Activated persulfate by DBD plasma and activated carbon for the degradation of acid orange II
Weigang CHEN (陳衛(wèi)剛),Haixia WU (武海霞),Jiawei FAN (樊佳煒),Zhi FANG (方志) and Shaohua LIN (林少華)
1 College of Urban Construction,Nanjing Tech University,Nanjing 211816,People’s Republic of China
2 School of Automation and Electrical Engineering,Nanjing Tech University,Nanjing 211816,People’s Republic of China
3 School of Civil Engineering,Nanjing Forestry University,Nanjing 210037,People’s Republic of China
Abstract
Keywords:DBD plasma,activated carbon,persulfate,sulfate radical,acid orange II
1.Introduction
Azo dyes are widely used in textile,paper and food industries and constitute about 70%of the world dye production.Due to azo dyes’complex chemical structures and high stability,it is very difficult to remove them in conventional wastewater treatment plants.The discharge of dye wastewater can result in reduction of water transparency and oxygen transfer rate into water,causing damage to ecological environment [1,2].Advanced oxidation technology is non-selective and an effective method for treating refractory pollutants,which is widely used in the treatment of dye wastewater[3].Dielectric barrier discharge(DBD)is a new type of advanced oxidation technology that combines physical and chemical processes,it can generate a substantial amount of activated species (such as OH·,H2O2and O3) as well as ultraviolet radiation via ionization,excitation and dissociation [4].DBD has been widely studied in water pollution abatement due to its stable discharge,high safety performance,and high-energy active particles [5–8].
Persulfate (PS) oxidation technology is a new advanced oxidation technology that has been developed in recent years.PS can be activated into strongly oxidized sulfate radical(SO4?·) (E0=2.5–3.1 V).Hydrogenation,addition,and electron transfer reactions are used to achieve the oxidative removal of refractory pollutants in PS oxidation [9].PS is classified into peroxydisulfate (PDS) and peroxymonosulfate(PMS).Researchers have used various methods to activate PDS and PMS,such as light[10],heat[11],transition metals[12],and activated carbon[13–15].Activated can function as both catalyst for PS activation and adsorbent for removal of some contaminants and by-products [15].
In recent years,some studies have used non-thermal plasma (NTP) to activate PS.Shang et al [16,17]examined the synergetic removal performance of acid orange 7 (AO7)by DBD plasma and PS.The decolorization efficiency of AO7 was substantially improved.The improvement efficiency depended on the addition dosage of PS,applied voltage,air discharge gap,and gas discharge atmosphere.Son et al[18]investigated the influence of added PDS or PMS on the removal efficiency of methylene blue in the submerged plasma irradiation system.Tang et al [19]investigated a process of PS activation in surface DBD plasma to facilitate the removal of tetracycline in water.It has been proved that introducing PS into the plasma setup could enhance pollutant decomposition and improve the energy efficiency of NTP.
In this paper,DBD plasma and HCl-pretreated granular activated carbon (HGAC) were used simultaneously to activate PDS to remove azo dye acid orange II (AOII).The overall objective of the present work is to evaluate the activation efficiency of the synergistic system for PDS.The effects of applied voltage,PDS dosage,HGAC dosage,initial pH value,and inorganic anions(Cl?,SO42?,HCO3?)on AOII removal in wastewater were investigated.The possible enhancement mechanism in the synergistic system DBD/HGAC/PDS was evaluated via active species scavenger experiments.The results of these experiments will be effective in the treatment of dyeing wastewater.
2.Materials and methods
2.1.Experimental device
Figure 1 shows the experimental device,which consists of a power supply (CTP-2000K,Nanjing Suman Plasma Co.,China),reactor,and auxiliary device.The plasma power supply voltage is 0–220 V,which is rectified by a voltage regulator to become an adjustable AC voltage with an amplitude of 0–30 kV and frequency of 5–25 kHz.The reactor comprises a quartz glass dish,high-voltage electrode,and ground electrode.Two cylindrical stainless-steel rods connected in parallel are used as high-voltage electrodes.Each rod is covered by a ceramic tube serving as dielectric barrier.The length and diameter of the rods are 50 and 20 mm,respectively.A stainless-steel plate with a diameter of 30 mm is attached onto the outside wall of the quartz glass vessel as a grounded electrode.The treated water was held by the vessel with 200 mm inner length,50 mm in height and 1.6 mm in wall thickness.
