Role of stem cell therapies in treating chronic wounds: A systematic review
Anjali C Raghuram, Roy P Yu, Andrea Y Lo, Cynthia J Sung, Melissa Bircan, Holly J Thompson, Alex K Wong
Anjali C Raghuram, Roy P Yu, Andrea Y Lo, Cynthia J Sung, Melissa Bircan, Alex K Wong, Division of Plastic and Reconstructive Surgery, Keck School of Medicine of USC, Los Angeles, CA 90033, United States
Holly J Thompson, Wilson Dental Library, Herman Ostrow School of Dentistry of USC, Los Angeles, CA 90089, United States
Abstract
Key words: Mesenchymal stem cells; Adult stem cells; Embryonic stem cells; Erythroid precursor cells; Stem cell therapies; Chronic wounds
INTRODUCTION
Cutaneous wound healing is a complex and intricate process contingent on a series of regulated factors that work in a concerted manner to repair skin injury and restore barrier function.Superficial wound healing tends to follow a predictable course, with aberrancies generally noted only in the case of underlying disease states, such as diabetes.However, impairment of the wound healing process can result in chronic, non-healing wounds.Chronic wounds that do not heal within an expected period of 3 mo[1]not only result in pain and disfigurement, but also impose a significant burden on patients and the healthcare system, with annual cost estimates approaching $30 billion in the United States alone[2].Chronic wounds are the consequence of local tissue hypoxia, bacterial colonization, and repetitive ischemia-reperfusion injury[3].Nonhealing wounds can originate from a variety of etiologies, including arterial disease, diabetes, vasculitis, venous valve insufficiency, prior irradiation[4], and skin malignancies[5].
Overcoming the factors that cause delayed wound healing involves an assessment of underlying pathology and consideration of advanced therapeutic agents.While a variety of wound care treatment options are available, few have demonstrated efficacy in the healing of chronic wounds and restoration of tissue to its pre-injury state.In contrast with normally healing wounds, chronic wounds exhibit increased levels of proinflammatory cytokines, reactive oxygen species (ROS), proteases, and senescent cells[6].Traditional chronic wound care protocols rely on the debridement of necrotic or infected tissue followed by the application of wound dressings and topical agents that serve to protect the wound from infection and accelerate healing.In the case of certain chronic wounds, such as diabetic foot ulcers, offloading with external compression is crucial in order to minimize pressure placed on the wound[7].Additionally, diabetic foot ulcers, venous leg ulcers, and open amputation wounds can be treated with negative pressure wound therapy to promote granulation tissue formation, wound area contraction, primary healing, and improved skin graft retention upon application to the wound bed[8].Management of the wound edge is further performed with electromagnetic therapy, laser therapy, ultrasound therapy, and systemic oxygen therapy[9,10].Although these therapeutic options are varied and can be tailored to each patient, these techniques have limited success and do not consistently facilitate complete wound closure.
Consequently, stem cell therapies have emerged as an exciting field of research because of their potential for the treatment of non-healing wounds.Notably, these wounds can be characterized by damaged or depleted stem cell populations[6,11].Stem cells possess the unique capacity to self-renew and differentiate into various cell types.Moreover, stem cells can upregulate the secretion of cytokines and growth factors necessary for immunomodulation and regeneration[12], which are two critical features inadequately addressed in chronic wound healing pathogenesis.Ranging from immature pluripotent cells to more differentiated, multipotent cells, stem cells comprise a budding area of interest for improved healing of chronic wounds without the associated risks of major surgical procedures and added donor-site morbidity.The aim of the following review is to systematically review current literature that addresses the role of stem cell therapies in treating varied chronic, non-healing wounds in order to provide plastic surgeons, clinicians, and other chronic wound care stakeholders a comprehensive guide from which to select optimal therapies for improved patient outcomes.
MATERIALS AND METHODS
Literature search
A systematic literature search was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines[13].Figure 1 depicts the algorithm for article identification, screening, and review.Inclusion and exclusion criteria are presented in Table 1.PubMed, EMBASE, Cochrane Library, Web of Science, and Scopus were queried to identify relevant publications, articles, and abstracts that reported stem cell applications from the years 2000 to 2019.The queries employed a combination of search terms, including “mesenchymal stem cells,” “adult stem cells,” “embryonic stem cells,” “erythroid precursor cells,” “stem cell therapies,” and “chronic wounds.” The search parameters are described in detail in Supplementary Table 1.To eliminate bias, four authors independently screened all articles for inclusion or exclusion, and in the case of a conflict, a fifth author screened pertinent articles as a tiebreaker.Retrieved publications included systematic reviews, literature reviews, case reports and series, retrospective and prospective studies, and clinical trials.All studies that contained material applicable to the clinical use of stem cell therapies were reviewed, and data were extracted using a standardized collection tool.A total of 43 articles describing the use of stem cell therapies for the treatment of chronic wounds were included in this review[14-56].
