Vedolizumab for ulcerative colitis: Real world outcomes from a multicenter observational cohort of Australia and Oxford
Samba Siva Reddy Pulusu, Ashish Srinivasan, Krupa Krishnaprasad, Daniel Cheng, Jakob Begun, Charlotte Keung, Daniel Van Langenberg, Lena Thin, Tamara Mogilevski, Peter De Cruz, Graham Radford-Smith,Emma Flanagan, Sally Bell, Soleiman Kashkooli, Miles Sparrow, Simon Ghaly, Peter Bampton, Elise Sawyer,Susan Connor, Quart-ul-ain Rizvi, Jane M Andrews, Gillian Mahy, Paola Chivers, Simon Travis, Ian Craig Lawrance
Abstract
Key words: Vedolizumab; Ulcerative colitis; Outcomes
INTRODUCTION
The aim of treatment in ulcerative colitis (UC) is to achieve sustained clinical, mucosal and histological healing[1,2]. The choice of treatment depends on several factors including induction, or maintenance, of disease remission, severity of disease, extent and location of bowel involvement, disease phenotype and individual characteristics of the drug and patient. The use of conventional medications may be limited either by a lack of efficacy (5-aminosalicylates) or side effects [steroids/azathioprine (AZA)/6 mercaptopurine (6MP)/methotrexate (MTX)][3]. Its’ use, however, is not without potential side effects including, development of opportunistic infections, reactivation of tuberculosis and an increased risk of melanoma[4].
Vedolizumab (VDZ), a humanised monoclonal antibody, selectively inhibits the migration ofalpha4-beta7inflammatory cells to the gastrointestinal tract, making it a biological agent without systemic immunosuppression and thus potentially reducing side-effects. In GEMINI 1, the randomised, double-blind placebo-controlled trial of VDZ in UC, the response rate for induction at week six was 47.1% with a response rate of 41.8% at week fifty two after eight-weekly VDZ treatments[5]. Patients enrolled in clinical trials, however, do not entirely represent the patients seen in routine clinical practice as demonstrated by a retrospective study where only 31% of 206 patients with moderate-to-severe inflammatory bowel disease (IBD) were eligible to participate in such a clinical trial[6].
Our aim was to assess the response and remission rates to VDZ in the real world, the time taken to achieve this, mucosal healing rates, adverse/serious events, the rates of colectomy and the predictors influencing remission in the first 12 mo of VDZ therapy through a multicenter consortium in a real-world setting.
MATERIALS AND METHODS
Study design
This was a multicenter retrospective review of prospectively collected data involving 14 IBD centers in Australia New Zealand inflammatory bowel disease consortium and data was also collected at a major IBD center in United Kingdom, thus reducing physician, site and country bias. All the centers involved in the study had a dedicated IBD team. In Australia, patients with UC refractory to conventional treatment, which was defined as failure of three, or more, mo of a 5-aminosalycylate and failure of three or more mo of an immunomodulator (AZA, 6MP or MTX) and 6 wk weaning dose of prednisolone that commenced at 40mg per day or more, were able to access VDZ from 2015 through the government funded pharmaceutical benefit scheme (PBS). In the United Kingdom, VDZ was given to patients at the physicians’ discretion if the conventional treatment and/or anti-tumor necrosis factor (anti-TNF) medications had failed to control the disease.
Consecutive patients with UC diagnosed as per the standard criteria[7]who received at least induction VDZ therapy were considered for the study. All patients who finished VDZ induction therapy were included in the study for analysis. VDZ was given as standard intravenous (IV) induction dosing of 300mg at 0, 2 and 6 wk followed by maintenance therapy of 8 weekly IV infusions. Patients continued to take, or wean off, steroids, 5-aminosalicylates (oral and rectal therapy) as deemed appropriate by the treating physician. Patients taking immunosuppressant medications, including AZA, 6MP, MTX orally, or rectal tacrolimus, continued on these medications under the treating physicians’ preference as guided by the disease control. There were no mandated changes to a patient’s regular IBD medications. The use of steroids and/or immunomodulators and their time of cessation was recorded for analysis.
A retrospective review of the IBD databases that contained prospectively-entered data included baseline patient demographics and disease characteristics classified by the Montreal classification[8], concomitant use of steroids and immunomodulator medications, prior exposure to anti-TNF medications, adverse events and colectomy rates.
Assessments tools and criteria
The Montreal classification was used to classify UC[8]. The Partial Mayo clinical score was used to assess disease control and is composed of 3 items, which includes stool frequency, rectal bleeding and the physician global assessment which were each scored individually from 0 to 3 at baseline, 3, 6 and 12 mo. The higher the score, more severe the disease and maximum score was 9. The Mayo endoscopic score (MES) is classified into four levels of severity from 0-4 based on mucosal friability, vascular pattern, friability and erosions. Mayo 0-1 was inactive disease while Mayo 2 and Mayo 3 were mild-moderate and moderate-severe disease respectively. Ulcerative colitis endoscopic index of severity (UCEIS) is composed of 3 items, which includes vascular pattern, bleeding and erosions/ulcers with score ranging from 0-2 for vascular pattern and scores 0-3 for bleeding and erosions/ulcers with higher scores indicating severe disease and the maximum score was 9. The response and remission to VDZ was assessed clinically using partial Mayo clinical score in both Australia and the United Kingdom sites. MES was used for assessing endoscopic appearance in Australia, and the UCEIS was used in the United Kingdom.
