and 2018 using the International Classification of Diseases, 9thRevision (ICD-9) codes (Supplementary table 1). The first diagnosis was defined as the index date.
Controls were hepatic disease-free MHS members, matched for age, sex and birth country at a ratio of 1:3. Sampling date in the control group was matched to the cirrhosis diagnosis date. All study patients were required to have at least three PTC measurements prior to index date.
Patients with known etiologies for thrombocytopenia other than cirrhosis (various diseases and medications), as indicated in the medical record, were excluded from analysis (ICD-9 codes in Supplementary Tables 2 and 3).
Clinical data
Cirrhosis etiology (viral, autoimmune/cholestatic, NAFLD) as well as data regarding the complications of cirrhosis and portal HTN (hepatocellular carcinoma, ascites, varices, hepatic encephalopathy, splenomegaly) were all based on ICD-9 codes (Supplementary Table 4). We calculated the longitudinal trends of PTC as well the following laboratory parameters throughout the preceding 20 years prior to cirrhosis diagnosis compared to healthy controls: Complete blood count, bilirubin (total), liver enzymes [aspartate aminotransferase (AST), alanine transaminase (ALT), gammaglutamyl transferase (GGT) and alkaline phosphatase], coagulation tests [prothrombin time (PT/INR), aPTT] and albumin were recorded (normal ranges are presented in Supplementary Table 5). APRI and FIB-4 were calculated for each patient according to the previously described formulas[15].
Ethical consideration
The study was approved by the MHS institutional review board (IRB). Since this is a retrospective study in which we used coded (anonymized) administrative data from electronic medical records, exemption from informed consent was granted by the IRB committee.
Statistical analysis
The statistical analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, United States). Significance was set atP< 0.05. Categorical variables are presented using frequencies and percent. Continuous variables are presented using mean (standard deviation) [median, interquartile range]. The non-parametric locally weighted scatterplot smoothing was used for the presentation of the PTC (as well as other laboratory measurements) throughout 15 years period. For the cirrhosis group, the measurements were prior to the cirrhosis diagnosis and for the control group, the measurements were prior to the sampling year of each individual. Multivariable logistic regression was performed using PROC GENMOD utilizing general estimation equation methodology for correlated data (i.e., several platelets measurements for each subject). The model included the platelets measurements, as well as the time gaps (in years) of each measurement from the diagnosis/sample year for the cirrhosis and control respectively. The number of measurements was also included in the model. Adjusted odds ratio as well as 95%CI were used to display the association between the study groups and the potential risk factors.
RESULTS
Characteristics of the study population
Characteristics of study population are presented in Table 1. The mean age in both groups was 56 (SD 15.8) and 54% were females. Most patients (25.7%) were diagnosed with cirrhosis in the years 2009-2012 while the least (14%) were diagnosed earlier between 2001 to 2004. Co-morbid conditions are presented in Table 2. Cases were more likely to be smokers (OR = 1.5; 95%CI: 1.39-1.6) as well as to be diagnosed with diabetes (OR = 1.17; 95%CI: 1.02-1.33), chronic kidney disease (CKD, OR = 1.24; 95%CI: 1.09-1.4) and tended to have higher prevalence of HTN (OR = 1.15; 95%CI: 0.96-1.37).
Cirrhosis etiology and complications
Of the cases with known etiology (n= 2058), the most common etiology for liver disease was viral infection (48%) followed by alcoholic liver disease (ALD, 24%) and NAFLD (20%). A total of 2768 cases had complications of liver cirrhosis, includingsplenomegaly (14.5%), varices (10.5%), ascites (8.5%) and hepatic encephalopathy (5.6%). Portal vein thrombosis was documented in 1.3% of cases and 10 patients (0.19%) had HCC (Table 3).