RESULTS
While stem cell therapies have been extensively explored inin vitroandin vivosettings, more recent investigative efforts have attempted to assess their clinical translatability.Transplanted cells deliver cytokines, chemokines, and growth factors, induce angiogenesis and innervation, and alter the wound inflammatory process[57].To better assess the advantages of these stem cell properties in treating patients’ chronic wounds, we extracted data from studies that explore stem cell therapies in a clinical setting.Table 2 presents these treatment options, characteristic surface markers, indications for use, and mechanisms of action.Table 3 delineates the clinical outcomes observed, considerations for stem cell therapy optimization, and pertinent challenges associated with each therapy’s usage.

Table 1 Inclusion and exclusion criteria
Adipose-derived stem cells
Adipose-derived stem cells (ADSCs) are mesenchymal stem cells that comprise a pluripotent, heterogenous cell population within human adipose tissue.The popularity of ADSCs can be attributed to their ease of harvest and limited donor-site morbidity; they can be isolatedvialiposuction aspiration or excision of fat samples[27].The most common approach for isolating ADSCs involves collagenase digestion followed by centrifugal density gradient separation[58].Isolated ADSCs can then be expanded in monolayer cultures on standard tissue dishes with a basal medium of 10% fetal bovine serum[59].Clinical use of ADSCs requiresin vitroexpansion that complies with good manufacturing practice (GMP) guidelines to ensure that no xenogeneic components are cultivated.As ADSCs are mesenchymal stem cells, their surface markers include CD90, CD105, CD73, CD44, and CD166 without the expression of hematopoietic cell surface markers CD34 and CD45[60].
ADSCs have demonstrated clinical efficacy in the treatment of chronic wounds secondary to severe radiation injury, chronic fistulae, and ulceration, which includes venous leg ulcers.Mechanistically, this stem cell population facilitates angiogenesis, augments the secretion of growth factors and cytokines, and allows for human dermal fibroblast proliferation through direct cell contact and paracrine activation during the re-epithelialization phase of wound healing[61].Notably, when combined with skin substitute containing human extracellular matrix (ECM), ADSCs permit regeneration of subcutaneous, dermal, and epidermal tissues[62].
ADSCs are among the more robustly explored stem cell therapy type in the clinical treatment of chronic wounds.They have demonstrated results of improved wound healing and closure, tissue ultrastructure and hydration, neo-vessel formation, and patient symptomatology of pain and claudication[14,63-70].In the treatment of chronic ulcers secondary to peripheral arterial disease, Marinoet al[71]observed decreased ulcer size, depth, pain, and improved transcutaneous saturation in all patients over the course of treatment.The versatility of ADSCs is further highlighted in treating chronic Crohn’s fistulas; treated patients experienced improved wound healing seen over 8 wk without the incidence of adverse events[72].Akitaet al[14]found that the use of an artificial dermis (Terudermis, Japan) scaffold protected ADSCs from infection and ambient dryness, while Larsenet al[43]found improved ulcer re-epithelialization and healing when ADSCs were administered on an OASIS wound matrix in conjunction with compression therapy.In addition, seeding ADSCs in hydrogel delivery vehicles by capillary force and then administering these hydrogel systemsin vivoeffectively enhances stem cell genetic expression and survival for improved wound healing outcomes[73].When harvesting ADSCs from subcutaneous adipose tissue, it is important to avoid penetration of the deeper visceral cavity or underlying major muscles, vessels, and nerves.Upon injection of ADSCs to chronically radiated wound beds, care must also be taken to avoid surface rupture or laceration[14].
Though their clinical potential has been favorably evidenced, there are some challenges to consider with the use of ADSCs.This cell population is not immortal and displays signs of aging and loss of chemokine markers with repeat culturing[50].Further, adipose tissue can widely vary in its metabolic activity and capacity for proliferation and differentiation depending on the site of tissue harvest and patient characteristics, such as age and sex[31].Marfia et al[45]identified that autologous ADSCs have an altered genotype in diabetic patients, which is characterized by decreased potency and expression of vascular endothelial growth factor-A (VEGF-A) and chemokine receptor CXCR4.Judicious use of ADSC therapy requires close patient surveillance for tumor formation as well as consideration of strategies to improve cell homing and transplantation[50].