Evaluation of clinical efficiency at 3, 6 and 12 mo
Induction:Clinical efficiency of VDZ induction therapy was assessed as either clinical response or clinical remission at 3 mo. A response to VDZ was defined as a decrease in the Partial Mayo score of ≥ 3 from baseline, while clinical remission was defined as Partial Mayo score of < 2.
Maintenance
Clinical efficacy of VDZ was also assessed at 6 mo and 12 mo of VDZ therapy. The Partial Mayo score again was used to assess clinical response and clinical remission. Data was collected if VDZ was ceased due to side-effects, or loss of response (LOR) that resulted in a switch of the therapy away from VDZ, or surgery if it was required.Endoscopic assessment of disease control was undertaken after approximately 6 mo of VDZ. An MES of 0 or 1 was defined as an endoscopic remission with a score > 1 indicating active disease. An UCEIS score of 0-1 was also defined as endoscopic remission with a score > 1 indicating active disease.
Corticosteroid and Immunomodulator therapy
Corticosteroid therapy was defined as the use of any oral steroid including prednisolone and budesonide. Immunomodulator therapy includes any oral or rectal immunomodulator which includes AZA, 6MP, MTX (oral or parenteral), tacrolimus (oral or rectal), ciclosporin and mycophenylate. Corticosteroid and immunomodulator use was assessed at baseline, 3, 6 and 12 mo.
Safety
The development of infusion reactions, adverse events and serious adverse events were all recorded. Infusion reactions were defined as any adverse event that occurred on the day, or the day after, the infusion. Adverse events were defined as any untoward medical occurrence not resulting in discontinuation of the VDZ or hospitalization. Adverse events were graded as serious if they resulted in discontinuation of VDZ, hospitalization of the patient, or patient death.
Statistical analysis
Data for each patient from their first dose of VDZ to either last infusion within the study period or cessation of the VDZ were included for analysis. All statistical analysis was conducted using SPSS version 24 (IBM Corp, released 2016). Statistical significance was set atP< 0.05. Patient demographic and disease profile information was described using frequency and percent for categorically classified variables, with mean and standard deviation and median and range used to describe scale variables. VDZ treatment outcomes are described at baseline, 3, 6 and 12 mo. Sample size varied across measures and was reported accordingly.
Two Cox Regression models and Kaplan Meier curves were performed separately for each site. The first model examined time (weeks) to remission. Time to censor was calculated as the difference between the date of remission (censor event) and the date the patient started the study. The second model examined time (weeks) to failure. Failure was defined as Partial Mayo clinical score ≥ 2 or MES ≥ 2, or the need for a change in the biologic agent, or requiring colectomy. Time to censor was calculated as the difference between this date of failure (censor event) and the date the patient started the study, truncated to 60 wk. Both models examined the covariates gender, disease duration (year), smoking (non–smoker/current smoker or ex smoker), disease location, age at which VDZ was given (year), and previous immunomodulator exposure. For the failure model, a remission covariate was also included (median split ≤ 13/> 13 wk of time to remission). For both models where remission or failure occurred at the start of the study, a small constant (0.01) was added to the time to event variable. Cox model proportional hazard assumption was tested using Schoenfeld residuals with no violations. A chi square analysis was used to investigate the remission and failure censor variables with anti-TNF, and site differences across categorical variables. Disease duration, age vedolizumab started, remission time and failure time were examined for normality using Shapiro Wilk and violations were noted. Therefore site differences for these continuous variables were examined using the non-parametric alternative Mann WhitneyUTest. Further Cox Regression models and Kaplan Meier curves were performed using the combined data set with site included as a variable.
RESULTS
Patient characteristics
Three hundred and thress UC patients (Australian= 210 and Oxford, United Kingdom,n= 93) from 15 centers (Australian= 14) were included in the study. Of the 303 patients, patient data was available in 278 at 3 mo, 250 patients at 6 mo and 209 patients at 12 mo. Of the 303 patients, 15 patients were in remission at the start of VDZ and VDZ was commenced due to side effects to the anti-TNF agents, these patients were analysed separately at each time point (Figure 1).
Patient demographics and clinical characteristics
Of the total 303 patients, 60% (n= 182) were anti-TNF na?ve and VDZ was their first biologic agent, while 40% (n= 121) had prior anti-TNF exposure with a secondary LOR in 20% (n= 61) and primary LOR in 15% (n= 45). VDZ was commenced in 5% (n= 15) of patients due to side-effects from anti-TNF therapy and these patients were in clinical remission at VDZ induction, so were analyzed separately. A total of 47% (n= 143) were female (Table 1).