2.2.Materials
All chemical reagents were analytical grade and used without further purification.AOII was purchased from Aladdin Chemistry Ltd Co.(Shanghai,China).Sodium persulfate was used as persulfate and purchased from Sinopharm Chemical Reagent Ltd Co.(Shanghai,China).Solutions used were prepared with pure water.The initial concentration and pH of AOII solution were 20 mg l?1and 5.4,respectively.
GAC was purchased from Zhuxi Activated Carbon Ltd Co.(Wuxi,China).Prior to use,the GAC was repeatedly rinsed with deionized water,and then immersed in 2 mol l?1of dilute hydrochloric acid for 24 h.Thereafter,the GAC was rinsed with deionized water until the pH of the supernatant was near neutral.Lastly,the GAC was dried in an oven at 105°C for 12 h and stored in an airtight container,recorded as HGAC.
2.3.Experimental method
Synchronous activation experiments were performed in the quartz glass vessel described above.A total of 100 ml AOII wastewater with a fixed amount of PDS or HGAC was placed in the vessel.The initial pH of solution was adjusted with 1 mol l?1HCl and 1 mol l?1NaOH.The bottom of the highvoltage electrode was brought into contact with the liquid surface in the vessel.Typically,1 ml sample was taken every 4 min and excess sodium nitrite was added and filtered through a 0.45 μm filter for analysis.Sole HGAC adsorption experiments,sole PDS oxidation experiments and HGAC/PDS experiments were also conducted in this reactor with the same condition in absence of DBD treatment.
2.4.Analytical method
The pH of the solution was measured using a pH meter(PHS-3C,Instrument Electric Scientific Instrument Ltd Co.Shanghai,China).The zero potential of HGAC was measured by the potentiometric mass titrations method [20].The absorbance of AOII was measured at a wavelength of 484 nm using a UV–vis spectrophotometer (T6 new century,General Analysis Instrument Ltd Co.Beijing,China).The applied voltage was measured using a voltage probe (P6015A,Tektronix,USA) and a digital oscilloscope (TBS-1202B,Tektronix,USA).
3.Results and discussion
3.1.DBD/HGAC/PDS synergistic system to remove AOII
The removal rates of AOII in solutions by sole PDS,HGAC adsorption,sole DBD,HGAC/PDS,DBD/PDS,DBD/HGAC,and DBD/HGAC/PDS processes are compared in figure 2.When the experiments were carried out for 28 min,the PDS system nearly had no removal of AOII,indicating that the oxidation performance of PDS is negligible when it is not activated.The removal rates of AOII by the HGAC adsorption and sole DBD were 14.3% and 28.2%,respectively.As a kind of NTP,DBD can generate kinds of oxidizing species,such as OH·,O3,H2O2,as well as some physical effects,which can degrade the organic contaminants in water [21].
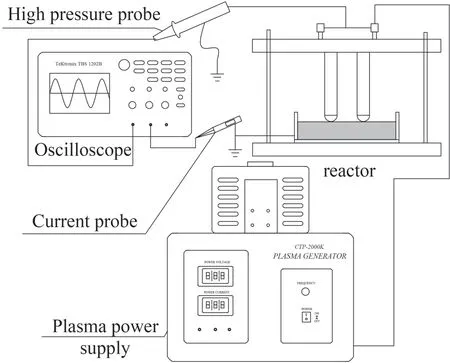
Figure 1.Schematic of the experimental apparatus.
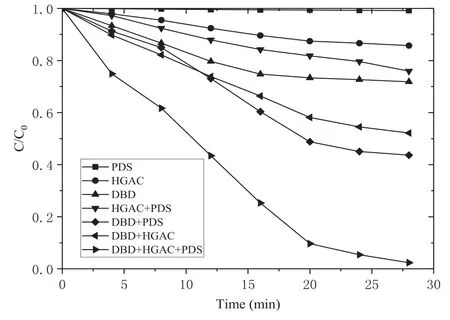
Figure 2.Removal of AOII in different systems(HGAC 1 g l?1,PS:AOII 200:1,16 kV).