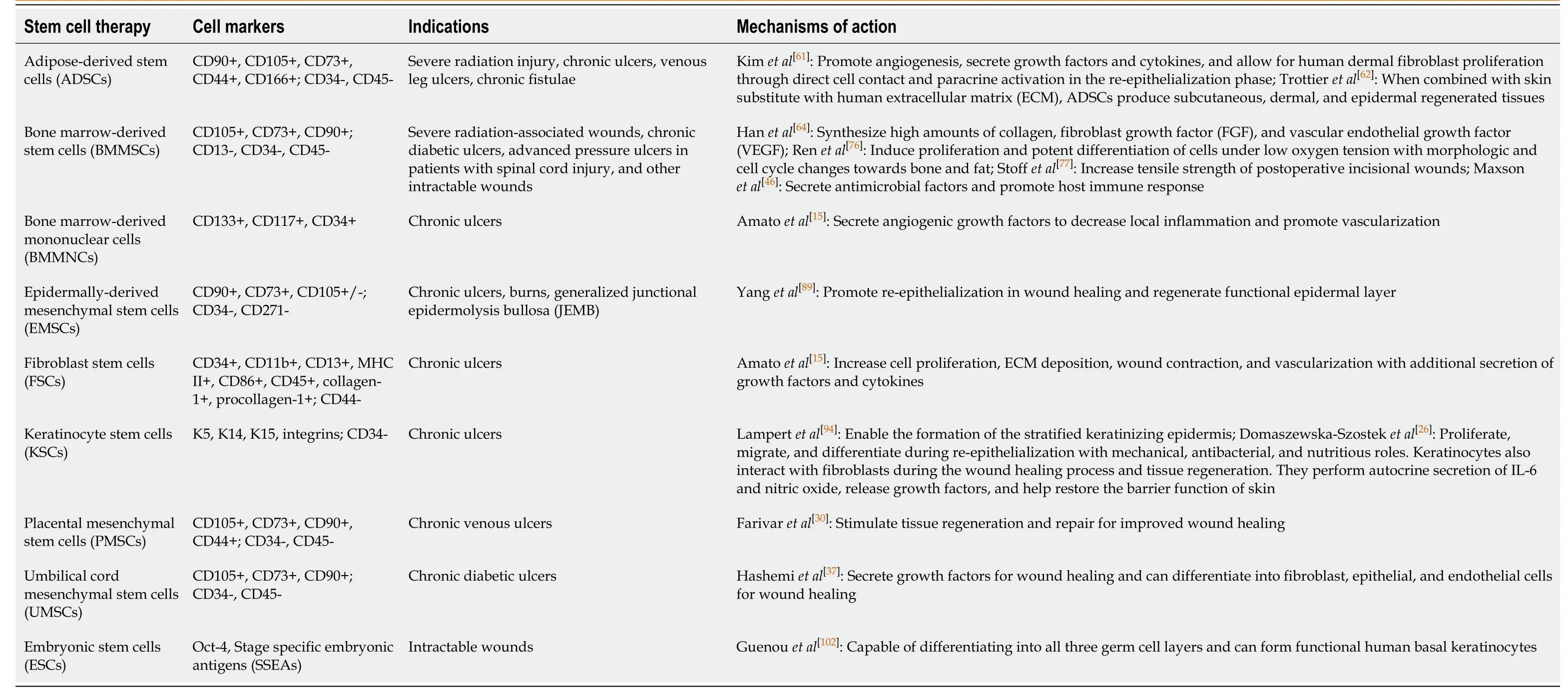
Table 2 Stem cell therapies

Table 3 Stem cell therapy clinical applications

et al[92]: Improved wound healing of 92% (n = 13 patients) when fibroblasts were cultured on a hyaluronic acid and atelo-collagen matrix; Marcelo et al[108]: More complete wound healing achieved when administered with autologous fibrin glue over the ulcer site.Keratinocyte stem cells (KSCs)Studies that demonstrated improved wound healing: Vanscheidt et al[95]: Complete and faster wound healing in 38.3% of patients receiving autologous keratinocytes, compared to 22.4% in the control group (n = 44 patients); Moustafa et al[96]: improved wound healing over 6 wk when administered as cultured autologous keratinocytes on cell-free discs (n = 12 patients); Bayram et al[99]: Reduction in diabetic foot wound size of 92% (n = 20 patients), compared to control wound size reduction of 30%.Studies that demonstrated complete ulcer healing: Hartmann et al[97]: Complete ulcer healing at a mean of 14.5 wk when administered as keratinocytes transplanted in a fibrin carrier (n = 4 patients); De Luca et al[98]: Complete ulcer healing in 20 wk (n = 20 patients) with a 30%-84.4% reduction in size after 3 wk (n = 4 patients); Beele et al[100]: Demonstrated complete venous leg ulcer closure within 1 wk (n = 11 patients) over a period of 4.1-24.9 wk with decreased pain.Stem cell delivery: Hartmann et al[97]: Better keratinocyte graft fixation and epithelial monolayer formation with low-density fibrin; Bayram et al[99]: More effective delivery when cultured keratinocytes are attached to microcarriers made of polyethylene and silica; Teepe et al[109]: Radial progression toward wound closure when administered as cryopreserved cultured allografts (n = 43 patients); Shukla et al[101]: Complete chronic wound healing over 12-48 wk when administered as keratinocytes along with epidermal cell suspension (n = 12 patients).Rezaie et al[50]: Difficult to isolate, short lifespan during serial cultivation, and possible tumor formation.Placental mesenchymal stem cells (PMSCs)Farivar