The median age at which VDZ was started was 35 years (range 16-84 years), while the median disease duration was 6 years (range 0.2-48 years) prior to the commencement of VDZ. A family history was present in 12% (n= 29) and 81% (n= 226) were non-smokers at the time of commencing VDZ. All patients were classified by the Montreal classification[8]and 56% were diagnosed with UC between the ages of 17-40 years (n= 170, A2) compared to younger than 17 years in 11% (n= 34, A1) and 33% older than 40 years (n= 99, A3). Disease extent was extensive in 56% of patients (n= 170, E3) with 38% with suffering left-sided colitis (n= 114, E2) and 6% patients with proctitis (n= 18, E1). Of all patients, 63% (n= 191) were on steroids at the commencement of VDZ, 53% (n= 162) were taking prednisone and 10% (n= 29) budesonide. 58% (n= 175) of the patients were on an immunomodulator, with the thiopurines (AZA/6MP) being the most commonly used in 45% (n= 136), followed byMTX, tacrolimus, ciclosporine and mycophenolate. The mean partial Mayo score was 5 (range 2-9) at commencement of VDZ.
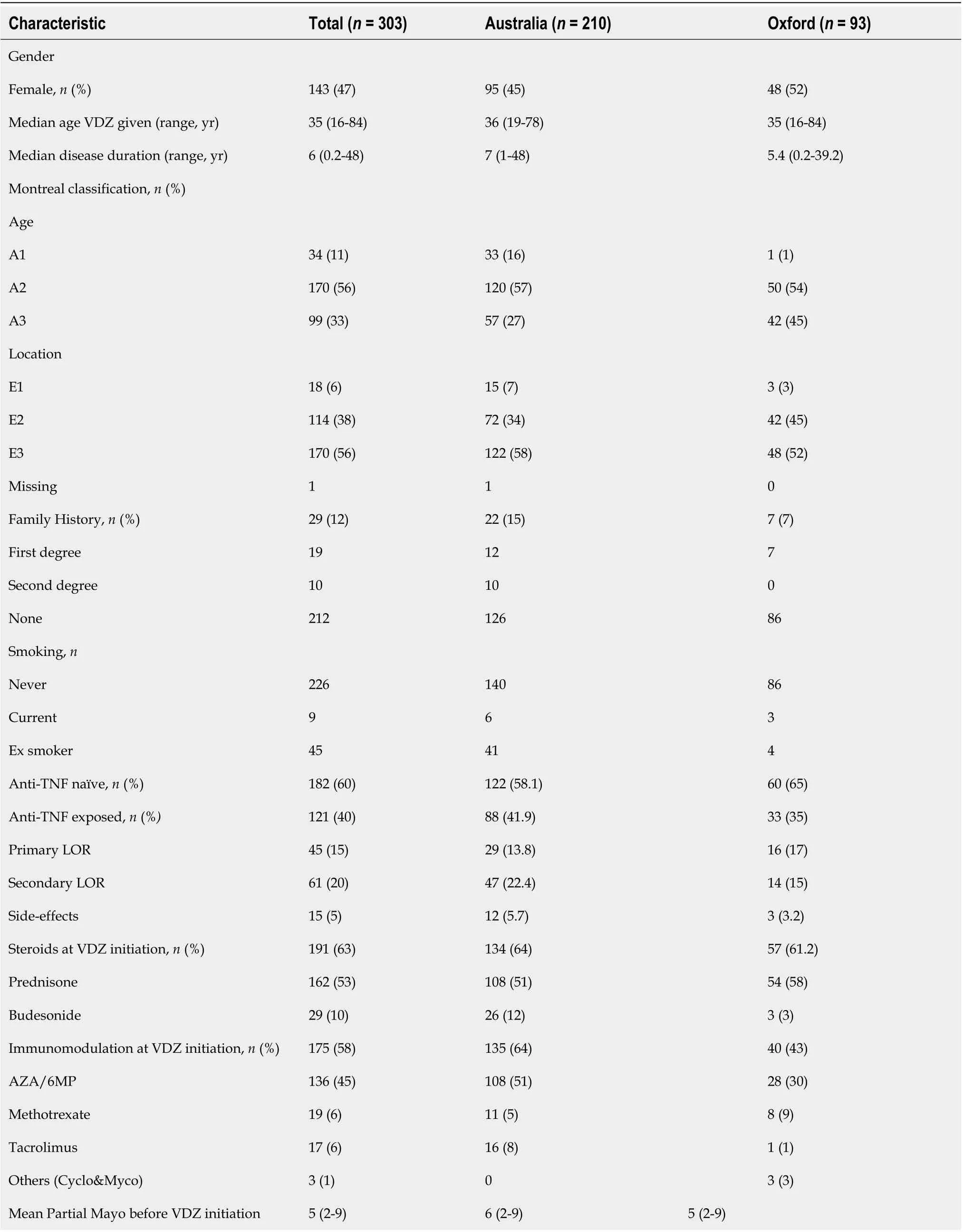
Table 1 Clinical characteristics of study population
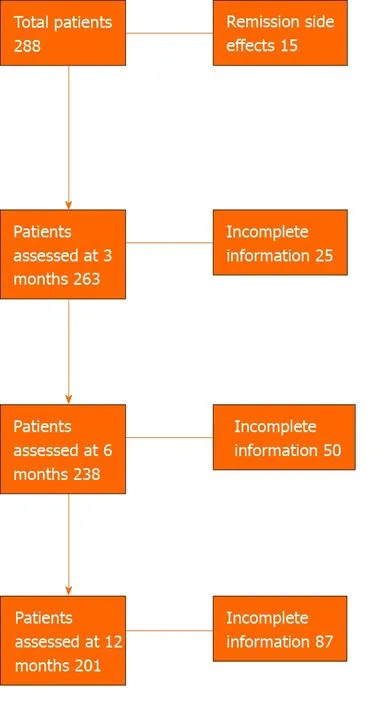
Figure 1 Flowchart.