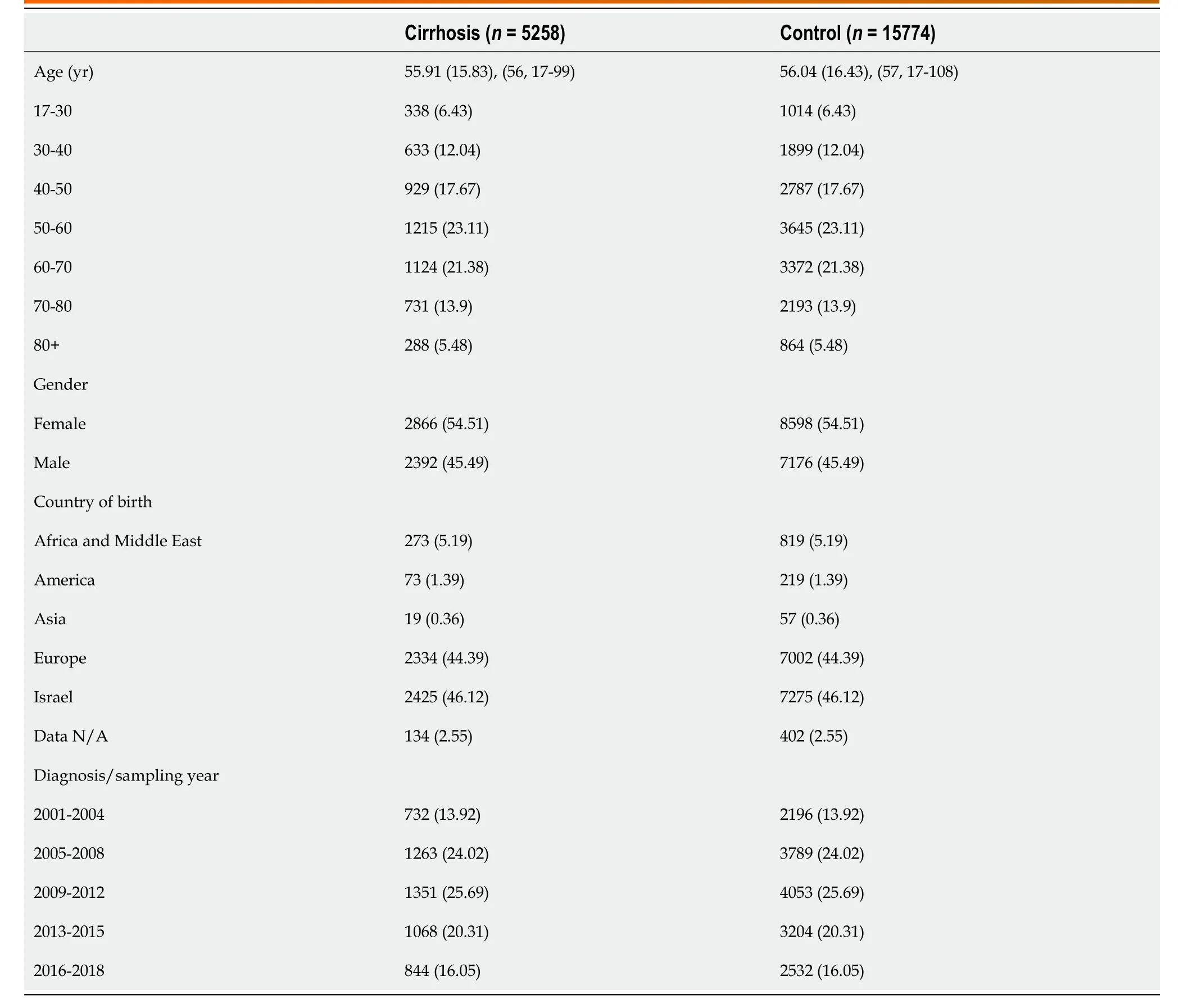
Table 1 Demographic characteristic of cases with cirrhosis and controls, n (%)
Platelets trends along 15 years prior to cirrhosis diagnosis and comparison to controls
In both groups, the mean time gap between the first PTC and the diagnosis/sampling year was similar (7.57-7.68 years) and the mean number of platelets measurements increased gradually from 2.5 (SD 2.1) to 6.4 (SD 5.7) close to the diagnosis date.