et al[30]: Complete chronic venous ulcer healing in 53% (n = 21 patients with 30 total ulcers) with 79% mean reduction in wound surface area over an average of 10.9 wk.Farivar et al[30]: Improved cell survival when MSCs are administered in cryopreserved, aseptic placental tissue (hVWM).Duscher et al[27]: Appropriate donor selection is necessary to avoid immune-mediated rejection or transmission of genetic diseases.More efficient cell isolation, culture, and expansion techniques are needed, along with close surveillance for malignant transformation.Umbilical cord mesenchymal stem cells (UMSCs)Hashemi et al[37]: Significant decrease in chronic diabetic ulcer size over 9 d with decreased wound healing time (n = 5 patients).Hashemi et al[37]: Improved tissue regeneration and wound healing when cells are seeded on an acellular amniotic membrane scaffold.Duscher et al[27]: Appropriate donor selection is necessary to avoid immune-mediated rejection or transmission of genetic diseases.More efficient cell isolation, culture, and expansion techniques are needed, along with close surveillance for malignant transformation.Embryonic stem cells (ESCs)Not reported in reviewed articles.Not reported in reviewed articles.Okano et al[104]: Patients will need to be monitored for tumorigenesis and teratoma formation.
Bone marrow-derived stem cells
Bone marrow-derived stem cells (BMMSCs) were originally isolated by Friedensteinet al[74]in 1966 and presently constitute a mesenchymal stem cell population that is typically harvestedviailiac crest aspiration.This aspirate is then subjected toin vitroselection, cell expansion in culture, and topical application to wounds for tissue regeneration.BMMSCs display the typical mesenchymal stem cell surface markers, including CD105, CD73, and CD90.Their clinical utility encompasses the treatment of severe radiation-associated wounds, chronic diabetic ulcers, advanced pressure ulcers, as seen in patients who have undergone spinal cord injury, and other forms of intractable wounds.
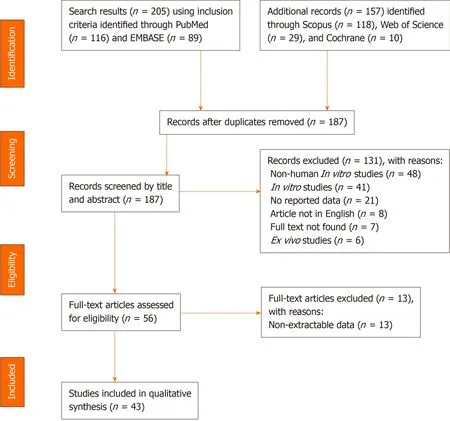
Figure 1 PRISMA Flowchart.