The removal rates of AOII reached 24.1%,56.4%,and 47.8% in HGAC/PDS,DBD/PDS,and DBD/HGAC system,respectively.In the HGAC/PDS system,HGAC can simultaneously be the activation site of PDS while adsorbing through reactions (1) and (2) [22]
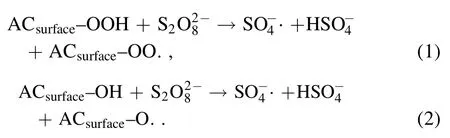
In the DBD/HGAC system,the presence of HGAC during the discharge process enhanced the AOII removal rate.Similar phenomenon has been reported by Wang et al[23]and Zhang et al [24].It has been demonstrated that carbon materials could result in the decomposition of O3to yield OH·and O2?·(reactions(3),(4)),and decomposition of H2O2to OH·and HO2· in solution (reactions (5),(6)) [23].Compared with HGAC adsorption and sole DBD treatment,synergistic effect on AOII removal was observed in the DBD/HGAC system.
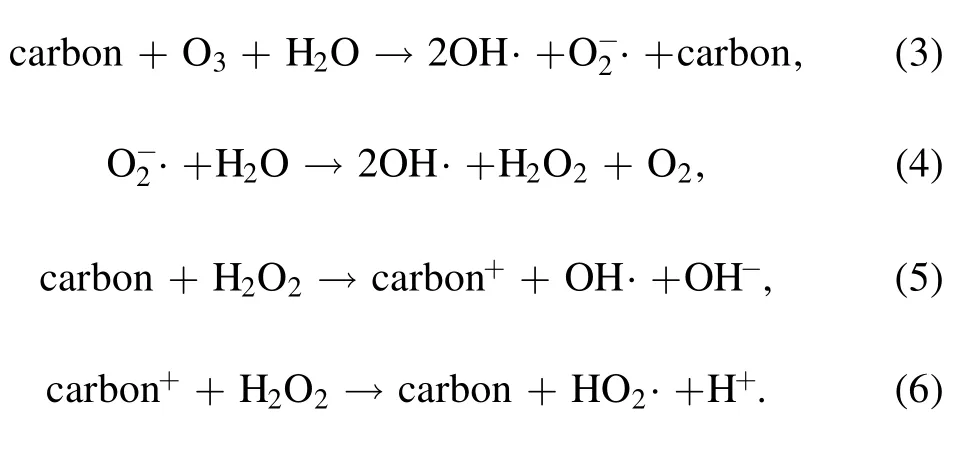
In the DBD/PDS system,ultraviolet light,hydrated electrons and high-energy electrons generated by DBD contribute to the activation of PDS to generate strong oxidizing SO4?·through the following reactions (7) [16]and (8) [17].Compared with the sole PDS and sole DBD process,the removal rate was promoted obviously in the DBD/PDS system.
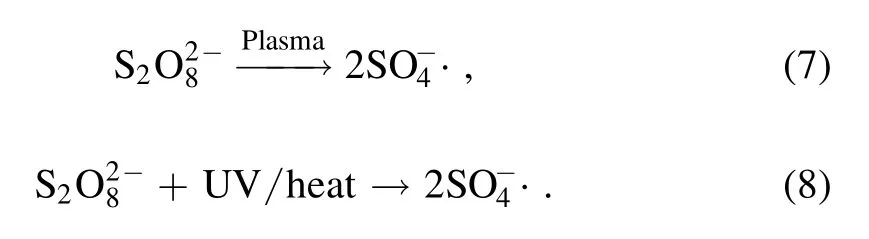
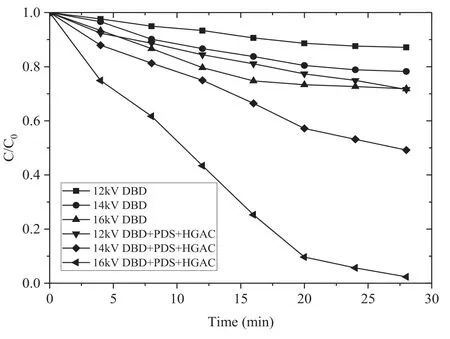
Figure 3.Effect of different applied voltages on the removal of AOII(HGAC 1 g l?1,PS:AOII 200:1).
