Exploring the food-gut axis in immunotherapy response of cancer patients
Edda Russo, Giulia Nannini, Monica Dinu, Giuditta Pagliai, Francesco Sofi, Amedeo Amedei
Abstract Nowadays, immunotherapy is widely used to treat different cancer types as it boosts the body's natural defenses against the malignancy, with lower risk of adverse events compared to the traditional treatments. The immune system is able to control cancer growth but, unfortunately, many cancers take advantage of immune checkpoints pathways for the immune evasion. An intricate network of factors including tumor, host and environmental variables influence the individual response to immune checkpoints’ inhibitors. Between them, the gut microbiota (GM) has recently gained increasing attention because of its emerging role as a modulator of the immune response. Several studies analyzed the diversities between immunotherapy-sensitive and immunotherapy-resistant cohorts, evidencing that particular GM profiles were closely associated to treatment effect. In addition, other data documented that interventional GM modulation could effectively enhance efficacy and relieve resistance during immunotherapy treatment. Diet represents one of the major GM determinants, and ongoing studies are examining the role of the food-gut axis in immunotherapy treatment. Here, we review recent studies that described how variations of the GM affects patient’s responsivity to anti-cancer immunotherapy and how diet-related factors impact on the GM modulation in cancer, outlining potential future clinical directions of these recent findings.
Key words: Immune response; Immunotherapy; Programmed cell death protein 1; PD-L1; Cancer; Gut microbiota; High fiber diet
INTRODUCTION
The role of the immune system in tumor control has been a topic of discussion for many years but the current opinion is that cancer is a genetic-based disease that arise when somatic cells acquire multiple mutations overcoming the barriers that normally keep them under control (see the cancer immunoediting, well reviewed by Schreibeet al[1]). The host immune system plays many roles in cancer prevention such as: (1) Suppression virus-induced tumors contrasting the viral infections; (2) Prevention of the setting of an inflammatory environment pro-tumorigenesis; and (3) Elimination of tumor cells that express ligands for receptors active on innate immune cells and tumor antigens recognized by the adaptive immune cells[2]. On the other side, cancer cells, thanks to their genetic instability, can escape from immunosurveillance and potentially lead to cancer. Therefore, a targeted immunotherapy acts as an innovative anti-cancer approach, able to boost the host anti-tumor immune response, and, concomitantly, help to “hit” recurrence mechanisms and cancer resistance[3].
Recent clinical and preclinical (in vitroand animal models) studies have revealed that the gut microbiota (GM) is implicated in modulating the efficacy and toxicity of anti-cancer treatment, like chemotherapy and immunotherapy[4]. The GM is a conglomerate of microorganisms composed of trillions of microbes, including viruses, bacteria, fungi and protozoa, with the greater population harbouring in the intestine. It is composed of two predominant phyla, namedFirmicutesandBacteroidetes(approximately 90% of the total bacteria), while the remaining 10% are partitioned betweenVerrucomicrobia,ProteobacteriaandActinobacteria[5]. GM plays an important role in host nutrition, metabolism and physiology, as well as the protection against pathogens and the development of the immune system. Notably, if on the one hand the microbiota participates in the maturation of the host's immunity, on the other hand its distribution is in turn modulated by the host's immune system[6]. In addition, the microbiota helps in aliment digestion from otherwise indigestible molecules and generates essential vitamins (K and B)[7]. Moreover, the intestinal dysbiosis (defined as a disequilibrium of the microbiota composition) has been found to be linked to the tumor initiation and progression[8]. Recent studies documented that anomalous microbiota-host interactions are associated with various neoplasia like gastric, colorectal and liver cancer[9,10]. Furthermore, a dysbiotic gut microbiota can also impact the tumor therapeutic outcome, with the latter deeply associated with the GM capacity to metabolize anti-tumoral molecules, as well as to regulate inflammation pathways and host's immune response[9]. In particular, the GM is able to influence anti-tumor immune responses through innate and adaptive immunity and the therapeutic response may be enhanced through its modulation[11,12].