No significant differences were observed between the Australian and Oxford patients for prior anti-TNF exposure (P= 0.36), sex (P= 0.3), family history (P= 0.43), and age at which VDZ was started (P= 0.35). Significant differences between the Australian and Oxford patients, however, were observed for smoking with more Oxford patients having never smoked (P< 0.001) but there was no difference in the current smokers at time of VDZ commencement. Immunomodulator usage at the commencement of VDZ was greater in Australian than Oxford patients (P< 0.001), and the disease duration in Australian patients was longer prior to VDZ commencement (P< 0.048).
Assessment at 3 mo, 6 mo and 12 mo
A total of 263 patients were not in remission at commencement of VDZ, and of these 79% (n= 208) achieved a clinical response and 56% (n= 148) achieved clinical remission by 3 mo. Three mo data was missing for 25 patients (9 Australia, 16 Oxford), and thus these were not included in the induction analysis but there was data for the clinical status of these patients at 6 and 12 mo.
At 3 mo, Australian patients were more likely to achieve response (P= 0.01), but were not more likely to achieve remission than Oxford patients (P= 0.58) (Table 2). Anti-TNF na?ve patients were more likely to achieve both response (P= 0.03) and remission (P< 0.001) at 3 mo compared to patients who had prior anti-TNF exposure. Within the anti-TNF exposed group, there was no significant difference between the patients who had a primary or secondary LOR to an anti-TNF agent in achieving clinical response (P= 0.9) or remission (P= 0.8) to VDZ at 3 mo.
Of the total 238 patients assessed at 6 mo, 62% (n= 147) were in remission and of the 201 patients assessed at 12 mo, 60% (n= 120) were in remission (Table 3). No significant difference was found between Australia and Oxford in the number of patients in remission at 6 mo (P= 0.3) or at 12 mo (P= 0.09). Anti-TNF na?ve patients were more likely to achieve remission both at 6 mo (P< 0.001) and 12 mo (P= 0.03) than those previously exposed. Within the anti-TNF exposed group, there was no significant difference between the patients who had primary or secondary LOR to anti-TNF agents in clinical remission at 6 mo (P= 0.2) or 12 mo (P= 0.3).