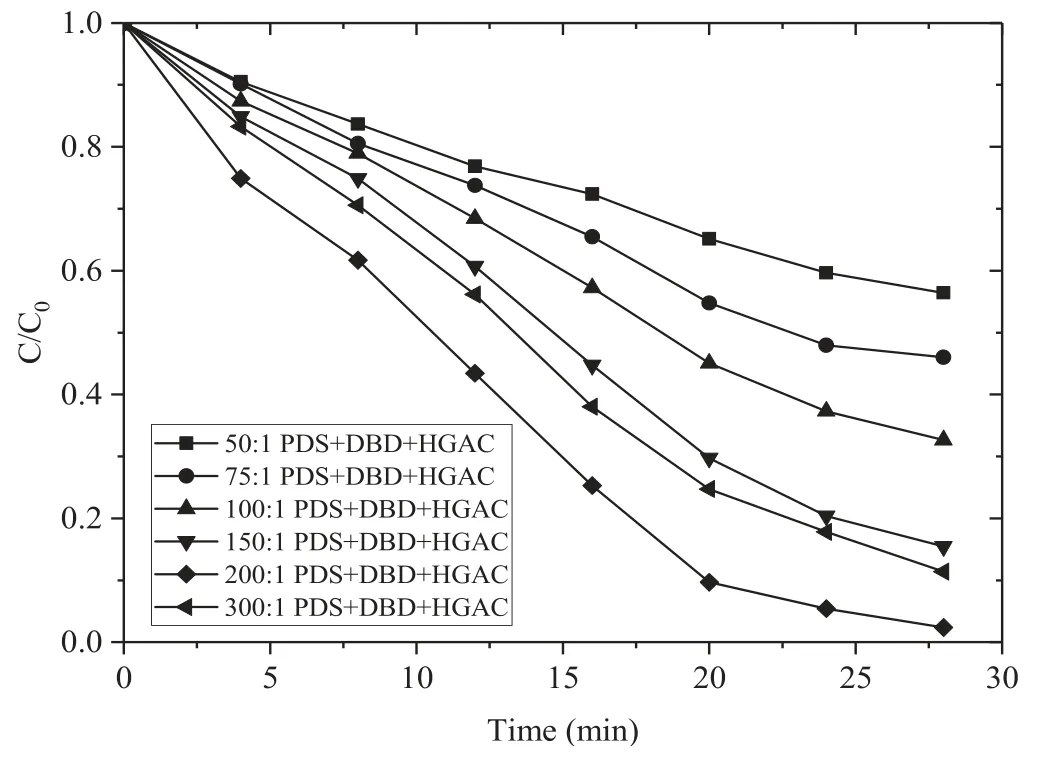
Figure 4.Effect of different PDS dosages on the removal of AOII(HGAC 1 g l?1,16 kV).
In the DBD/HGAC/PDS synergistic system,the removal rate of AOII reached 97.6%after 28 min treatment.Compared with the HGAC/PS,DBD/PS,and DBD/HGAC systems,the removal rate of AOII increased by 73.5%,41.2%,and 49.8%,respectively,and 54.7% higher than the sum of the three separate systems.Except the PDS activation by plasma and by HGAC,the discharge reaction of DBD facilitates the specific surface area of activated carbon and can increase the acidic oxygen-containing functional groups of activated carbon [25].Therefore,the adsorption for AOII and activation for PDS by HGAC was facilitated.The more production of OH·and SO4?·caused by the synergistic effects in the DBD/HGAC/PDS system promotes high removal of dyes in a short period of time.
