Current state of minimally invasive treatment of locally advanced non-small cell lung cancer
Daniel P. Dolan, Aaron R. Dezube, Scott J. Swanson
Division of Thoracic Surgery, Brigham and Women’s Hospital, Boston, MA 02115, USA.
Abstract Locally advanced non-small cell lung cancer (NSCLC) has historically been defined as Stage III by the IASCLC staging. While the workup for these patients has been standardized, the treatment algorithms remain unclear. The use of neoadjuvant chemotherapy, radiotherapy, and now immunotherapy still awaits results in terms of optimal regimen. Surgery for local disease control is routinely used and this group of patients have historically been treated with open thoracotomy for resection. Only in the last 10-20 years have minimally invasive surgical methods been applied for treatment. Video-assisted and robotic-assisted thoracoscopic surgery have retrospectively been shown to be safe and effective with equivalent or better perioperative outcomes, long-term overall and disease-free survival, mediastinal lymph node staging to open thoracotomy, and the ability to operate on patients who are too sick for thoracotomy. This review shows that minimally invasive surgery for treatment of locally advanced NSCLC disease should now be routinely offered to patients as the initial surgical method of resection.
Keywords: Locally advanced, minimally invasive surgery, video assisted thoracoscopic surgery, non-small cell lung cancer
INTRODUCTION
Locally advanced non-small cell lung cancer (NSCLC) has been variably defined in the literature from Stage III alone in the 7th edition International Association for the Study of Lung Cancer (IASCLC) staging to the inclusion of the stage groupings of II, IIIA, IIIB, and the newly created IIIC in the 8th edition of the IASCLC Tumor Node Metastasis (TNM) staging[1,2]. This further breakdown in the 8th edition TNM staging was ref l ective of the different prognosis for T3 and T4 tumor size associated with N3 nodal disease without metastases. This change means Stage III in the 8th edition of the TNM staging range in size from≤ 1 cm to > 7 cm with nodal involvement ranging from none to metastases in the contralateral mediastinal or hilar area, ipsilateral or contralateral scalene area, or supraclavicular lymph nodes[3]. The new Stage III subgroups were observed to have the following 5-year survival for clinical and pathologic staging,respectively: IIIA 36% and 41%, IIIB 26% and 24%, and IIIC 13% and 12%[2].
This has led to an update in the clinical practice guidelines available to clinicians. The workup is the same for all Stage III tumors including pulmonary function tests (PFTs), bronchoscopy, evaluation of mediastinal lymph node evaluation, FDG PET/CT, and MRI or CT of the head[4]. The difference lies in how to proceed afterward. The European Society of Medical Oncology (ESMO) describes a three-pathway approach,whereas the National Comprehensive Cancer Network (NCCN) guidelines describe many more options for management based on the type of Stage III NSCLC cancer[1,4]. ESMO focuses on nodal status based on preoperative imaging and, while the NCCN guidelines start similarly, the nuance lies with T status, location of primary tumor, presence of multiple tumors, N status, and determination of resectability. Both guidelines are in general agreement that N3 patients and patients deemed unresectable proceed with non-surgical multimodality treatment as their primary management. Incidental or occult N2 disease not previously diagnosed remains a debated topic with NCCN stating that surgery can proceed and then use adjuvant therapy or surgical resection can be halted and neoadjuvant treatment administered before definitive resection[4]. ESMO suggests proceeding with surgery and then adjuvant treatment[1]. Both guidelines agree that patients with N0-N1 disease can proceed to surgery fi rst, with caveats in NCCN guidelines regarding location in the thoracic cavity and presence of invasion.
Mediastinal staging is critical as the presence of N2 disease even with tumors of T stage T1a to T1c fall into Stage IIIA[2]. Staging techniques fall into the three broad categories: imaging, endoscopic, and surgical.De Leyn et al.[5]in their “Revised ESTS guidelines for preoperative mediastinal lymph node staging for NSCLC” provided an overview of available techniques including Chest CT scan, PET-CT scan,transbronchial needle aspiration, endoscopic ultrasound with aspiration, endobronchial-TBNA,cervical mediastinoscopy, video-assisted thoracoscopic (VATS) biopsy, video-assisted mediastinal lymphadenectomy, or transcervical extended mediastinal lymphadenectomy[5]. The NCCN recommends any patient suspected of having nodal disease to be biopsied by endoscopic or surgical means[4].