Consistent with other mesenchymal stem cell types, BMMSCs have immunomodulatory properties[46]and promote angiogenesis[75].These stem cells synthesize high amounts of collagen, fibroblast growth factor (FGF), and VEGF[64], allow for cell proliferation and differentiation under low oxygen tension conditions[76], and demonstrate increased tensile strength when applied to postoperative incisional wounds[77].When clinically administered, BMMSCs have shown complete closure, dermal rebuilding, reduced scarring, and successful cell engraftment in non-healing wounds[78].Particularly in the case of chronic ulcers and wounds resultant from traumatic, thermal, electric, or infectious etiologies, this stem cell therapy is shown to significantly reduce wound size[29,35,54,79-82].For chronic diabetic ulcers, BMMSC therapy results in improved limb perfusion, ankle-brachial indices, transcutaneous oxygen pressure, and magnetic resonance angiography analysis[79].Sarasúaet al[33]found that BMMSC treatment of longstanding stage IV pressure ulcers in patients with spinal cord injury decreased the mean hospital stay from 85.16 to 43.06 d, when compared with standard treatment modalities alone.In addition, patients given the BMMSC treatment did not evidence any ulcer recurrence over a follow-up period of 19 mo.
In order to improve the delivery and outcomes associated with BMMSC therapy, these cells can be cultured and placed in an artificial collagen dermis as a composite graft, which can improve skin regeneration processes[83].Additionally, when BMMSCs are cultured and administeredviaa fibrin spray, chronic wound or ulcer size is reduced significantly by 40% over a period of 20 wk, with no adverse events reported[29].To enhance their favorable therapeutic profile, BMMSCs can be administered along with platelets, fibrin glue, and bone marrow-impregnated collagen matrix; this strategy has resulted in significant diabetic wound closure in patients with formerly recalcitrant wounds[84].Similar to ADSCs, BMMSCs necessitate careful monitoring of patients for possible tumor formation as well as appropriate but not excessive culturing in order to avoid transplantation of aged cells with inferior homing abilities and loss of chemokine markers[50].
Bone marrow-derived mononuclear cells
Bone marrow-derived mononuclear cells (BMMNCs) are a heterogeneous group of cells that include mature B cells, T cells, monocytes, and a smaller proportion of progenitor cells, including hematopoietic stem cells, mesenchymal stem cells, endothelial progenitor cells, and embryonic-like cells[85].BMMNCs offer ease of harvest, processing, and administration, making them a favorable option for clinical testing.Cell surface markers include CD133, CD117, and CD34, and BMMNCs have been clinically applied most frequently in the treatment of chronic ulcers.Their most notable property is the ability to secrete angiogenic growth factors that decrease local inflammation and promote vascularization[15].
BMMNCs simultaneously accelerate the rate of wound healing and decrease wound area compared to non-stem cell therapy treatment[24,86].When applied with epidermal grafting for the treatment of chronic diabetic foot ulcers, Yamaguchiet al[87]observed significant ulcer healing without incidence of osteomyelitis or the need for patient amputation.Moreover, when administered in conjunction with high density nanofat and negative pressure wound therapy, Denget al[25]reported improved patient wound healing outcomes as well as decreased lymphocyte recruitment, higher collagen deposition, and increased vessel growth.
Epidermally-derived mesenchymal stem cells
Epidermally-derived mesenchymal stem cells (EMSCs) categorize the mesenchymal stem cell population present within the epidermis that is responsible for homeostasis of the superficial skin layers[88].This population includes interfollicular, sebaceous gland, and bulge area stem cells.EMSCs are characterized by the cell surface markers CD90 and CD73, with variable expression of CD105.Clinically, this stem cell therapy has been employed to treat chronic ulcers, burns, and non-healing wounds secondary to junctional epidermolysis bullosa (JEMB).EMSCs promote both re-epithelialization in wound healing and regeneration of a functional epidermal skin layer[89].Of note, if EMSCs undergo gene correction, they promote enhanced functional epidermal regeneration in JEMB ulcers[90].Tenget al[52]found that cultured epidermal autografts enriched with EMSCs on an ECM-compatible substrate not only replenished EMSCs in chronic wounds, but also stabilized the ECM substrate within the wound site.As a stem cell category, EMSCs can be manipulated and stimulatedviabiomaterial-based approaches to modify their spatial and temporal cues for precise niche conditions that are beneficial in wound healing and skin regeneration[27].
Fibroblast stem cells
Fibroblast stem cells (FSCs) are part of a relatively novel focus in regenerative medicine and can be generated by reprogramming adult fibroblasts into an immature, pluripotent state[91].These autologous induced pluripotent stem cells (iPSCs) are furthermore nonimmunogenic.The addition of fibroblasts to the chronic wound healing environment addresses the deficiency of appropriately functioning fibroblasts in the setting of this chronic inflammatory state.In non-healing wounds, fibroblasts exhibit premature, stress-induced cellular senescence with decreased proliferative potential, impaired reactivity to growth factors, and abnormal protein production[26].Fibroblasts and their stem cell equivalent states have been employed in the treatment of chronic ulcers, and their characteristic cell surface markers include CD34, CD11b, CD13, MHC II, CD86, CD45, collagen-1, and procollagen-1.This cell population functions to promote proliferation, ECM deposition, wound contraction, vascularization, and secretion of growth factors and cytokines[15].