Nowadays, it is of utmost importance to improve the therapeutic outcome of patients, developing novel approaches to modulate the GM by strategies without any associated risk, as dietary intervention[13]. In general, diet has a direct role in cancer prevention, prognosis and therapies, deeply supported by animal model, epidemiological and clinical studies. Concerning nutritional patterns, diets rich in plant-based foods such as vegetables, fruit, legumes and whole grains are known to have beneficial effects due to the significant amount of fiber and antioxidant compounds, which exert an inhibitory effect on several carcinogenic pathways, including the carcinogens’ activation, cell cycle regulation, cell signaling, angiogenesis and inflammation[14]. On the contrary, a high intake of refined cereals, sugar-sweetened beverages, alcohol, red and processed meat seems to have detrimental effects, deriving from the large amount of simple sugars, salt, ethanol metabolites, saturated and trans fatty acids, which are related to pro-inflammatory effects, and alterations to cell proliferation and apoptosis[15,16]. Thus, healthy dietary habits are not only useful for weight control, but indirectly influences cancer risk since there are increasing evidence on the link between obesity and carcinogenesis. The obesity is associated with inflammation, endocrine and metabolic alterations, which increase cell proliferation and exert anti-apoptotic effects (in other words, the tumor cells do not self-destruct even after serious DNA damage)[17]. While nutritional problems such as obesity can induce carcinogenesis, cancer therapies can promote both malnutrition and weight loss. In cancer patients, weight loss can be caused by several factors, such as low energy intake, deficiency in nutrient absorption, tumor-induced catabolism and treatment side effects, resulting in therapeutic failure and poor quality of life[18]. Therefore, it seems clear that proper nutrition in cancer patients is crucial not only to prevent the malnutrition risk, but also to improve the therapy effectiveness. Indeed, recent studies suggest that diet plays a crucial role also in the treatment of cancer patients, due to the chemo-protective effects of some foods - such as omega-3 fatty acids or polyphenolic compounds - and the synergistic effect in suppressing cell proliferation[19]. On the other hand, some foods can also generate toxicity or interact with a particular type of treatment such as oral chemotherapy[16]. Therefore, it appears strategical to implement both personalized pharmacological and non-pharmacological interventions taking into account not only the histopathological cancer characteristics but also the environmental factors and the nutritional requirements of patients. Interestingly, as previously reported, diet components could act on cancer treatment also indirectly by changing the GM composition[13]. Based on the “food-gut” axis, this review aims to discuss the emerging link between immunity, diet and GM with a special focus on immunotherapy (using the immune checkpoint inhibitors - ICIs), along with recent observations that could connect dietary-related modulation of microbiota with anti-cancer therapies outcome.
“MICROBIOME-IMMUNITY AXIS” IN CANCER IMMUNOTHERAPY AND NEW INSIGHTS ON THE IMPACT OF DIETARY FACTORS
Regarding the GM impact on the host immune system, the current years witnessed the publication of marking breakthrough, profoundly correlating the patients' microbiota profile with the positive outcome of cancer immunotherapy using the ICIs[20], Chimeric antigen receptor T cells (CAR-T) and vaccines[21]. ICIs are one of the main current therapeutic anti-cancer strategies[22]. Cancer cells presented on their surface immune checkpoint molecules that interact with CD8+T cells, leading to tumor escape from immune system cells[23]. ICIs inhibit the interactions between molecules of the immune checkpoint and inhibitory T cellular receptors. Currently, the most adopted ICIs are monoclonal antibodies acting towards the programmed cell death protein 1 (PD-1) and its PD-L1 Ligand[24]. The food and drug administration (FDA) has approved several proteins programmed for PD-1 and PD-L1 in the treatment of solid and haematological malignancies.
As far as it is concerned CAR-T, CAR technology was first reported in 1993 when Zelig Eshhar and coll. transduced T cells with chimeric genes encoding single-chain antibodies linked to a transmembrane region and an intracellular domain encoding the signalling adaptor for the T cell receptor[25]. CAR-T cells, currently approved in haematological malignancies, are autologous T-cell re-directed towards tumor-specific antigen. CAR-T cells contain signalling domains involved in T-cell activation as CD3ζ, CD28, 4-1BB, ICOS, and OX40. A second generation CAR connects an antigen-binding domain to the T-cell signalling domain coupled to the intracellular signalling domain of a costimulatory moleculee.g., CD28. CAR-T cell constructs may contain multiple signalling domains, that favour activation and durability of CAR-T cells, permitting therefore the implementation of the effector functions of a CAR T-cells MHCindependent[26-28]. In August 2017, the FDA approved CAR T-cells directed against CD19 for the treatment of acute lymphoblastic leukemia (ALL) and then axicabtagene ciloleucel to treat R/R large B-cell lymphoma in October 2017[26,27,29-31].