The platelets trends along the study years (total 250646 platelets measurements) stratified by the study groups are presented in Figure 1. The mean PTC in the cirrhosis group decreased from 240000/μL starting 15 years prior to cirrhosis diagnosis to approximately 190000/μL close to the diagnosis date. In the control group, the PTC remained stable throughout the years (240-250000/μL). In addition, for each subject in both groups, the mean PTC was calculated per year; the difference in mean PTC per year between groups is presented in Supplementary Table 6.
Males had lower baseline PTC in both groups (Supplementary figure 1). In the cirrhosis group, males had a mean PTC of 210000 decreasing to 170000/μLvsfemales, with a mean number of 250000 decreasing to 215000/μL. In the control group, the same pattern was observed: males had a mean PTC of 225-230000/μL compared to females with ranges of 250000/μL. However, despite the sex differences, the trend of gradual decrease in PTC prior the diagnosis of cirrhosis was seen in both sexes. Additional sub-grouping was performed in order to assess whether age had a modifying effect within each sex. Among younger patients (17-40), PTC was constantin both males and females throughout the years, while among older patients, a trend of gradual decrease in PTC was seen in both sexes prior to cirrhosis diagnosis.

Table 2 Comparison between the number of platelets measurements and co-morbidities between cases with cirrhosis and controls, n (%)

Table 3 Distribution of cirrhosis etiology and complications among cirrhotic patients (n = 5258)
In contrast, there was no significant change in hemoglobin levels in both groups (range of 12.8-13.4 g/dL). But, there was a steep decrease in white blood cell (WBC) counts prior the diagnosis of cirrhosis compared to controls, which had an increase of these values (Supplementary figures 2 and 3).
Platelets trends among cirrhosis patients by etiology and complications

Figure 1 Trends in platelet counts across 15 years prior to cirrhosis diagnosis among cases and controls (n total = 21032, 250646 platelets measurements). Done with locally weighted scatterplot smoothing trend. The mean platelet counts in the cirrhosis group decreased from 240000/μL to 190000/μL, starting 15 years prior to cirrhosis diagnosis, compared to stable values in the control group.
In the cirrhosis group, the trend in PTC was calculated and compared among the most common etiologies of liver cirrhosis in the cohort (viral and ALD). There was a gradual decrease in PTC prior to cirrhosis diagnosis, within the normal ranges in both etiologies. However, cirrhotic patients with ALD had lower mean platelets levels compared to those with viral liver disease, starting 15 years prior to cirrhosis diagnosis (230-180000/μLvs200-170000/μL) (Supplementary figure 4A and B). Stratification of cases by complications of cirrhosis and portal HTN revealed a steeper decrease in PTC in cirrhotic patients who had esophageal varices, ascites and hepatosplenomegaly compared to cirrhotic patients with no such complications (Figure 2A and B).
Trends of liver enzymes and other laboratory markers of liver function
The trends of liver enzymes during the years prior to the diagnosis of cirrhosis (or sampling year for the control group) were calculated (Supplementary Figures 5-8). Compared to controls, whose enzymes levels remained stable and within the normal range, there was a gradual increase in both ALT and AST in cirrhotic patients during the 15 years preceding the diagnosis of liver cirrhosis, whose mean levels were both above the normal range: ALT increased from 50 U/L 15 years prior diagnosis to 75 U/L close to the diagnosis date; AST increased from 40 U/L to 70 U/L close to the diagnosis date.
Similarly, a gradual increase in both cholestatic enzymes could be seen during the years prior to the diagnosis of cirrhosis: Alkaline phosphatase increased from 75 U/L 15 years prior diagnosis to 135 U/L close to the diagnosis dates; GGT increased from 60 U/L to 200 U/L close to the diagnosis date. As for the control group, there was a gradual mild increase in both enzymes over the years.