Clinically, FSCs have been shown to be more effective when co-delivered or cocultured with additives, such as fibrin glue[57].Broweret al[20]found that commercially available 2-chamber fibrin sealants, containing fibrinogen and thrombin, enhanced the adherence, proliferation, and migration of fibrocytes.Furthermore, fibroblasts have also demonstrated improved wound healing when cultured on hyaluronic acid and atelo-collagen matrices[92]prior to application to chronic wound sites.
Keratinocyte stem cells
Keratinocytes comprise the majority of cells within the human epidermis and promote re-epithelializationviaproliferation, migration, and differentiation[93].Keratinocyte stem cells (KSCs) reside in the basal epidermis, hair follicles, and sebaceous glands and can serve to replenish depleted keratinocyte populations found in chronic wounds.Characteristic cell surface markers of KSCs include K5, K14, K15, and integrins, and KSCs have been most widely applied in the treatment of chronic ulcers.These stem cells enable the formation of a stratified, keratinizing epidermis[94], contribute to the process of re-epithelialization, and offer antibacterial and nutritious roles[26].When differentiated into keratinocytes, these cells interact with fibroblasts and perform autocrine secretion of interleukin-6 (IL-6) and nitric oxide, release growth factors, and facilitate the restoration of the skin’s barrier function.
When administered to patients with chronic wounds, keratinocytes promote complete wound healing over a shorter period of time[95-99]compared to standard wound healing therapy, with additionally decreased local wound pain[100].Moreover, keratinocytes can be cultured on cell-free discs[96], administered as cryopreserved cultured allografts, or suspended with epidermal cells in order to improve chronic wound healing outcomes[101].Graft fixation can be performed with low-density fibrin to ensure better cell survival and epithelial monolayer formation, thereby optimizing keratinocyte therapy[97].Bayramet al[99]found that cultured keratinocytes attached to microcarriers of polyethylene and silica resulted in better cell delivery than keratinocytes administered alone.When considering KSC or keratinocyte therapy, it is important to recognize that these cells can be difficult to isolate, display a short lifespan throughout serial cultivation, and necessitate patient monitoring for tumor formation[50].
Placental mesenchymal stem cells
Placental mesenchymal stem cells (PMSCs) are another member of the mesenchymal stem cell category with clinical benefits.PMSCs stimulate the wound healing process through the release of trophic mediators, promotion of new vessel formation, recruitment of endogenous progenitor cells, and facilitation of cell differentiation, proliferation, and ECM formation[27].As with other mesenchymal stem cells, PMSC surface markers include CD105, CD73, CD90, and CD44.PMSCs have been clinically investigated in the treatment of chronic venous ulcers with resultant higher quality tissue regeneration and repair[30].
When administered as cryopreserved placental tissue containing PMSCs, Farivaret al[30]demonstrated improved chronic venous ulcer healing, compared to standard therapy, with a significant reduction in size from baseline.PMSC therapy can be optimized when the cells are cryopreserved in aseptic placental tissue to protect placental tissue components, including growth factors and collagen-rich extracellular membranes, from degradation[30].These stem cells require careful donor selection to avoid immune rejection or genetic disease transmission.Additionally, patient surveillance is imperative to monitor for malignant transformation[27].
Umbilical cord mesenchymal stem cells
Umbilical cord mesenchymal stem cells (UMSCs) represent another mesenchymal stem cell population aptly isolated from umbilical cord-lining tissue.Notably, umbilical cord epithelial cells have stem-cell like properties and can form stratified epithelium[27].As with placental-derived mesenchymal stem cells, UMSCs are extracted from an extra-fetal source, and donors must be appropriately selected to avoid immune-mediated rejection or transmission of genetic diseases.Characteristic cell surface markers include CD105, CD73, and CD90.UMSCs secrete growth factors for wound healing and are capable of differentiating into fibroblast, epithelial, and endothelial cell subtypes for improved wound healing[37].
UMSCs have been employed in the treatment of chronic diabetic ulcers and significantly decrease both ulcer size and time required for wound healing to occur[37].When seeded on an acellular amniotic membrane scaffold, UMSCs promote tissue regeneration and improve wound healing outcomes.This scaffold not only confers anti-adhesive, bacteriostatic, and epithelialization properties, but also attenuates the wound pain reported by patients[37].In refining the applications for UMSCs, it is necessary to develop more efficient techniques for cell isolation, culture, and expansion.Lastly, it is essential to monitor patients for the possibility of tumor formation[27].