In spite of promising clinical outcomes obtain with immune checkpoint blockade and CAR therapies, the results of therapeutic vaccination against established tumours proved to be only in part positive, with clinical benefit for cancer's patients. However, as well elucidate from Melief and colleagues, the suboptimal vaccine design and the presence of an immunosuppressive tumor microenvironment are the reasons for the shortage of cancer eradication[32].
However, not all patients with cancer may undergo immunotherapy as some of them are resistant to therapy[33]. In the last years there has been a growing interest in identifying the immune properties of the cancer microenvironment including the characterization of tumor infiltrating lymphocytes (TILs) that seems to play an important role in response to checkpoint inhibition[34].
Recent evidence of the interaction between specific microbes and ICIs are documented in mouse models studies[35-37], suggesting that efficacy of checkpoint blockade through PD-1 and CTLA-4 pathways could be improved through microbiota modulation (Figure 1).
As early as 2015, Sivan and coll. observed melanoma growth in mice with distinct microbiota profiles and noticed differences in anti-tumor immune-mediated response, especially tumor-specific T cell responses and intra-tumoral CD8+ T cell accumulation. Interestingly this different response was reset upon cohousing or after fecal microbiota transplantation (FMT)[35]. In same time, a study analysed various data from patients with different neoplasia, including the antibiotic therapy when starting a CTLA-4 treatment[36]. The result showed that bacteria belonging to the generaBacteroidesandBurkholderiawere associated to the anti-tumor action of the microbiota. In response to these bacteria genera, innate immune cells release IL-12 that may activate the adaptive immune response, thus stimulating T cells. In order to endorse these data, microbes were transferred directly in germ-free mice or indirectly, by giving them feces enriched inBacteroidesof Ipilimumab-treated patients. In all cases, the response to the checkpoint inhibitors was improved[36].
Similarly, in a different study, the GM analysis of patients who positively responded to PD-1 blockade showed an enrichment inCollinsella aerofaciens, Enterococcus faecium and Bifidobacterium longum. When responders’ fecal samples were transferred to germ-free mice, a slower tumor growth and improved therapeutic effects were observed, compared to mice receiving non-responders’ fecal samples. In addition, an increase in CD8+T cells and a decrease in Tregs (Regulatory T cells) in the tumor microenvironment was observed[38].
These preliminary mouse studies endorsed the critical GM role in tumor ICIs therapy and encouraged clinical pursuits to determine the influence of the microbiota on human ICIs-based therapies.
Routy and coll. demonstrated that primary resistance to ICIs can be associated to anomalous GM profiles showing that anti-PD-1/PD-L1 treatment was more efficient in patients with advanced epithelial tumor that did not receive antibiotics, compared with the outcome of those receiving antibiotics[20]. This result underlines that antibiotic treatment is able to destroy the gut microbiota, impairing immune checkpoint blockade response. In particular, whole metagenomic sequencing analyses in fecal samples, collected at diagnosis, revealed that patients responding to PD-1 blockade had different GM composition, rich inAkkermansiaandAlistipes. In addition, before the anti-PD-1 therapy, germ-free mice were treated with FMT using fecal samples from responding patients and immune response was found to be enhanced, while immune response of germ-free mice treated with FMT from non-responders was restored by oral supplementation ofAkkermansia muciniphila. These bacteria induced the efficacy of PD-1 blockade in an IL-12-dependent way by augmenting the enrollment of CXCR3+CCR9+CD4+T cells into mouse tumor beds[20].
Gopalakrishnan and coll. analysed oral and gut microbiota of a cohort of 112 metastatic melanoma patients under anti-PD-1 therapy. The analysis of systemic immune responses revealed that patients whose gut samples where enriched in
Clostridiales,Ruminococcaceae, orFaecalibacteriumhad more effector T cells (CD4+and CD8+) in the peripheral blood and a protected cytokine response to anti-PD-1 treatment, while patients with abundance ofBacteroidaleshad higher frequencies of Tregs, with a diminished cytokine response[39]. Immune profiling indicated an enhanced anti-tumor and systemic immunity in responding melanoma patients with a beneficial intestinal bacterial flora, as well as in germ-free mice receiving FMT from responding patients.
A recent study analyzed patients with non–small cell lung cancer undergoing Nivolumab therapy. The higher diversity of fecal microbiota before and after anti-PD-1 therapy was related with prolonged “progression-free survival time” compared with the lower GM diversity[40]. Furthermore, patients responding to Nivolumab therapy showed higher diversity of enteric microbiota at baseline, which sustained stable composition during therapy[40].