Of the 15 patients who were in remission at the start of VDZ, where the indication was side effects to anti-TNF agents, 66% (n= 10/15) at 3 mo, 58% (n= 7/12) at 6 mo, 50% (n= 4/8) at 1 year were still in clinical remission.
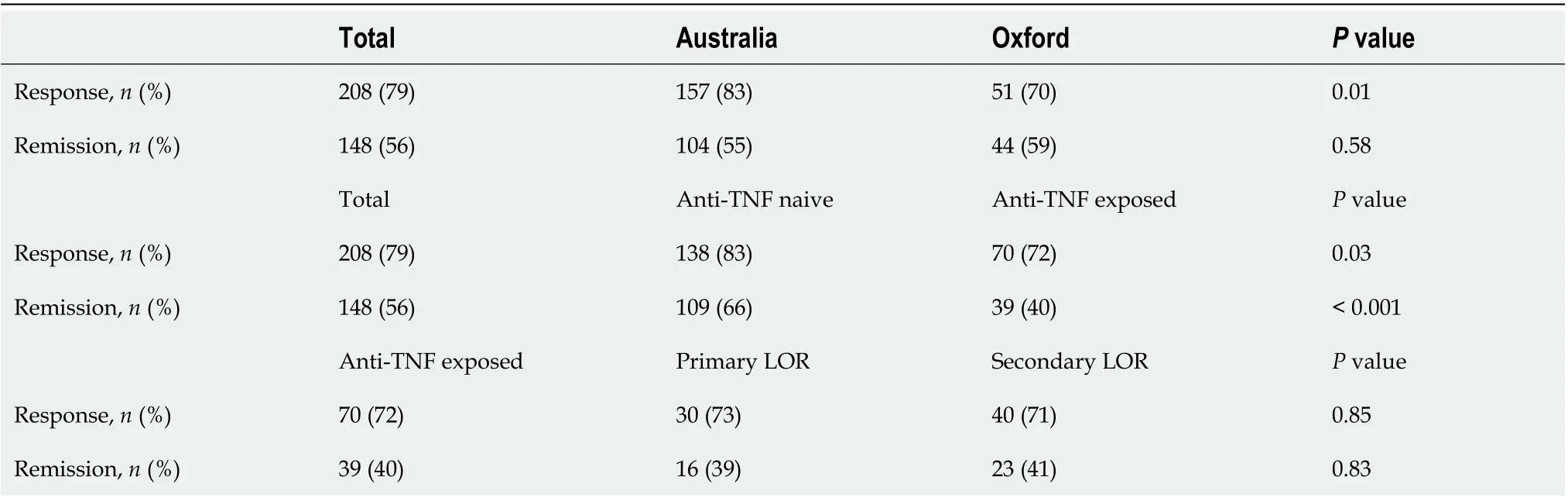
Table 2 Response and remission at 3 mo of vedolizumab therapy
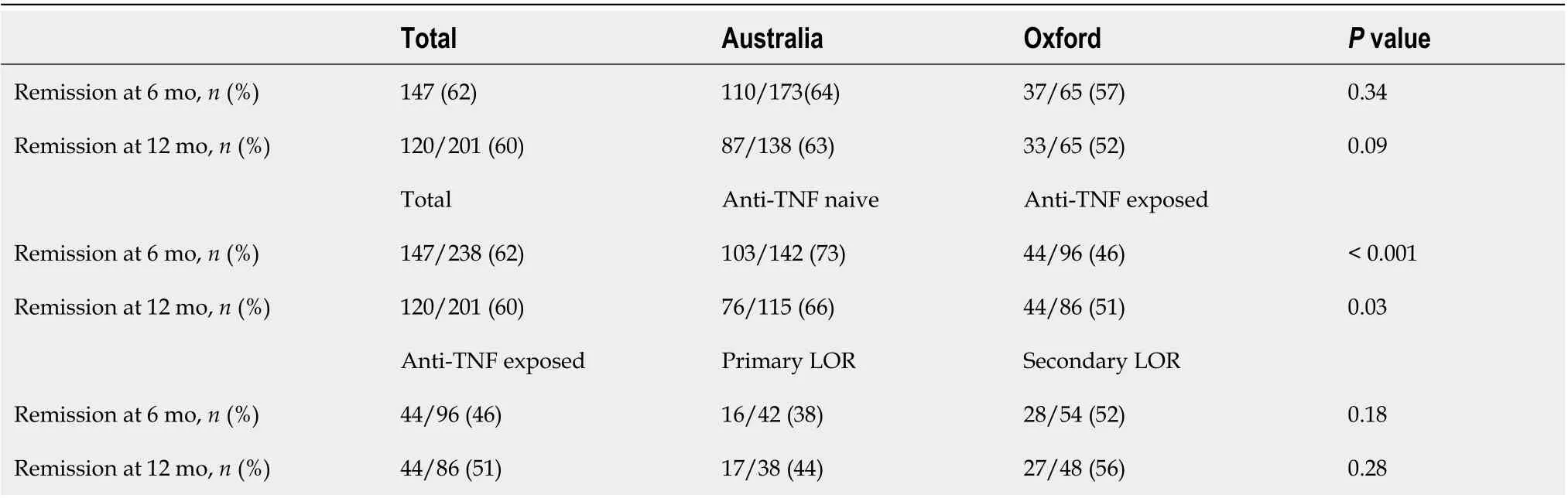
Table 3 Remission at 6 mo and 12 mo of vedolizumab therapy
Endoscopy
A total of 108 patients had endoscopy at 6 mo, 78 in Australia and 30 in Oxford. Of the Oxford patients, 43% (13/30) had an UCEIS of ≤ 1 indicating endoscopic remission. Of the Australian patients, 69% (54/78) had an MES of 0-1 indicating endoscopic remission. A significantly greater percentage of patients achieved endoscopic remission in Australia compared to Oxford (P= 0.01). A MES score of 0 was achieved in 31% (24/78) of the Australian cohort. A MES ≥ 2 was reported in 31% (n= 24) of Australian patients and a UCEIS ≥ 2 was reported in 57% (n= 17) of Oxford patients.
Time to remission
A total of 224 (73.9%) patients were censored as being in “remission”. While controlling for the site, univariate cox regression models for time to remission found no significant associations for gender (P= 0.3), disease duration (P= 0.6), smoking status (P= 0.9), age at which VDZ was given (P= 0.7), immunomodulator exposure (P= 0.8) and these were also not significant when considered with anti-TNF exposure. The final model included anti-TNF exposure and the site with the Log Rank (Mantel-Cox) reporting a significant difference (P< 0.001) between time-to-remission for anti-TNF exposure, with anti-TNF na?ve patients 1.8 times more likely to achieve clinical remission (95%CI: 1.3-2.3) (Figure 2).