However, occult N2 disease can still be found even after these techniques. Risk factors that have been identified with occult N2 metastases include larger tumor size and central location as well as high tumor standardized uptake value seen on fluorodeoxyglucose (18F) PET/CT and tumor histology such as adenocarcinoma with micropapillary features[6-9].
Our review aims to provide a summary of the latest body of knowledge on identification, medical treatment.and surgical approaches to locally advanced NSCLC disease, with a focus on emerging minimally invasive approaches to treatment including video-assisted thoracoscopic surgery and robotic-assisted lung resection.
An extensive literature search was performed by two independent co-authors. PubMed and Cochrane Library were searched from their inception until December 2019. Published manuscripts regarding the management of locally advanced NSCLC were reviewed with regards to the following: tumor characteristics(size, location of tumor, metabolic activity, nodal involvement, clinical and pathologic staging, and fi nal histology), surgical vs. nonsurgical treatment, neoadjuvant or adjuvant therapy around surgery, extent of resection (sublobar, lobectomy, and pneumonectomy), and method of resection (open, VATS, and robotic).We also examined references of articles that we discovered using the previous criteria for additional studies that may not have been found in our initial search. Additionally, articles deemed relevant and not identified in the above-mentioned searches were included after review and consensus by the authors. We excluded all studies that were case-reports, small case-series, or had questionable data analysis.
NEOADJUVANT AND ADJUVANT TREATMENT STRATEGIES
Management of the subset of patients with locally advanced NSCLC remains difficult given their heterogenous presentations and lack of clear consensus regarding optimal management. Additionally,important distinction should be made between those for whom medical therapy is definitive compared to those considered for surgical resection. Finally, those found to have occult N2 disease following surgery represent a unique treatment dilemma. Current treatment modalities include chemotherapy, radiation,surgery, and immunotherapy with the recent introduction of immune checkpoint inhibitors such as PD-(L)-1 inhibitors. Given the complexity of treatment, a multidisciplinary plan is preferred to optimize care.
Unresectable NSCLC
For unresectable NSCLC as defined by unresectable, node-positive Stage II and Stage III or greater, initial therapy has previously been chemoradiation alone with the American Society of Clinical Oncology endorsing the American Society for Radiation Oncology Evidence-Based Clinical Practice Guidelines which recommend concurrent chemoradiotherapy[10]. In the past decade, attention has turned to the use of targeted immune therapy as an alternative or in addition to chemotherapy. To date, targeted immunotherapy (excluding check-point inhibitor) has not been shown to improve overall survival in phase III trials for locally advanced NSCLC including most notably the START trial[11]and INSPIRE trial[12]for unresectable NSCLC.
Immune checkpoint inhibitors of PD-(L)1 have shown promising results in management of NSCLC. The recent PACIFIC randomized control trial demonstrated that Stage IIIA patients unable to undergo surgery had not only improved progression free survival (23.2 months vs. 14.6 months with placebo; P < 0.001), but also overall survival as high as 66% at 24 months with chemoradiation therapy followed by immunotherapy(durvalumab) as compared to chemoradiation alone[13,14]. Currently, the NCCN recommends this treatment algorithm as standard of care in unresectable disease[4].
Resectable NSCLC
For potentially resectable NSCLC, the consensus is less clear. All guidelines agree that surgical treatment alone for IIIA NSCLC continues to have a poor 5-year survival and unimodality therapy is not recommended. These findings were demonstrated by two landmark randomized control trials (RCTs),now over two decades old, which demonstrated that the addition of induction chemotherapy to surgery improved overall survival and disease-free survival (median survival 26 months vs. 8 months and median disease-free survival 20 months vs. 5 months for chemotherapy plus surgery compared to surgery alone,which established the standard of care; P < 0.001) in Stage III NSCLC patients[15,16].