Regarding markers of the synthetic functions of the liver, a gradual increase in bilirubin levels occurred within the normal range, in the cirrhosis group compared to controls. Albumin levels decreased in both groups, but remained within the normal range (Supplementary Figure 9).
Trends in fibrosis scores
We calculated FIB-4 and APRI for both cirrhotics and controls. In the preceding years before cirrhosis diagnosis, FIB-4 and APRI increased gradually, ranging from 1.3 to 3 and 0.48 to 0.93 respectively, compared to controls whose scores either increased minimally or remained stable respectively throughout the years (Figure 3A and B).
Association between PTC and cirrhosis in multivariate analysis
Since cases were matched with controls by age, gender, birth country and time period (i.e.cirrhosis diagnosis year and the sampling year for the control group), these factors were adjusted by selection. After adjusting for the platelet’s measurements time points and the number of measurements, we found that for every 50 units decrease in the platelets, the odds of cirrhosis increase by 1.3-fold (95%CI: 1.25-1.35).
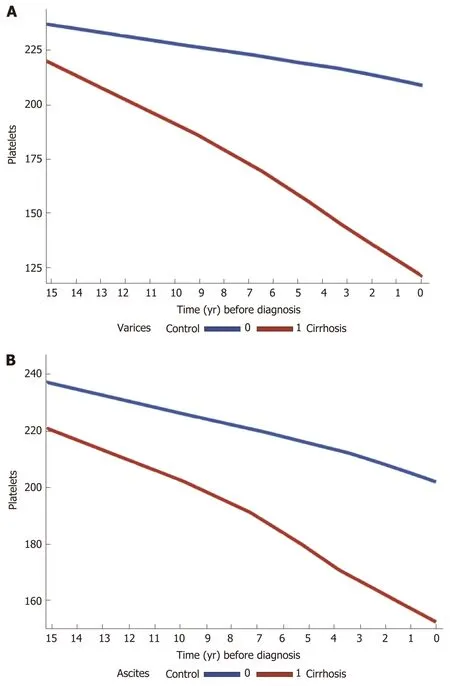
Figure 2 Trends in platelet counts across 15 years prior to cirrhosis diagnosis among cases and controls, stratified by cirrhosis complications A: Varices (n = 551) and B: Ascites (n = 450), both done with locally weighted scatterplot smoothing for trend. In patients with complications of portal hypertension (varices and ascites), platelet counts decline is steeper compared to those with no such complications.
DISCUSSION
Based on a large, well-characterized cohort, the results of this nested case-control study indicate that liver cirrhosis is characterized by a longitudinal decrement in platelet counts, within the normal limits, that may start 15 years prior to diagnosis. This trend is consistent regardless of sex, etiology of liver disease and observed after the age of 40.
In the cirrhosis group, more patients had diabetes mellitus (DM), CKD, a tendency for HTN and were smokers compared to controls. The relationship between these metabolic factors and CLD or cirrhosis is well established. However, their effect on PTC is unclear. We did not find a clear relationship between the presence of DM or HTN and lower PTC in the literature. The exact pattern of PTC in patients with CKD is controversial but several studies revealed a decrease in PTC and platelets dysfunction in renal failure[19-21]. The trend of PTC decrease was consistent in both sexes although males had generally lower PTC values compared to females among both cases and controls. The literature on thrombocytopenia in liver disease does not show clear gender predominance so we can assume that this difference, in both groups, is physiological[22-25].