Embryonic stem cells
Embryonic stem cells (ESCs) constitute the latest and perhaps most up-and-coming category in stem cell therapy development.ESCs are pluripotent stem cells derived from the blastocyst stage of embryos and, given the correct conditions, are capable of differentiating into any cell in all three germ cell layers[42].These ESC-derived cell lineages include hematopoietic stem cells, differentiated T cells, and epidermal cells.Characteristic cell surface markers include octamer-binding transcription factor 4 (Oct-4) and stage specific embryonic antigens (SSEAs).Because of their pluripotent nature, ESCs have the unique potential to serve as a therapeutic modality in the treatment of multiple disease processes, including chronic wounds.Preclinical studies have demonstrated that ESCs can differentiate into fully functioning human basal keratinocytes, which can further develop into stratified epidermis[102].In vivostudies in mice have also shown that when compared to endothelial progenitor cells derived from cord blood, human ESCs exhibit improved dermal regeneration and reepithelialization in chronic wounds.
The clinical use of ESCs is, however, limited by the ethical controversy surrounding their procurement.ESC therapy has been widely debated because currently, human ESCs cannot be obtained with causing significant damage to the human embryo.Proponents of ESC therapy maintain that this treatment will advance medical science and that the politicization of scientific research stymies this progress[103].On the other hand, induced pluripotent stem cells (iPSCs) offer an effective alternative to ESC therapy that demonstratein vitroandin vivopotential in wound healing while circumventing the ethical concerns of ESCs.These iPSCs can be generatedviaprogramming of differentiated adult keratinocytes with embryonic-like stem cell properties.The process of reprogramming keratinocytes involves retroviral transduction of essential transcription factors, such as c-Myc, Klf4, Oct-3/4, and Sox2[48].Nonetheless, the use of either iPSC or ESC therapy will require the medical community to address their respective safety concerns.Despite their associated risk for tumorigenesis and teratoma formation, these cells offer a promising option for the treatment of chronic wounds because of their ability to differentiate into all three germ cell layers[104].
DISCUSSION
The role of stem cell therapies in the treatment of chronic, non-healing wounds is continuously being refined within the scope of tissue engineering.Stem cells promote restoration of impaired signaling pathways for growth factors, delivery of important cytokines and chemokines, induction of vascularization and innervation, and more precise control of the inflammatory processes underlying chronic wounds[105].Considerations with therapy usage involve donor variables, such as site and cell availability, patient age, and patient sex, as well as risks for diminished stem cell efficacy associated with repetitive culturing and possible malignant transformation.Nonetheless, stem cell therapy can be optimized extensivelyviaa wide range of codelivery techniques in the form of scaffolds, hydrogels, and other carriers with or without wound healing additives.
As with any systematic evaluation of the literature, there are some important limitations to consider with this review.Compared to preliminary, preclinical work, there are fewer clinical studies exploring the full scope of stem cell therapies for the treatment of chronic wounds.Given the lack of clear guidelines regarding therapy use for certain patient demographics, underlying health statuses, and presence of comorbid diseases, it is difficult to generalize the applicability of one therapy to another chronic wound etiology that has not been previously tested.Therapeutic success is determined on a case-by-case basis.
However, there are ongoing investigative efforts to improve understanding of stem cell therapies.Recently, the use of electrospun fiber scaffolds for stem cell therapy culture has been explored with the goal of enhancing cell proliferation and differentiation.This technique additionally opens up new possibilities for controlled fiber morphology and structure to generate layered skin substitute dressings[32].Though clinical studies and trials have supplemented our knowledge of stem cell therapy usage for chronic, non-healing wounds, further studies are needed in order to more comprehensively examine the breadth of this therapeutic modality and more closely personalize wound care regimens for each individual patient.As these treatments become more advanced by optimizing wound healing outcomes while minimizing donor morbidity, stem cell-based therapy is likely to establish itself as a mainstay of chronic wound care and management.