Time to failure
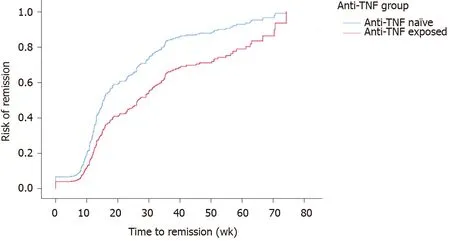
Figure 2 Cumulative remission rate of anti-tumor necrosis factor na?ve patients to vedolizumab therapy (vs anti-tumor necrosis factor exposed patients, P < 0.001).
A total of 84 (27.7%) patients were censored as ‘failure’. Controlling for site, univariate cox regression models for time-to-failure found no significant associations for gender (P= 0.4), smoking status (P= 0.3), age at which VDZ was given (P= 0.9), immunomodulator exposure (P= 0.2). These factors remained not significant when considered with anti-TNF exposure. Disease duration was significant (OR = 0.95, 95%CI: 0.93-1.00P= 0.048), however, was no longer significant when considered with anti-TNF exposure. The final model included anti-TNF exposure and site with the Log Rank (Mantel-Cox) reporting significant difference (P= 0.011) between time-to-failure for anti-TNF groups with anti-TNF exposure patients 1.8 times more likely to lose response (95%CI: 1.16-2.75) (Figure 3).
Safety
The tolerability of VDZ was high with only 8% (n= 25) of all patients reporting an adverse event. Infections (7%,n= 21) were by far the most common adverse event. Two patients reported a serious infection, one was Haemophagocytic syndrome due to Cytomegalovirus and another was from Klebsiella sepsis, both from Australia and both patients were on dual immunosuppression thorough out the study period. A total of 9 patients who received VDZ reported respiratory complications of whom 4 patients reported sinusitis, 2 patients an upper respiratory tract infection, one patient each of nasopharyngitis, pharyngitis and pneumonia. Gastrointestinal infections were reported in 8 patients. Clostridium difficile was the most common gastrointestinal infection (4 patients) followed by Strongyloides (one patient), Campylobacter (one patient), and Salmonella (one patient). A buttock abscess was reported in one patient. Oral thrush was reported in an Oxford patient was attributed to VDZ use. The other complications due to VDZ use reported in our cohort during the study period include rash in one patient, delayed hypersensitivity in one patient and arthralgia and headaches in another patient (Table 4). No deaths were attributed to VDZ use.
A colectomy was undertaken in 11% (n= 32) patients by 12 mo. No significant difference (P= 0.25) in the number of patients requiring colectomy in Australia (n= 19/210) and Oxford (n= 13/93) was observed. Anti-TNF exposed patients (23/121) were more likely to require colectomy compared to anti-TNF na?ve (9/182) by 12 mo (P= 0.0005) but when patients with primary and secondary LOR to anti-TNF agents were compared, no significant difference was noted (Table 5).
DISCUSSION
In this study of 303 UC patients treated with VDZ from 15 specialist IBD centers in two countries, VDZ was noted to be both safe and effective. This study, and the GETAID studies[9], are the only studies where VDZ data is collected from multiple centers encompassing two countries, thus effectively reducing physician, site and country biases.
The key findings in this cohort were that the week 12 response in UC was 79% while remission rates were 56%, 62% and 60% at 3, 6 and 12 mo respectively. No differences were observed between the two countries in achieving remission at all time points. Anti-TNF-exposed patients, however, were almost twice as likely to lose response to VDZ compared to anti-TNF na?ve patients but no difference in VDZ outcomes were observed between patients who had a primary and secondary LOR to anti-TNF agents.Adverse events were observed in 8% of patients and 11% patients required colectomy by 12 mo.