Historically, the most debated topic has been the role of surgery in the management of this subset of Stage III lung cancer, IIIA. Initial RCTs such as Intergroup 0139 trial, which enrolled over 400 patients with Stage IIIA NSCLC due to N2 disease to either chemoradiotherapy or surgery, found surgery was not associated with an improvement in overall survival [5-year survival rate, 27% vs. 20%; odds ratio (OR) 0.63; 95%CI:0.36-1.10]. The intergroup 0139 trial did however fi nd a sevenfold increase in the control of the primary tumors and an improvement in 5-year progression-free survival (PFS, 22% vs. 11%). Of note, in this study,survival was impacted by the high rate of pneumonectomies but there was a clear survival with benefit with surgery for patients requiring lobectomy[17]. At the same time, the EORTC 08941 study found no difference in overall survival in those who received surgery or radiation following induction chemotherapy[18]. The latter study was limited as it only enrolled patients with unresectable disease and the rate of incomplete resection was greater than 50%. Most recently, the ESPATUE trial found in IIIA (N2 disease) that 5-year overall survival and progression free survival were equivalent in those who received surgery versus definitive chemoradiotherapy following induction therapy[19]. In those patients identified as having N2 disease intraoperatively, current NCCN guidelines suggest that those with negative preoperative nodes with one single positive node found at time of surgery are resectable candidates[4]. However, the decision to stop and proceed with neoadjuvant therapy upfront continues to be debated amongst clinicians.
The use of targeted immunotherapy as part of multimodality therapy with surgery is less well known.The most recent systematic review of nine eligible trials (eight with surgically resected locally advanced NSCLC) utilizing immunotherapy (excluding immune checkpoint inhibitors) totaling 4940 randomized participants found no statistical survival benefit in overall survival in their pooled meta-analysis (HR =0.94; 95%CI: 0.83-1.06; P = 0.35), and progression free survival (HR = 0.93; 95%CI: 0.81-1.07; P = 0.19;high-quality) when compared to conventional therapy except for checkpoint inhibitors such as PD-(L)-1 inhibitors for which results are promising[20]. Recently, R0 resection has been demonstrated as still being possible in the majority of cases (95%) after immunotherapy, with two recent pilot studies demonstrating no delay in surgery following neoadjuvant nivolumab[21-23]. Unfortunately, no RCT results are yet available that have examined incorporation of immunotherapy with surgically resectable disease, with four studies(NCT01857271, NCT02201992, NCT02347839, and NCT02595944) created to examine this question with one trial [Erlotinib Hydrochloride Before Surgery In Treating Patients with Stage III Non-Small Cell Lung Cancer (EVENT trial) NCT02347839] closed to poor accrual already (https://clinicaltrials.gov/ct2/show/NCT02347839).
In terms of timing of therapy, current guidelines recommend neoadjuvant therapy followed by possible surgery in the appropriate candidate for curative resection if N2 disease is recognized upfront[4].Trimodality therapy, consisting of chemotherapy, radiation, and surgery, has been associated with improved median survival and in certain cases has been shown to demonstrate a survival benefit even with Stage IIIB disease (P < 0.001) and N3 (P = 0.010) in non-randomized trial[24]. In this regard, one recent meta-analysis by McElnay et al.[25]demonstrated improved survival with neoadjuvant chemoradiation compared to neoadjuvant chemotherapy alone prior to surgery (HR 0.87 vs. HR 1.1, although neither reached statistical significance). However, one phase III trial found no survival benefit with induction chemoradiation compared to induction chemotherapy alone followed by surgery[26]. To date, there continues to be a lack of consensus regarding utilization of trimodality therapy.
When examining forms of adjuvant therapy, the role of postoperative adjuvant radiation (PORT) is not clear. Initial studies demonstrated a modest benefit in Stage IIIA disease with adjuvant radiation treatment but had limited reduction in local recurrence or survival benefit in early stage disease[27]. The ANITA III trial is the only RCT to demonstrate increased survival in N2 disease with the addition of adjuvant radiation to chemotherapy (median, 47 months if given radiation vs. 24 months in those without radiation given adjuvant chemotherapy; 23 months vs. 13 months with or without adjuvant radiation in those not given adjuvant chemotherapy)[28].