Finally, a study of Tanoue and coll. has identified 11 healthy human associated bacterial strains that induce IFN-γ CD8+T cells and are effective at inhibiting growth of colorectal cancer if used with ICIs[41].
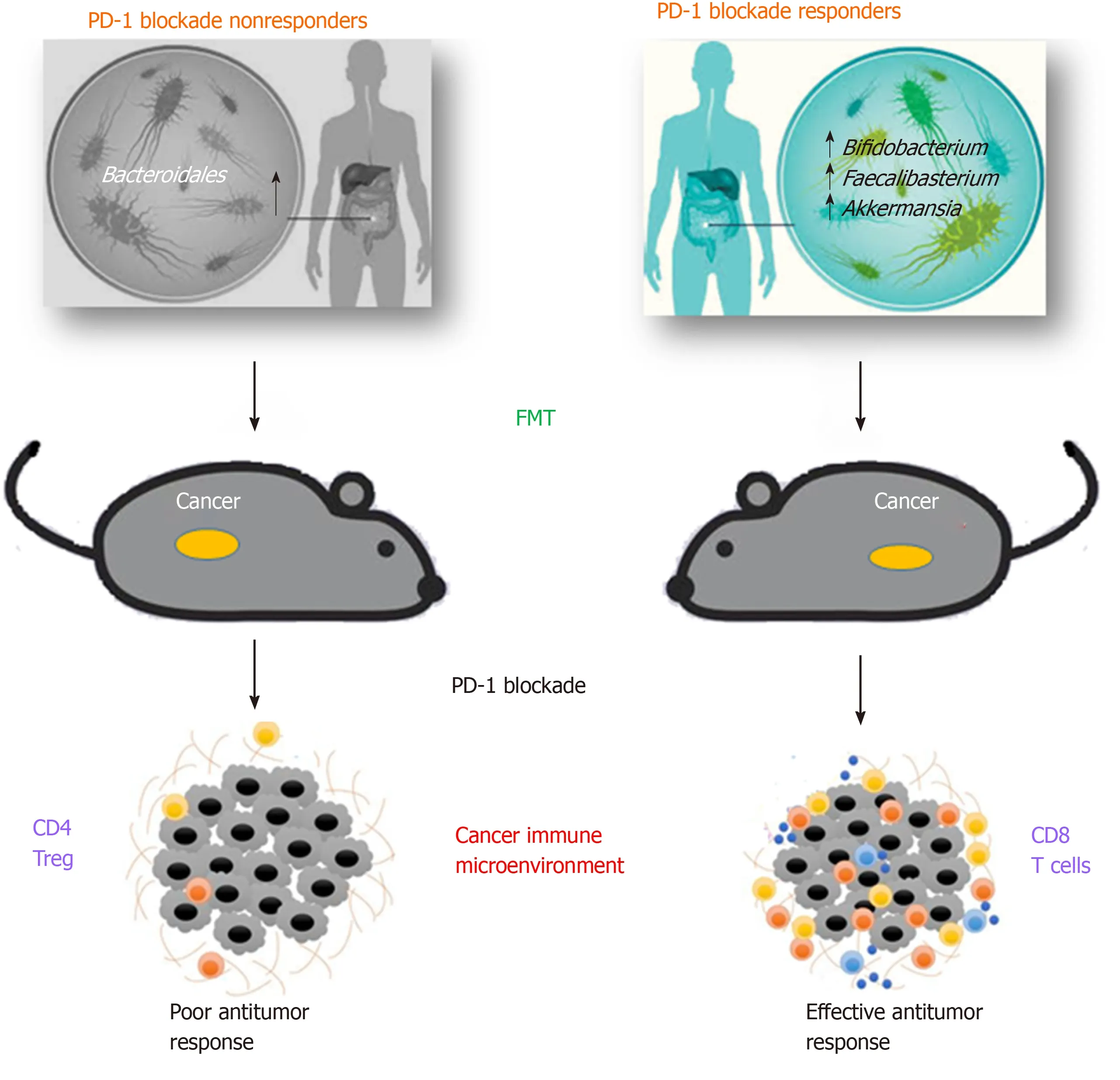
Figure 1 Gut microbiota impact on the efficacy of programmed cell death protein 1 blockade. Specific gut microbiota profiles correlates with response to programmed cell death protein 1 (PD-1) blockade in tumor patients. Fecal microbioma transplantion (FMT) from responders into mice, improve responses to anti-PD-1 treatment and correlated with increased anti-cancer CD8+ cells in the tumour environment. Mice receiving FMT from non-responders’ patients did not respond to anti-PD-1 therapy, and tumour microenvironment is enriched of immune suppressive CD4+ regulatory T cells. PD-1: Programmed cell death protein 1; FMT: Fecal microbioma transplantion; Tregs: Regulatory T cells.