Table 4 Complications of vedolizumab therapy
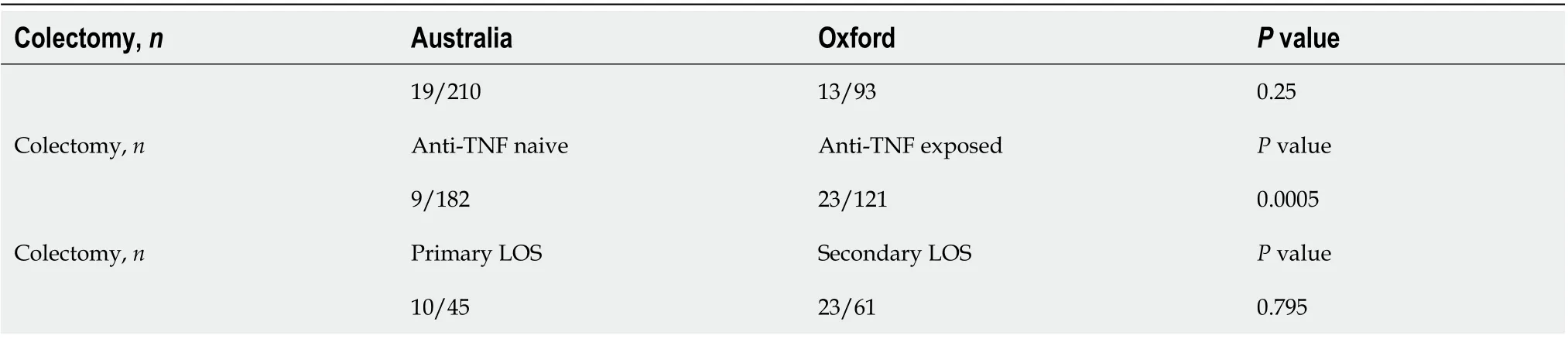
Table 5 Colectomy at 12 mo of vedolizumab therapy
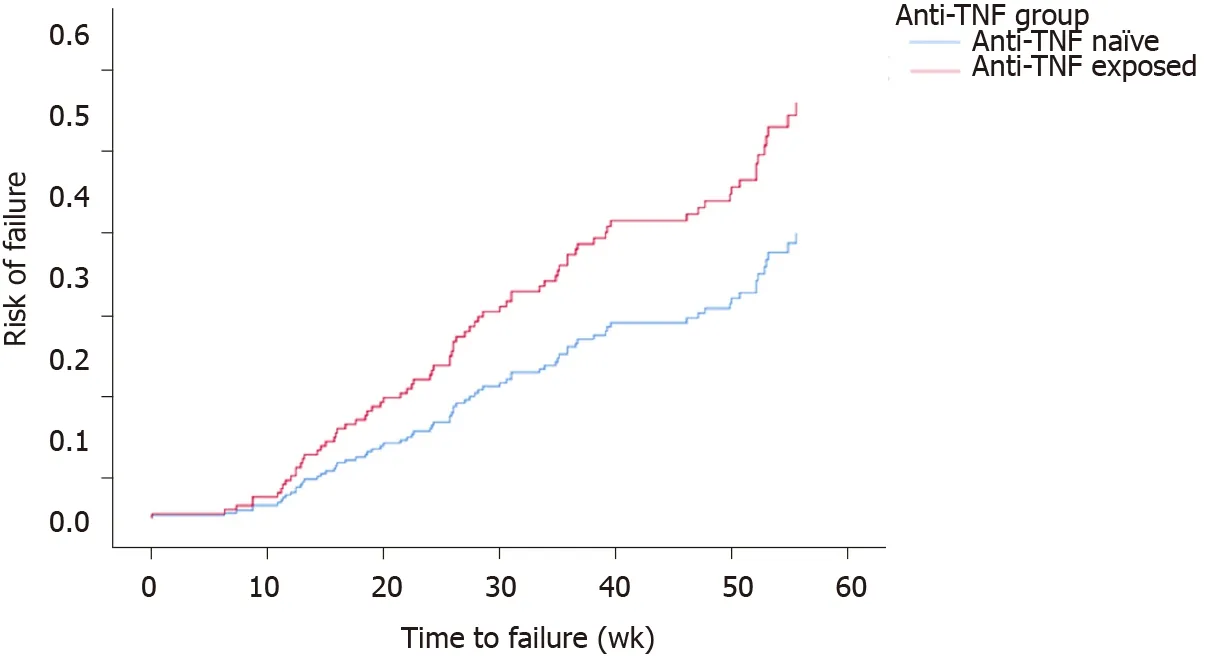
Figure 3 Cumulative loss of response of anti-tumor necrosis factor exposed patients to Vedolizumab therapy (vs anti-tumor necrosis factor na?ve patients, P = 0.011).
Our results were comparable to prior work from other real world studies. A Swedish real world study observed that 64% of UC patients achieved clinical remission at the end of the follow-up period[10], while a French study demonstrated that 40.5% were in steroid-free clinic remission at week 54[11]. The discrepancy in the clinical response rates could be explained by the difference in clinical characteristics of the patients entering the study and also different clinical criteria (steroid-free remission in French study) used to assess the patients. Similarly, other real world data have shown that prior exposure to anti-TNF agents reduces VDZ effectiveness, but no difference in VDZ outcomes when the patients had either primary or secondary LOR to anti-TNF agents is in line with our findings[12]. The adverse event profile with VDZ treatment was also similar to what has been previously reported[13].
Mucosal healing in UC is associated with improved long-term clinical outcomes and STRIDE guidelines identified it as a therapeutic goal[1]. With MES as an endpoint, we report mucosal healing rates of 69% in Australian Cohort at 6 mo compared to 50% in ACT1, 46% in ACT2 at week 30 with Infliximab[14], 59% in PURSUIT at week 30 with Golimumab[15], 25% in ULTRA2 at week 52 with adalimumab[16], 51% in GEMINI1 with VDZ at week 52[5].The high mucosal healing rates observed in our study could be due to high concomitant immunomodulator use in Australia(64%), however further prospective studies are needed to prove the role of immunomodulator use with VDZ.