For those who may be candidates for adjuvant radiation, survival differences occur based on degree of resection. In a non-clinical trial, PORT was associated with improved survival in R1 resection[29].In contrast, a recent meta-analysis found patients treated with PORT have worse survival after R0 resection[30]. Only one recent study noted a survival benefit in R0 patients if given sequentially following chemoradiation, which has not yet been confirmed by RCT[31]. The NCCN guidelines currently recommend those found to have occult N2 disease after resection should either receive chemotherapy for R0 resection or combined chemoradiation for R1 or R2 resection[4].
OPEN THORACOTOMY VS. MINIMALLY INVASIVE SURGERY FOR LOCALLY ADVANCED DISEASE (INCLUDING ROBOTICS)
Thoracotomy has been the standard surgical approach to thoracic surgery, but the past 30 years has seen the development of VATS. While this modality has been further advanced to include robotics, some contention remains whether VATS is equivalent in terms of safety, lymph node evaluation, and outcomes to open thoracotomy[32].
Perioperative outcomes
Contemporary studies have demonstrated equivalent or better perioperative outcomes for VATS and RATS[33-36]. Huanget al.[33]performed one of the earlier studies that called attention to VATS treatment in locally advanced NSCLC. They reviewed 43 patients with Stage IIA-IIIB per UICC 7th edition staging who underwent neoadjuvant therapy from 2006 to 2012 and proceeded on to VATS. Overall, 97.7% of the patients’ resections were completed VATS. Blood loss was 253.57 ± 117.08 mL for 28 lobectomies, 5 double lobectomies, 5 wedge resections, 4 pneumonectomies, and 9 sleeve resections. No perioperative deaths were reported. While this study lacked a comparison group, the overall conclusion was that VATS was safe and feasible in this group of patients[33]. Parket al.[34]soon followed up on this report with a 428-patient study,397 thoracotomyvs. 17 RATS and 14 VATS (referred to as MIS collectively), who had been diagnosed as clinical Stage II and IIIA and underwent surgery after induction therapy. From 2002 to 2013, they noted a conversion rate from MIS of 26% with R0 resection rate of 97% MISvs. 94% open (P= 0.71). Complications were similar between groups at 32% and 33% (P= 0.99), with more of the open complications related to the cardiovascular system, 11%. Four perioperative deaths were noted in the open group with none in the MIS group. Median length of stay was 4 days in MISvs. 5 days in open (P< 0.001). This allowed them to conclude that perioperative outcomes for MIS were equal or better than open surgery[34]. Veronesiet al.[35]built on this and, similar to Huanget al.[33], focused on RATS for locally advanced NSCLC. In total, 223 patients were retrospectively collected from multiple international sites who were diagnosed as Stage III preoperatively or intraoperatively. They divided the groups into neoadjuvant (15%), adjuvant (63%), and no neoadjuvant/adjuvant treatment (22%). Overall, 10.3% of patients experienced Clavien-Dindo Grade III-IV complications with no difference noted between groups (P= 0.14). Overall, 9.9% of cases were converted large tumor size and > 2 positive lymph nodes significantly associated on univariate analysis,which did not carry over to multivariable analysis. Mean hospital length of stay was 5.3 days (P= 0.641)[35].Lastly, Gonfiottiet al.[36]reported their retrospective review of the Italian VATS Group database, including 3720 early stage patients and 454 locally advanced stage patients who all underwent VATS. They defined locally advanced as cT2b to cT4 in the 7th edition staging and/or received neoadjuvant treatment. They noted a lower estimated blood loss for the advanced stage patients at 169.44 ± 63.69 mL than prior studies but greater than early stage, 186.69 ± 69.65 mL (P= 0.038)[31,34]. Conversions were more common in the advanced stage group (13.0%vs. 9.3%,P= 0.018); however, bleeding was more commonly the reason for the early stage group, 33.4% (102), while tumor extension was the predominant cause for locally advanced tumors, 25.4% (15). Complication rate was higher in the locally advanced group which was significant,37.0%vs. 30.4% (P= 0.040). Thirty-day mortality was not significantly different between locally advancedvs. early stage, 1.5%vs. 1.6% (P= 0.880), nor was length of stay, 7.96 ± 10.10vs. 7.35 ± 29.39 (P= 0.660)[36].Taken together, these data indicate that perioperatively the outcomes for MIS methods, including for locally advanced NSCLC, is safe with equivalent or better perioperative outcomes.