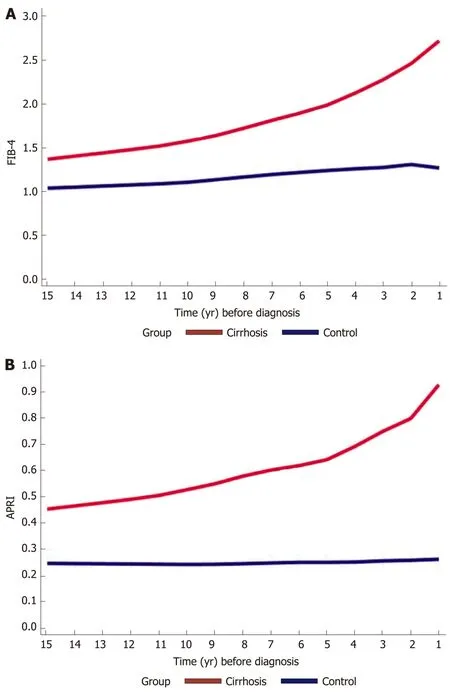
Figure 3 Trends in fibrosis-4 and aspartate aminotransferase-to-platelet ratio index scores across 15 years prior to cirrhosis diagnosis among cases and controls. Trends in A: Fibrosis-4 and B: Aspartate aminotransferase-to-platelet ratio index scores, both done with locally weighted scatterplot smoothing trend. There is a gradual increase in both scores in the cirrhosis group compared to controls, ranging from 1.3 to 3 and 0.48 to 0.93 respectively.
We compared other laboratory parameters that are related to CLD and portal HTN and examined the trends in the years prior to the diagnosis of cirrhosis. ALT, AST and GGT where above the upper normal limit (UNL) in the years preceding the diagnosis of cirrhosis, markedly so in the last two years before diagnosis, compared to controls in which liver enzymes were in the normal range. In both groups, there was a slight gradual increase in bilirubin and a slight decrease in albumin. These laboratory changes are probably physiological; several studies show small but incremental increases in bilirubin and a fall in serum albumin concentration that occurs with increasing age[26-29]. There was no change in the hemoglobin levels, while WBC decreased in cirrhotics compared to controls.
Due to the nature of the study we cannot ascertain why specific blood tests were taken and if liver disease was suspected by the treating physician which triggered more laboratory testing. Compared to the liver enzymes that were above to UNL and should have theoretically alerted the treating physician to the presence of liver disease, at any given time or visit, the PTC during this period decreased within the normal range and were thus easy to miss.
The most common etiologies for cirrhosis in our cohort were viral hepatitis, ALD and NAFLD. The trend of decrease in PTC in our study was consistent regardless the etiology of liver cirrhosis. It was previously suggested, that different etiologies may be associated with different hepatic damage mechanisms relating to platelets which play a role in the induction of hepatic fibrosis[30,31]. A previous study in NAFLD, showed that PTC were decreased in cirrhotics over a 5 year follow up compared to healthy controls[14]. Additionally, a negative correlation between the PTC and the severity of liver fibrosis in NAFLD patients has been demonstrated; a linear decrease of the PTC was correlated with increasing histological fibrosis stage. A similar trend occurs in chronic HBV and HCV infection; previous studies showed that liver fibrosis in HCV patients, assessed by biomarkers and FibroScan?, was negatively correlated with PTC. Moreover, patients with advanced liver fibrosis had significantly lower PTC[32-36].
The platelets decrease trend in our study was most pronounced in patients with complications of liver cirrhosis and portal HTN with the steepest decline in patients who had varices. Studies show that together with liver and spleen stiffness measurements, PTC correlates with significant portal HTN and particularly in the presence of varices. The risk of having varices increases with decreasing PTC and have been used to assess the presence of varices non-invasively[37-40].
Our results suggest for the first time that the platelets trends can be used for the prediction of liver cirrhosis regardless the underlying etiology. In multivariate regression analysis, we found that for every 50 units decrease in the PTC, the odds of cirrhosis were 1.3 times higher. When looking at the normal range of PTC, a gradual decrease in PTC, still within the normal range, from 380 to 180 over time would signify 5.2- fold increase in the risk of being diagnosed with cirrhosis compared to individuals with no change in the PTC. Indeed, the progression to cirrhosis usually takes years to develop and may be missed due to lack of clinical symptoms or laboratory aberrations before significant portal HTN appears.