We chose week 12 to assess the response and remission, in contrast to GEMINI 1 time of assessment for response at week 6[5]. This was done in accordance with Australian PBS criteria, which stipulates that patients must be in remission after induction at clinic review before applying for maintenance VDZ. It appears, however, that the full effect of VDZ may take longer than 12 wk as a longer duration of treatment is associated with a higher rate remission at 6 (62%) than at 3 mo (56%). This study thus suggests that the PBS funding criteria may need to be re-attended to benefit the patients. Compared to its predecessor Natalizumab, analpha4antagonist, VDZ is a more selective integrin antagonist blocking onlyalpha4beta7and thus does not effect lymphocyte trafficking to central nervous system, thereby theoretically eliminating the risk of progressive multifocal leukoencephalopathy (PML), a catastrophic side effect of Natalizumab[17]. No case of PML occurred in our study or any other previous studies with VDZ[18,19].
More Australian patients compared to Oxford patients achieved clinical response at 3 mo (83%vs70%P= 0.01) and endoscopic remission at 6 mo (69%vs43%P= 0.01). This may be due to more patients on concomitant immunomodulation in Australia compared to Oxford (64%vs43% at VDZ initiation). Further analysis of our data and more prospective studies need to be done to define the role of concomitant immunomodulation with VDZ.
Our observed rate of VDZ efficacy at 12 mo in UC (60%) was comparable to the rates reported with anti-TNF agents[20,21]. While there are no head to head randomized control trials comparing VDZ and infliximab in UC, VDZ showed a significantly better durable clinical response (OR = 3.18, 95%CI: 1.14-9.20) and clinical remission (OR = 2.93, 95%CI: 1.03-8.28) when compared to infliximab in a network meta-analysis[22]. With no major safety concerns[23], in the treatment algorithm ladder of UC, we argue that VDZ should be considered as the first biologic when conventional treatments fail due to its gut selectivity. This is most relevant in patients at high risk of serious infections such as the elderly, those with chronic obstructive airway disease or cardiac disease. If cost allows, it should even be considered before conventional treatment such as AZA due to the same feature and with more and more biosimilars reducing the cost of biologics we may see this in future.
VDZ is also an attractive option in patients who have failed prior anti-TNF agent. Anti-TNF therapy is effective for the treatment of moderate to severe UC, however a significant proportion of patients either fail to respond to anti-TNF therapy termed or lose response with time[24]. Second and third anti-TNF agents can be used in such patients, however, it is a game of diminishing returns, as Golimumab efficacy data has shown that clinical response diminishes with each subsequent anti-TNF agent[25]. Rather than giving another anti-TNF agent, VDZ provides a unique mechanism of action with gut selectivity and less side effects. VDZ does work in anti-TNF refractory IBD patients[26]and our study supports this with 51% of TNF exposed patients achieving remission at 12 mo with VDZ therapy.
There are several limitations to our study, the most significant of which is retrospective review of the data (although the data in Australia pertaining to each VDZ application to PBS was collected prospectively). Different endoscopic assessment scores were used in Australia and United Kingdom, although a significant correlation was found between the two scores in a recent study[27]. There was consistency, however, in the clinical and endoscopic outcomes across the institutions. One another limitation is we did not report the number of patients who were steroid free and on immunomodulators at different time points in our study although our aim is to look at that in future by obtaining further information from the centers.
As more and more biologic agents become available for the treatment of IBD, the role of VDZ needs to be defined. Real-world data is important in developing treatment algorithms, which will ultimately help physicians make important treatment decisions in complex IBD patients. This study has shown that VDZ is safe and effective in achieving clinical and endoscopic remission in UC patients.
ARTICLE HIGHLIGHTS

ACKNOWLEDGEMENTS
Australia New Zealand inflammatory bowel disease consortium Australian centers involved in the study include St John of God Hospital Subiaco (Perth), Mater Hospital (Brisbane), Eastern Health (Melbourne), Fiona Stanley Hospital (Perth), Austin Health (Melbourne), QIMR Berghofer Medical Research Institute (Brisbane), St Vincent’s Hospital (Melbourne), Northern Health (Melbourne), The Alfred Hospital, (Melbourne), St Vincent’s Hospital (Sydney), Flinders Medical Centre (Adelaide), Liverpool Hospital (Sydney), Royal Adelaide Hospital & University of Adelaide (Adelaide), Townsville Hospital (Townsville). John Radcliffe, Oxford (United Kingdom) also contributed for the study.
 World Journal of Gastroenterology2020年30期
World Journal of Gastroenterology2020年30期
- World Journal of Gastroenterology的其它文章
- Comment on pediatric living donor liver transplantation decade progress in Shanghai: Characteristics and risks factors of mortality
- Risk prediction platform for pancreatic fistula after pancreatoduodenectomy using artificial intelligence
- Predictive model for acute abdominal pain after transarterial chemoembolization for liver cancer
- Accelerating the elimination of hepatitis C in Kuwait: An expert opinion
- Role of minimally invasive surgery for rectal cancer
- Differential diagnosis of diarrhoea in patients with neuroendocrine tumours: A systematic review