Lymph node evaluation
Tianet al.[37]focused on lymph node evaluation after neoadjuvant treatment with VATS compared to thoracotomy. For 127 patients, 56 VATS and 71 open from 2000 to 2016, they did propensity matching between the two surgical groups to get 28 pairs to evaluate the sufficiency of mediastinal lymph node dissection between VATS and open. All cases were lobectomies or larger resections. They found no difference in the completeness of resection (P= 0.611), but a nonsignificant difference in adequacy of mediastinal lymph node dissection. The guidelines they quoted required evaluation of three hilar and interlobar lymph nodes and three mediastinal lymph nodes from three stations. They noted that 60.7%of the open cases did not meet this guideline while 75.2% of VATS cases did. Most importantly, however,when the lymph node numbers and stations sampled were compared, there was no statistically significant difference between the two groups. They proceeded to apply multivariable logistic regression and did not find side or surgical technique to be significant predictors for sufficient lymph node dissection; upper or middle lobe location did note a 3.843 hazard ratio for sufficient lymph node dissection (P = 0.002)[37].Park et al.[34]also demonstrated no difference with their MIS comparison to open, and, although nonsignificant, it trended towards a higher median for lymph node stations sampled in the combined MIS group (RATS and VATS) than the open cohort, 5 (3-7) vs. 4 (1-9) (P = 0.081)[34]. When Gonfiotti et al.[36]compared their locally advanced NSCLC VATS resections to their early stage NSCLC VATS resection, they had more total lymph nodes sampled (15.69 ± 10.47 vs. 13.48 ± 8.18, P < 0.001), more N1 stations sampled(7.55 ± 6.96 vs. 6.38 ± 4.30, P < 0.001), and more N2 stations sampled (8.27 ± 6.62 vs. 7.02 ± 5.58, P < 0.001)[36].All this evidence indicates that VATS is at least equivalent to open in terms of lymph node sampling for locally advanced NSCLC.
An additional benefit of VATS as the primary surgical modality is that it can serve as a restaging method before definitive resection. CALGB 39803 was a prospective phase II trial designed to evaluate the possibility of restaging Stage III NSCLC patients, 7th edition TNM staging, after they had undergone neoadjuvant therapy for N2 disease burden. The study was multi-center and ran from 1998 to 2003. The protocol mandated histologically confirmed N2 NSCLC disease and a two-cycle course of platinum-based chemotherapy and/or 40 Gy or more of radiotherapy. Patients then underwent a VATS restaging procedure focusing on signs of pleural carcinomatosis, malignant effusion, or any positive mediastinal node with at least three sampled. Of 68 patients who were evaluated, 20 had no nodal tissue present due to neoadjuvant therapy, 7 had negative nodes, 16 had persistent N2 disease, and 4 had progression to carcinomatosis. This gave a feasibility rate of 69% (95%CI: 57%-80%) for VATS as a restaging modality[38]. While this study was done, as noted by the authors, before the more regular use of EBUS, this demonstrates that VATS can be used as a restaging modality prior to committing to an open thoracotomy.