Diagnosis and staging of liver fibrosis are vital part of the clinical management of CLD of any etiology as it is associated with poor outcomes. Although liver biopsy is recommended as the gold standard for the diagnosis and staging of fibrosis, due to its invasive nature and other disadvantages, indirect assessments of liver fibrosis have been developed and are widely used. These include blood-based biomarkers (APRI, FIB-4, enhanced liver fibrosis, Fibro Test) and image-based techniques (US, transient elastography, shear wave elastography, Magnetic resonance elastography) as well as innovative methods that uses combined modalities including advanced magnetic resonance imaging sequences like diffusion-weighted magnetic resonance imaging and genetic testing[41,42].
Fibrosis risk scores have been developed based on readily available clinical and laboratory parameters that are simple to use at point of care, and can be implemented into computerized medical systems. However, current risk scores have several limitations; they incorporate PTC in the formulation, however, they do not consider progressive, longitudinal changes in PTC and use a single platelets value each time they are used[15,16,43-45].
Among others, FIB-4 have been most extensively studied and validated in diverse populations for the prediction of advanced fibrosis. Two cut-off values were defined; FIB-4 score ≤ 1.3 can be regarded as having a low risk for advanced fibrosis while score > 3.25 represents advanced fibrosis or cirrhosis. It was published previously, that intermediate FIB-4 values of 1.45–3.25 have negative predictive value of 89% for excluding advanced fibrosis and patients in this range would require a liver biopsy to assess the fibrosis stage. Thirty to forty percent of patients have an indeterminate score, and in these cases, additional testing is needed[35,46].
In our study, we show that along with the increase in AST (above the ULN) and age, a longitudinal PTC decrease before the diagnosis of cirrhosis, still within the normal range was associated in high FIB-4 and APRI scores, which were mostly in the range of 1.4-3.25, reaching values compatible with advanced fibrosis. Together with the intermediated values of FIB-4, the longitudinal PTC decrease, even within the normal levels, may reflect progressing fibrosis and can predict cirrhosis development. These combined changes may be picked up by computers and alert the treating physician of an ongoing liver disease before advanced fibrosis takes place, enabling therapeutic and preventing measures.
We acknowledge several limitations of this study. The main limitation is the retrospective nature of the study, with its built-in weaknesses of data collection and selection bias. Due to the nature of the study, we could not know why specific data was ordered/collected; especially which circumstances have led to the diagnosis of cirrhosis. Additionally, clinical events may have been missed or only partly followed up, so that the diagnosis of cirrhosis could have been missed or not recorded. Additionally, we could not look at radiology or endoscopic results of each patient in order to identify signs of CLD, cirrhosis and portal HTN. All diagnoses were made exclusively according to ICD-9 codes. However, although we might have missed a large number of undiagnosed cirrhotic patients, our sample is large and representative enough to offer sound observations.
The number of platelets measurements and distribution in the preceding years before cirrhosis/sampling date was not equal. Cirrhotic patients had more PTC generally with the highest platelets measurements close to the diagnosis date. We hypothesize that there was a recognizable change in the medical condition of the patient which lead to more frequent tests. Due to the nature of this study, this information is not available. However, adjustment for the number and timing of testing did not attenuate the association.
Other limitations should also be noted. A relatively large proportion of patients in the cirrhosis group had missing data regarding the etiology of cirrhosis. However, this should not have an effect on the general observation.
This study also holds important strengths. We present longitudinal changes in PTC, compared to previous studies in which PTC were presented as a single measurement in a certain point of time. By using continuous and repeated measurements for the same individual, this method represented dynamic changes in laboratory data that indicated a trend before the diagnosis of cirrhosis. This method could potentially be used for longitudinal assessment of fibrosis regression following therapeutic interventions such as antiviral therapy or life style changes for NAFLD.