However, enteric microbiota is also able to induce toxicity to the immune checkpoint blockade. This effect was firstly observed in animal models and then in cohorts of patients[42-44]. The microbiota taxa associated in both toxicity and response to ICIs has been identified within theFirmicutesphylum andRuminococaceaefamily[39,42,43]. On the contrary, the microbiota taxa lacking response to ICIs have been identified within theBacteroidalesorder, but a higher richness in these taxa commonly diminish the incidence of toxicity[39,42,43].
In addition, the preliminary result of the first clinical study on the impact of the food-gut axis in the relationship between GM and response to anti-PD-1 checkpoint inhibitors reported that diet and supplements might have an effect on the patient’s responsivity to anti-cancer immunotherapy, probably due to the modulation of their intestinal microbiome[45]. The analysis of fecal samples from 113 melanoma patients under anti-PD-1/PD-L1 therapy confirmed that higher microbial diversity might be associated with a positive response to treatment. Participants provided information about their dietary habits and use of probiotic supplements but only 40% of them reported to have effectively taken the supplements. Moreover, researchers found that a high fibers’ consumption (including fruits, vegetables and whole grains) is positively linked with the presence of microorganism associated to a good anti-PD-1 treatment response in previous studies[45]. On the contrary, a diet with high consumption of sugars and processed meat was negatively linked with these species. In particular, an enrichment ofRuminococcaceaefamily was associated with better therapy response, whileBacteroidalesbacteria were associated to poorer response. Furthermore, data from a subset of 46 patients treated with anti-PD-1 immunotherapy (Pembrolizumab or Nivolumab), showed that patients who consumed a high-fibers’ diet were about five times as likely to respond to anti-PD-1 therapy compared to patients who consumed a low-fibers’ diet. Surprisingly, the consumption of probiotic supplements was linked to less gut bacteria diversity[45].
Given these results on the potential importance of fibers in the dietary survey, the same group of researchers is also starting a controlled diet trial, comparing melanoma patients eating a healthy, high-fibers’ diet to those eating a healthy diet but without a high-fibers’ component during their immunotherapy treatment (NCT03950635).
On the other side, the link between dietary fibers and immunity could be the shortchain fatty acids (SCFAs), which are produced by fiber-fermenting bacteria and are the primary nutrient source for gut enterocytes. A very recent work of Coutzac and coll. showed that systemic microbial SCFAs’ modulate anti-CTLA-4-induced immune responses and its antitumor efficacy[46]. In particular, they demonstrated that, in mice, butyrate stops upregulation of CD80/CD86 on DC and ICOS on T cells, and accumulation of tumor specific T cells and memory T cells lead by anti-CTLA-4. As in mice, Coutzac’s group provided evidence that high butyrate was associated with less accumulation of memory and less ICOS induction on T cells in metastatic melanoma patients treated with anti-CTLA-4. This study supplies a new vision on the relationship between the GM composition GM and the clinical response to anti-CTLA-4viamicrobiota-derived metabolites.
A very recent communication (ASCO-SITC Clinical Immuno-Oncology Symposium, February 2020), showed that cancer patients who were eating more fibers, fruits, vegetables, and whole grains had an increased abundance of pro-response and fiberfermenting bacteria. Of note, patients who consumed a high-fibers’ diet as a habitual diet were nearly five times more likely to respond to PD-1-based immunotherapy and had significantly improved progression-free survival[47]. The researchers also studied the synergy between the GM signature and dietary fiber intake. They found that patients most likely to respond to immunotherapy are those who have both “beneficial” bacteria and a fiber-rich, plant-based diet needed to feed those bacteria. The next step is to observe whether GM modulation with a controlled feeding study can influence immune response.
TRINITY CONNECTION: DIET, GUT MICROBIOTA AND IMMUNE RESPONSE
To date, most of the information regarding the GM modulation of the immune system through the food –gut axis comes from studies on germ-free animal models. The data showed that nutrients play a considerable role in shaping the GM composition, while the composition and GM products influence the development of the host immune system and the inflammatory response (Figure 2). At the same time, the immune system has developed multiple strategies to maintain its symbiotic relationship with commensal microbes, making the interaction between microbiota and immunity bidirectional[48].