3.2.Effect of applied voltage
To evaluate the effect of different applied voltages on AOII removal,experiments were conducted in sole DBD and DBD/HGAC/PDS system.Obviously shown in the figure 3,as the applied voltage increases,the removal rate of AOII is improved in the two systems.In the absence of PDS and HGAC,when the applied voltages were 16 kV,14 kV and 12 kV,the AOII removal rates after 28 min treatment were 28.2%,21.7% and 12.9%,respectively.The removal rate of AOII was 97.6% with applied voltage of 16 kV in the DBD/HGAC/PDS system,which was 69.4%higher than that in the sole DBD system.Compared with no PDS and HGAC addition,the AOII removal was substantially increased after the addition of the two substances.As the applied voltage increased,the number of the energy injected in the reactor improved,which is beneficial for the SO4?· formation and AOII removal.On the other hand,the oxidizing substances(i.e.H2O2,O3,O·,and OH·) are considerably generated owing to the increase of the applied voltage.The collisions between the AOII molecules and active particles are improved [26].
3.3.Effect of PDS dosage
Figure 4 shows the effect of different PDS dosages on the removal of AOII in the DBD/HGAC/PDS system.Evidently,as the molar ratio of PDS to AOII increased from 50:1 to 200:1,the removal rate of AOII increased significantly.However,when the molar ratio of PDS to AOII was 300:1,the removal rate decreased.After 28 min treatment,the PDS dosages with molar ratio were 50:1,75:1,100:1,150:1,200:1,and 300:1,the removal rates of AOII reached 43.6%,53.9%,67.4%,84.5%,97.6%,and 88.6%,respectively.
When the molar ratio of PDS to AOII was below 200:1,SO4?· and OH· generated by the synergistic system increased with the increasing addition of PDS,thereby leading to improve the removal of AOII.Though the augmented amount of PDS is good to the enhancement of active species formation,the recombination feasibility of SO4?·would be greater at the higher PDS concentration,resulting in the balance between the production and inhibition reactions of reactive radicals [19].Excessive PDS will react with SO4?· and OH·generated in the reaction,and excessive SO4?·will occur with own quenching and react with OH·,resulting in further reduction of oxidants,as shown in reactions (9)–(12) [27].Thereby an appropriate addition dosage of PDS is required in DBD/HGAC/PDS at a high removal rate of AOII
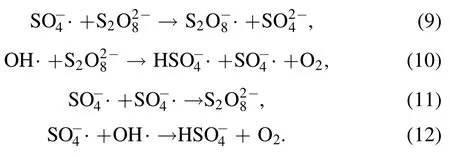
3.4.Effect of HGAC dosage
Figure 5 shows the removal rate of AOII under different HGAC dosages in the DBD/HGAC/PDS system.As the HGAC dosage increases,the removal rate of AOII also increases.When the HGAC dosages were 0.2,0.5,1,and 1.5 g l?1,the removal rates of AOII after 28 min treatment reached 52.7%,78.0%,97.6%,and 98.5%,respectively.The AOII removal enhancement is attributed to the improvement of active substances.The reason is that as the HGAC dosage increases,the total surface area increases which leads to generate more OH·[28].Moreover,the carboxyl groups and acidic oxygen-containing functional groups of HGAC also increase as the PDS activation site increases.This situation is considerably favorable for PDS to generate more SO4?·.However,the addition of 1.5 g l?1of HGAC compared to the addition of 1 g l?1did not result in a significant increase in the removal rate.It is due to that some of HGAC can gather by itself in the system,leading to reduction of contact area with PDS.
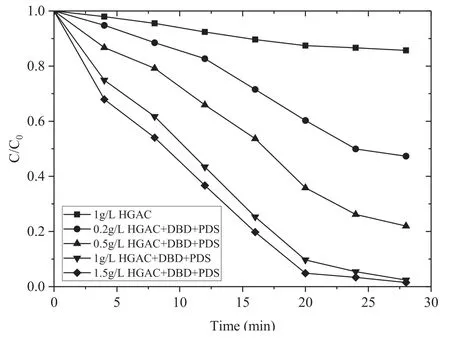
Figure 5.Effect of different HGAC dosages on the removal of AOII(PS:AOII 200:1,16 kV).