The recent interest in Big Data Mining, which is aimed at identifying patterns that are often unrecognizable during routine clinical management, enabled us to use the MHS database which offers high-quality data from electronic medical records, automatic data capture, and a central laboratory. The large number of members in this insurance group enabled the inclusion of a large study population both overall and in matched groups during a long period of time. Patients with various diseases (hematological/viraletc) and medications that could affect the PCT were excluded from the study so that the change in PTC could be attributed with high probability to the ongoing liver disease. Furthermore, our cohort represents a cohort of cirrhotic patients in a community and likely avoids selection bias seen in cohorts from tertiary referral centers.
CONCLUSION
Years before the diagnosis of liver cirrhosis is made there is a progressive decline in platelet counts, within the normal range, matched to a gradual increase in fibrosis scores. These changes may be identified by machine learning algorithms and alert the treating physicians of an early liver disease and may enable early therapeutic and preventive interventions before serious complications occur.
ARTICLE HIGHLIGHTS
Research objectives
To explore whether big data analysis of PTC trajectories over time, can predict advanced liver fibrosis and cirrhosis complications across the different etiologies of liver diseases.
Research methods
A nested case-control study with diagnosed cirrhosis patients and matched controls, utilizing the Maccabi Health Services database was performed. The trends of PTC, liver enzymes, bilirubin, international normalized ratio, albumin and fibrosis scores [fibrosis-4 (FIB-4) and aspartate transaminase-to-platelet ratio index] throughout the preceding 20 years prior to cirrhosis diagnosis were calculated and compared to healthy controls. The association between PTC, cirrhosis complications and fibrosis scores prior to cirrhosis diagnosis was investigated.
Research results
Cirrhosis cases (n= 5258) were compared to controls (n= 15744) matched for age and sex at a ratio of 1:3. The leading cirrhosis etiologies were viral, alcoholic and fatty liver disease. The mean PTC decreased from 240000/μL to 190000/μL up to 15 years prior to cirrhosis diagnosis compared to controls who’s PTC remained stable around the values of 240000/μL. This trend was consistent regardless of sex, cirrhosis etiology and was more pronounced in patients who developed varices and ascites. Compared to controls whose values remained in the normal range, in the cirrhosis FIB-4 increased gradually from 1.3 to 3 prior to cirrhosis diagnosis. Additionally, in multivariable regression analysis, a decrease of 50 units in PTC was associated with 1.3 times odds of cirrhosis (95%CI: 1.25-1.35).
Research conclusions
This study indicates that a progressive decline in platelet counts, within the normal range, is associated with a gradual increase in fibrosis scores, starting up to 15 years before the diagnosis of cirrhosis.
Research perspectives
Progressive PTC decline in the preceding years before the diagnosis of liver cirrhosis, when still within the normal limits, may be identified by machine learning algorithms and alert the treating physicians of an early liver disease and may enable early therapeutic and preventive interventions before serious complications occur.
ACKNOWLEDGEMENTS
The author is grateful to the staffs in Maccabi Health Services for their valuable assistance with this work.
 World Journal of Gastroenterology2020年38期
World Journal of Gastroenterology2020年38期
- World Journal of Gastroenterology的其它文章
- Role of artificial intelligence in the diagnosis of oesophageal neoplasia: 2020 an endoscopic odyssey
- Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma
- Tacrolimus and mycophenolate mofetil as second-line treatment in autoimmune hepatitis: Is the evidence of sufficient quality to develop recommendations?
- Comparative study between bowel ultrasound and magnetic resonance enterography among Egyptian inflammatory bowel disease patients
- Monitoring hepatitis C virus treatment rates in an Opioid Treatment Program: A longitudinal study
- Endoscopic ultrasound-measured muscular thickness of the lower esophageal sphincter and long-term prognosis after peroral endoscopic myotomy for achalasia