Long-term outcomes
Yang et al.[39]published, in 2016, Duke University’s retrospective review of 111 cases of Stage IIIA pN2 NSCLC, 7th edition IASCLC staging, who had received induction chemotherapy with or without radiation and then proceeded on to lobectomy. Cases were from 1996 to 2012 with a distinct trend towards increased VATS in later years. They found patients who had undergone VATS had significantly better 5-year overall survival than open surgery, 56.6% vs. 31.4% (P = 0.007). No significant difference was noted in recurrence free survival between VATS and open groups, 27.3% vs. 22.3% (P = 0.17)[39]. Yang et al.[40]followed up on this by focusing on VATS vs. thoracotomy after preoperative chemotherapy for any stage NSCLC, including 203 thoracotomy and 69 VATS patients from 1996 to 2012. On univariate analysis, they found significantly better 3-year overall survival for VATS patients vs. open, 61% vs. 43% (P = 0.010), but no difference with multivariable analysis despite a trend towards significance, HR 0.56 (0.32-1.01) (P = 0.053). Recurrence free survival was no different on univariate or multivariable analysis, 36% vs. 27% (P = 0.12) and HR 0.68(0.42-1.09) (P = 0.11). They proceeded with propensity matching on preoperative variables and found no difference on multivariable analysis between VATS and open for overall survival or for recurrence free survival, HR 0.88 (0.39-1.97) (P = 0.76) and HR 0.91 (0.46-1.83) (P = 0.80)[40]. Matsuoka et al.[41]from Japan published their institution’s experience with 132 patients who had undergone induction therapy before VATS or open and followed them out to 5 years. For the 97 patients they defined as locally advanced Stage II/III, the 5-year overall survival was not statistically different in the VATS vs. open groups,but precise values were not reported (P = 0.227)[41]. Lastly, Park et al.[34]demonstrated similar fi ndings in their RATS and VATS vs. open study with 3-year overall and recurrence free survival being no different,48.3% vs. 56.6% (P = 0.84) and 49.0% vs. 42.1% (P = 0.19), respectively[34]. Taken together, all these studies demonstrate that even in long-term outcomes VATS or RATS is as good as or better than thoracotomy.
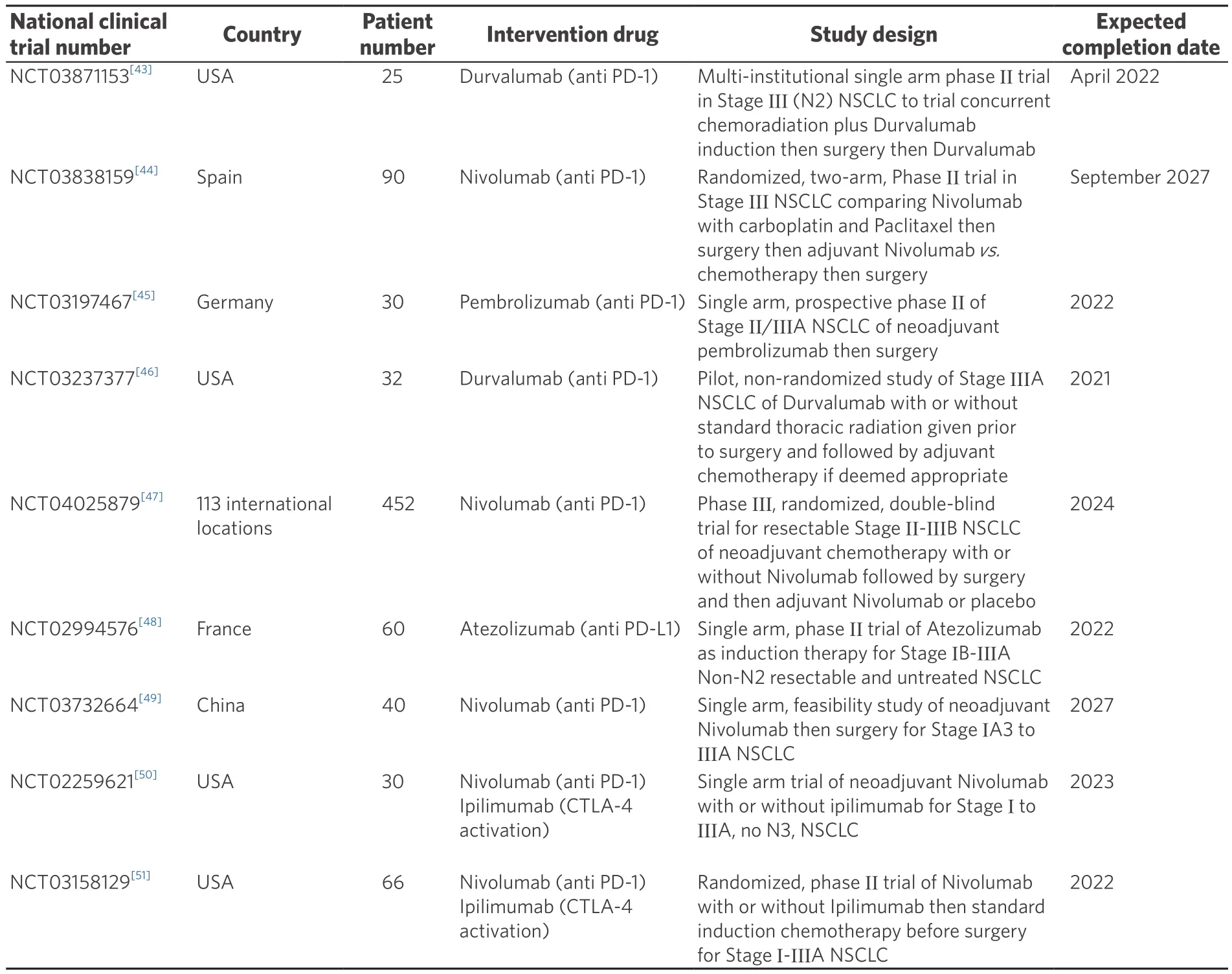
Table 1. Actively recruiting clinical trials of neoadjuvant immunotherapy before surgery
FUTURE DIRECTIONS
Immunotherapy, alone or in combination with traditional chemoradiotherapy, is emerging as one of the next frontiers alongside different methodologies of radiation treatment that could change surgical management of locally advanced NSCLC[42]. There are currently multiple ongoing trials examining the use of immunotherapy regimens for NSCLC [Table 1][43-51]. However, there remains a lack of evidence regarding the safety of pulmonary resection after immunotherapy with only one retrospective study examining surgery after immunotherapy and a Cochrane review on immunotherapy after surgery[9,18].