The influence of dietary habits on the GM composition and functionality has been widely investigated and many studies have shown that dietary changes rapidly alter the GM[49,50]. In animal models, the consumption of a western diet rich in sugar, salt and fat and low in fibers was associated with changes in microbial composition, gene expression and metabolic pathways after only one day and led to an increase in adiposity in two weeks[51]. In humans, the transition from a plant-based to an animalbased diet has resulted in a significant increase in β-diversity nearly after 24 h, with a decrease in the faecal levels of carbohydrate fermentation metabolites and an increase in amino acid fermentation metabolites[49]. GM alterations after short-term dietary interventions, however, seem to be transient and generally do not persist for more than a few days[52,53]. Long-term diets, on the other hand, have a greater influence on the GM, and habitual diet has been shown to be associated with a distinct intestinal enterotype[54]. In particular, the African diet, high in fibers, has been associated with the enterotypePrevotella, while the Western diet, rich in animal proteins and fat, has been associated withBacteroidesenterotype[55,56]. Further studies have confirmed these findings, supporting the hypothesis that diets rich in fermentable nutrients may induce an increase in beneficial bacteria such asBifidobacteriaandEubacteria, while diets rich in animal proteins and saturated fatty acids and low in fibre, increase the abundance of potentially unhealthy bacteria such asBacteroidesandClostridia[57].
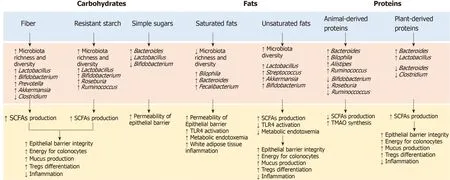
Figure 2 Relationship between dietary factors, microbiota and immune response. SCFAs: Short-chain fatty acids; TLR4: Toll-like receptor 4; TMAO: Trimethylamine N-oxide; Tregs: Regulatory T cells.
To date, the content, quantity and type of fibers are among the most commonly accepted determinants of the GM composition[57]. Accumulated evidence has shown that fibers’ intake is associated with increased abundance of potentially beneficial species such asAkkermansia municiphiliaandRoseburiaspp, which ferment dietary fibers and produce SCFAs (acetate, propionate and butyrate). As previously reported, SCFAs are important for maintaining epithelial barrier function and regulating mucosal and systemic immunity[58]. In addition, SCFAs modulate cytokines’ production and reduce oxidative DNA damage. While the SCFAs’ impact on intestinal epithelial cells refer to their role as an energy source, the effects on the immune system are mediated by the interaction with the G-protein coupled receptor 43 (GPR43) and by the inhibition of the production of histone deacetylase[59]. This inhibition promotes T cell differentiation towards regulatory T cells (Tregs), able to produce the antiinflammatory interleukin-10 (IL-10) and other molecules such as CTLA-4 and tumor growth factor-β (TGF-β)[60]. The role of Tregs in maintaining the immunological selftolerance and in safeguarding the immune balance at the sites of environmental exposed surfaces is therefore crucial and recent studies suggested Tregs as an attractive target for T-cell-based immunotherapies[60]. Bacteria involved in the response to these therapies resemble those eating and secreting mucin (e.g.,Akkermansia municiphilia, Bifidobacterium longumandFaecalibacterium prausnitzii), which maintain barrier integrity and improve ICIs’ efficacy. The abundance of these bacteria, as demonstrated by several studies, is influenced by dietary factors such as fibers and proteins’ intake[61].
Moreover, the fibers can influences the immune systemviathe synthesis of glycans by the bacteria of theBacteroidetesphylum. The commensal bacteriaBacteroides fragilis, for example, produce a particular immunosuppressive glycan, the polysaccharide A, which works as a toll-like receptor 2 (TLR2) ligand and promote Tregs’ differentiation[62].Bacteroides fragilisalso induces an IL-10 response in intestinal T cells, preventing the expansion of Th (helper)17 cells and the potential damage to the mucosal barrier[63].
In addition to fibers, other nutrients are involved in modulating the immune system, either directly, by interacting with the host through the intestinal immune system, or indirectly, by modulating the GM composition. Omega-3 fatty acids, for example, modulate the inflammation by inducing a transient increase in some SCFAsproducer bacterial genera and by interacting with the G protein-coupled receptor 120 (GPR120)[64]. GPR120 is primarily expressed by the macrophages, and binding with omega-3 fatty acids supress the production of tumor necrosis factor alpha (TNF-α) and IL-6, decreasing the macrophage-induced tissue inflammation. Similarly, saturated fatty acids, proteins and vitamins influence the host inflammatory responses through the TLR4 activation[65], the increase in trimethylamine-n-oxide (TMAO) synthesis and the expression of mucin 2[66,67], and the induction of tolerogenic dendritic cells and Tregs’ differentiation, respectively[68].