ARTICLE HIGHLIGHTS
Research background
Chronic wounds are defined as those that do not heal within a period of 3 mo,resulting in significant patient morbidity and healthcare burden.Due to local tissue hypoxia, bacterial colonization, ischemia-reperfusion injury, and diminished stem cell populations, these wounds do not progress through the normal wound healing phases.Further, non-healing wounds are attributable to a host of etiologies, including arterial disease, diabetes, vasculitis, venous valve insufficiency, irradiation, and malignancy.Their complex pathophysiology poses a formidable treatment challenge,and presently, there are ineffective techniques to facilitate wound closure and improved patient symptomatology.Stem cell therapies have therefore emerged as a unique therapeutic approach to modulate the chronic wound environment in favor of healing.In this systematic review, we evaluate literature over the past two decades to ascertain clinical findings associated with stem cell therapies for treating chronic wounds.
Research motivation
While adipose-derived stem cells (ADSCs) and bone marrow-derived stem cells (BMMSCs) have been tested the most frequently in clinical settings, it is unclear how other emerging stem cell therapy types function in healing chronic wounds.It is critical that we comprehensively consider a variety of stem cell therapies for the treatment of a diverse scope of non-healing wounds in order to provide maximal clinical benefit to wound care specialists and providers.
Research objectives
To investigate the scope of a variety of stem cell therapies, including adipose-derived stem cells (ADSCs), bone marrow-derived stem cells (BMMSCs), bone marrowderived mononuclear cells (BMMNCs), epidermally-derived mesenchymal stem cells (EMSCs), fibroblast stem cells (FSCs), keratinocyte stem cells (KSCs), placental mesenchymal stem cells (PMSCs), umbilical cord mesenchymal stem cells (UMSCs), and embryonic stem cells (ESCs), for the treatment of chronic, non-healing wounds.
Research methods
We performed a systematic review of the literature according to the 2009 PRISMA guidelines.Five authors conducted a search in five databases (PubMed, EMBASE, Cochrane Library, Web of Science, and Scopus) to identify relevant publications, articles, and abstracts reporting clinical stem cell therapy use for chronic wounds from the years 2000 to 2019.
Research results
A total of 43 studies were included in this review.The studies reported that ADSCs and BMMSCs have been tested in the widest scope of clinical applications, including the treatment of severe radiation-associated wounds, venous leg ulcers, chronic fistulae, chronic diabetic ulcers, and advanced pressure ulcers from spinal cord injury.Enhanced testing of other stem cell therapy types has provided informative guidelines for therapy optimization, including seeding stem cells into artificial dermal, wound matrix, and hydrogel scaffolds for improved cell survival and proliferation in the wound beds.FSCs and KSCs can be delivered with additives, including fibrin, to strengthen cell properties of adherence, migration, and epithelial monolayer formation.Improved wound healing with each of the stem cell therapy types was determined on the basis of histological and functional parameters.No studies reported significant complications with clinical use of any of the investigated therapies.
Research conclusions
Stem cells promote healing of chronic wounds by restoring impaired signaling pathways for growth factors, ensuring delivery of important cytokines and chemokines, inducing vascularization and innervation, and modulating inflammatory processes.Selecting optimal therapy for various wounds is contingent on patient variables, including age, sex, and stem cell donor site, as well as processing variables, such as culturing after cell harvest and potential for malignant transformation.Additional clinical studies are required to replicate the strength of the literature findings for ADSCs and BMMSCs and substantiate use of a wider scope of stem cell therapies for treating non-healing wounds.
Research perspectives
There is limited clinical evidence examining the use of EMSCs, FSCs, KSCs, PMSCs, UMSCs, and ESCs for the treatment of chronic wounds, though these stem cells have demonstrated potential in preclinicalin vitroandin vivowork.Further studies exploring the use of these therapies for clinically diverse patient wounds would be highly informative.In addition to aforementioned artificial dermal, wound matrix, and hydrogel scaffolds, there is ongoing development with newer cell delivery constructs, such as electrospun fiber scaffolds to facilitate creation of layered skin substitute dressings.As the etiology of chronic wounds varies from patient to patient, it is necessary to personalize therapeutic approaches.The goal of future investigations will be to further realize improved patient wound closure and histologic markers of wound healing, as well as decreased pain, disfigurement, and healthcare system burden.
 World Journal of Stem Cells2020年7期
World Journal of Stem Cells2020年7期
- World Journal of Stem Cells的其它文章
- Mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles: Potential roles in rheumatic diseases
- Application and prospect of adipose stem cell transplantation in treating lymphedema
- Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling
- Involvement of glycated albumin in adipose-derived-stem cell-mediated interleukin 17 secreting T helper cell activation
- Adipose-derived stem cell therapy shows promising results for secondary lymphedema
- Vitamin D and calcium signaling in epidermal stem cells and their regeneration