CONCLUSION
The treatment of locally advanced NSCLC continues to evolve. Work is ongoing regarding immunotherapy and the best approach: neoadjuvant vs. adjuvant treatment. Additionally, minimally invasive surgical methods continue to evolve and become refined as surgeons increase their experience and technology improves. Although open thoracotomy has previously been the standard for locally advanced NSCLC,VATS is slowly becoming more common as studies show similar long-term outcomes and equivalent or better perioperative outcomes. In our own, unpublished experience, we observed similar rates of complications versus open surgery and shorter length of stay as previously reported but a better rate of proceeding on to adjuvant therapy holding with the concept of faster recovery for less invasive surgery[52].This indicates to us that, by performing more cases of locally advanced NSCLC in a minimally invasive manner, we can help patients proceed more quickly to indicated therapy.
While further work is needed to elucidate the appropriate management of locally advanced NSCLC, in terms of neoadjuvant and adjuvant treatment, the minimally invasive surgical approach to this condition has now come into its own. With perioperative, operative, and long-term outcomes now equivalent or better than open thoracotomy, we recommend that experienced surgeons offer minimally invasive VATS approach as the primary surgical method for locally advanced NSCLC.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study and performed data analysis and interpretation: Dolan DP, Dezube AR, Swanson SJ
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Dr. Swanson is a consultant for Covidien and Ethicon. Remaining authors declared that there are no conf l icts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
? The Author(s) 2020.
- Mini-invasive Surgery的其它文章
- Endometrioma surgery and possibilities of early disease control
- Early experience of uniportal video assisted thoracoscopic surgery in a New Thoracic Unit in Hospital Kuala Lumpur, Malaysia
- Robotic thymectomy for myasthenia gravis
- Transcatheter mitral valve implantation: different fi xation techniques
- Quality of life, pain, and functional respiratory recovery after lobectomy for early stage non-small cell lung cancer: a review of the literature comparing minimal invasive and open procedures
- Transanal total mesorectal excision for rectal cancer: state of the art