Finally, the use of probiotics and prebiotics has also been studied in relation to the GM modulation and the immune system. Although some evidence suggest that their consumption can restore innate and adaptive immunity, human supplementation trials have produced conflicting results[69,70]. Diet supplement of microbiota may be a double-edged sword as potential risks of bacteremia, sepsis, and multi-organ failure associated with the use of probiotics have been reported, especially in immunecompromised populations or in subjects with metabolic hyper-inflammatory conditions[71]. Despite of that, probiotics’ administration in multiple trials has shown beneficial effects on ameliorating diarrhea and other gut-related damages following anti-cancer therapy, thus re-establishing a healthy GM composition[72].Lactobacilliwere administrated to cancer patients to re-populate the compromised patients’ GM, thus re-establishing the levels and functionality of the commensal bacteria, depleted after the treatments[73]. Regardless the beneficial effects, larger and controlled clinical trials are further needed to truly endorse both the efficacy and the safety of administering selected species of probiotics during or following anti-cancer treatments. Therefore, as previously suggested, a safer way to modulate the GM could be through dietary interventions or supplementation with prebiotics such as oligosaccharides, fructans, and galactans, which are known to increase the proportion of potentially beneficial bacteria (e.g.,Bifidobacteria) and the production of SCFAs.
FUTURE STRATEGIES TO IMPROVE THE RESPONSE TO IMMUNOTHERAPY THROUGH THE FOOD-GUT AXIS
Based on the aforementioned results, the GM profile might be studied as a predictive or prognostic biomarker for anti-cancer treatment efficacy. As previously stated, higher diversity in the intestinal microbiota was associated with a better response rates to immune checkpoint blockade[39], which suggests that assessing community richness and proportion of “detrimental” and “beneficial” bacterial, prior to immunotherapy, might be crucial for the outcome trend and treatment decision. In the next future, the GM profile could be coupled with other known parameters related to outcome such as cancer mutational burden and T cell infiltration. Moreover, GM composition could be used to indicate additional interventionsviathe microbiota to improve immunotherapy effectiveness or alternatively decrease therapy related toxicity.
However, the role of external influencers, such as medications, probiotics, prebiotics and postbiotics’ administration and dietary pattern on the GM modulation and the potential need to monitor these items during cancer therapy, have to be thoroughly investigated.
In order to favour the expansion of beneficial bacteria or, conversely, “starving” detrimental bacteria, adjunctive therapeutic strategies, as preparative regimens (viadietary supplementation) prior to modulation of the gut microbiota, could be considered as upcoming ways to increase the efficacy of primary cancer immunotherapy (Figure 3).
The administration of beneficial or immune-potentiating probiotic, provided as an immunotherapy adjuvant, offers a more achievable system of microbial manipulation in the clinical setting. Probiotics are inexpensive and are generally regarded as safe. In addition, the genetic manipulation of the microbes is a strategy that alters the bacterium’s existing capacities through the insertion of new genes in order to improve beneficial properties[74]. Moreover, in preclinical experiments, some phages have been already used to reduce pathogenic microbes in the commensal community, and could be further engineered to target strains efficacy[74]. Clinical trials to evaluate the impact of probiotics’ administration on treatments with checkpoint inhibitors have been initiated[75]. Notably, a double-blind clinical trial in colorectal cancer, revealed the positive effects of the probioticLactobacillus johnsoniion the GM composition[76]. In addition, a preoperative probiotic therapy withSaccaromices bulardiiinduced downregulation of pro-inflammatory cytokines within the colonic mucosa, with lower IL-10, IL-1β, and IL-23A mRNA levels[77]. This last result proved that probiotic administration could influence the local immunity. An ongoing protocol for patients with stage I-III breast cancer is actually analysing the effects of probiotics on CD8+T cell infiltrate (NCT03358511). Furthermore, a research using the keywords “cancer”, “probiotics” and immunotherapy returned three ongoing studies from the website http://www.clinicaltrials.gov. Especially, the BISQUIT study (NTC03870307) is a phase-II randomized protocol of the use of pre-and probiotics during the definitive treatment of chemotherapy-radiotherapy for patients with localized anal canal squamous cell cancer (ACSCC). The aim is to increase the effectiveness of conventional treatment. ACSCC is, in many cases, a virus-associated tumor and therefore potentially immunogenic; immunotherapy is a promising strategy in ACSCC, so pre- and probiotics stimulating the immune system through the GM modulation, might improve oncological outcomes.
Concerning prebiotics, in theory, if they are administered to the immunotherapy and consequently lead to the growth of beneficial microbes, they could have a helpful impact on rebalancing the intestinal microbiota inducing the host to react favourably to immunotherapies. The dietary supplementation with plant polysaccharide inulin as prebiotic has already revealed an increase ofBifidobacteriumandFaecalibacteriumspecies, previously associated with immunotherapy positive response[78]. Dietary fibers have also shown to induce a decrease in the abundance of immune-promotingF. prausnitzii[79].
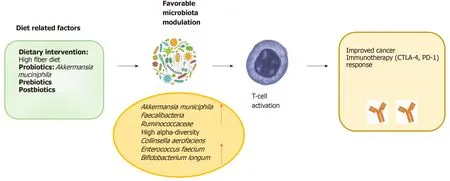
Figure 3 Potential strategies to shaping the gut microbiota through the “food- gut axis” and enhance immune checkpoint inhibitors’ response. In order to obtain a favourable microbiota modulation, adjunctive therapeutic strategies, as preparative regimens (via prebiotics, prebiotics, postbiotics or dietary supplementation) could be considered as upcoming ways for improving the efficacy of immunotherapy response. PD-1: Programmed cell death protein 1.
Dietary interventions, having a low cost and safe profile, represent a straightforward opportunity for studying the association of microbiota and downstream immune manipulation in tumor. As previously reported, the literature in this field has suggested the responsiveness of particular taxa and their induced metabolites to several immune parameters and nutrients and provides exploratory insight into how dietary modulation could impact the intestinal microbiome and immune health[80,81], with parallels that can be drawn in the immunotherapy. Interestingly, a very recent study revealed that dietary deprivation of non-essential aminoacids improved anti-PD-1 immunotherapy in a mouse model of colon cancer[82].
Finally, studies show that some nutrition formulation called “immunonutrients” containing essential aminoacids, omega-3 fatty acids, SCFAs and nucleotides could induce particular effects on immune system. Nowadays, these new developed formulas have improved outcome in tumor patients receiving chemo-radiotherapy, decreasing acute toxicity and modulating the immune and inflammatory response.
Immunonutrition, defined as a modulation of the immune system provided by specific interventions that modify dietary nutrients, is an emerging field in oncology, and further research is needed, especially focusing on its effects in cancer immunotherapy[83,84]. The combination of immunotherapies with the support of immunonutrition may provide future therapeutic approaches.
CONCLUSION
In the past decade, immunotherapy propelled the advancement of oncology therapeutics. However, only in the very recent few years we have gained significant insights into the role of GM in cancer immunotherapy response, giving new alternative explanations on patients’ resistance acquisition.
There is also evidence that the food-gut axis is involved in the modulation of gut microbiota, resulting in synergistic effects during cancer immunotherapy. The first clinical trials on dietary modulation of the microbiota involving cancer patients undergoing immunotherapy are now ongoing, giving further opportunities to improve efficacy and relieve resistance.
Further investigations should determine the bacterial combination pools needed to help the anti-cancer response and how to develop such combinations and, in addition, the optimal dietary strategies to enhance the immunotherapy strategies, modulating the intestinal microbiota.
ACKNOWLEDGEMENTS
The authors thank Dr. Elisangela Miceli, PhD for her English language revision.
 World Journal of Gastroenterology2020年33期
World Journal of Gastroenterology2020年33期
- World Journal of Gastroenterology的其它文章
- Bowel function and quality of life after minimally invasive colectomy with D3 lymphadenectomy for rightsided colon adenocarcinoma
- Treatment repurposing for inflammatory bowel disease using literature-related discovery and innovation
- Effects of denosumab treatment in chronic liver disease patients with osteoporosis
- Neutrophil to lymphocyte ratio and albumin bilirubin grade in hepatocellular carcinoma: A systematic review
- Radiomics of rectal cancer for predicting distant metastasis and overall survival
- Liver fat accumulation measured by high-speed T2-corrected multi-echo magnetic resonance spectroscopy can predict risk of cholelithiasis