3.5.Effect of initial pH value
Solution pH value is an important parameter,which will not only affect the production of active species in the discharge process but also affect the PDS activation performance.Figure 6 shows that removal rates in neutral and acidic solution conditions were faster than that in alkaline solution condition in the DBD/HGAC/PDS system.After 28 min reaction,the removal rates of AOII reached 86.3%,97.6%,98.8%,80.3%,and 74.3%when the pH values of the solution were 3.0,5.4,7.0,9.0,and 11.0,respectively.Shang et al found that the acid orange decolorization efficiency in basic and neutral solutions was lower than that in acidic solution by DBD and PS synergetic treatment [16].Tang et al found that there was little difference in the tetracycline degradation efficiencies among acidic,neutral and alkaline conditions treated by discharge plasma coupled with PDS[19].In acidic condition,O3is the dominant active species for the organic compounds degradation in the plasma system without PDS.However,O3mainly exists as a molecular state at low pH value[29].On the other hand,the secondary radicals NO·,NO2·,OH· generated by air discharge in acid condition can react with organic compounds further [28].When PDS was added in solution,SO4?· is dominant and becomes more important than other radicals in acid solution,which is responsible for organic removal in the synergic system [30].
With the pH value rises,O3would be rapidly decomposed to OH· (reactions (13),(14)) [31].On the other hand,SO4?·generated by the PDS activation will react with OH?to form OH·(reaction(15)).Thereafter,OH·will be converted into H2O2and·O?through reactions (16),(17),and H2O2will reduce the oxidation potential of S2O82?[32].In addition,a substantial amount of OH?under alkaline conditions will reduce the carboxyl and acidic oxygen-containing functional groups of HGAC.Hence,the free radicals generated by PDS activation are reduced
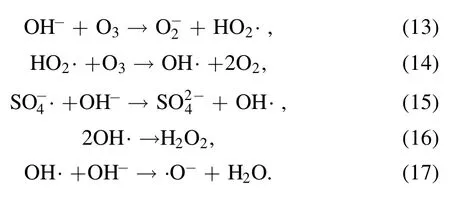
From another aspect,the pHpzc(potential of zero charge)of HGAC is 5.9.In acidic condition(pH <pHpzc),there are lots of positive charges on the surface of HGAC,which is beneficial to adsorb anion dye AOII molecule.At basic pH values,a net positive charge is presented in HGAC and the repulsive electrostatic interactions between the negatively charged on the HGAC surface and the anion dye AOII molecule [33].Hence,the reactions between the catalytic produced active specials and AOII molecule are hindered in alkaline condition.
3.6.Effect of different radical quenchers
To analyze the main reactive species for AOII removal in the DBD/HGAC/PDS system,MA (methanol),TBA (tertbutanol),and phenol with a molar ratio of 1000:1 to AOII were added as free radical quenchers under the initial experimental conditions.MA is a free radical quencher and can react with OH· and SO4?·.The reaction rate constants are 9.7×108l mol?1s?1and 3.2×106l mol?1s?1.TBA is an effective quencher for OH· and the reaction rate constant is 3.8×108–7.6×108l mol?1s?1.However,the reaction with SO4?·is considerably slower at approximately 4×105–9.1×105l mol?1s?1[34].Phenol can immediately react with OH·and SO4?· simultaneously and the reaction rate constants are 6.6×109l mol?1s?1and 8.8×109l mol?1s?1[35].Figure 7 shows the removal effects of AOII with the addition of MA,TBA,and phenol in the DBD/HGAC/PDS system after 28 min of reaction.Evidently,the removal rates were 57.9%and 86.5%,which were decreased by 39.7% and 11.1% after the addition of MA and TBA.This result indicates that SO4?·and OH· radicals play an important role in the removal of AOII.The presence of phenol inhibited the removal rate of AOII by 81.1%,which was substantially higher than that of MA and TBA.The result may be related to the hydrophilicity of MA and TBA.Compared with phenol,MA and TBA are not easily adsorbed on the surface of HGAC.Hence,quenching SO4?· formed on the surface of HGAC is impossible.This finding also proves that the free radicals formed on the surface of HGAC also play an important role in the removal of AOII.
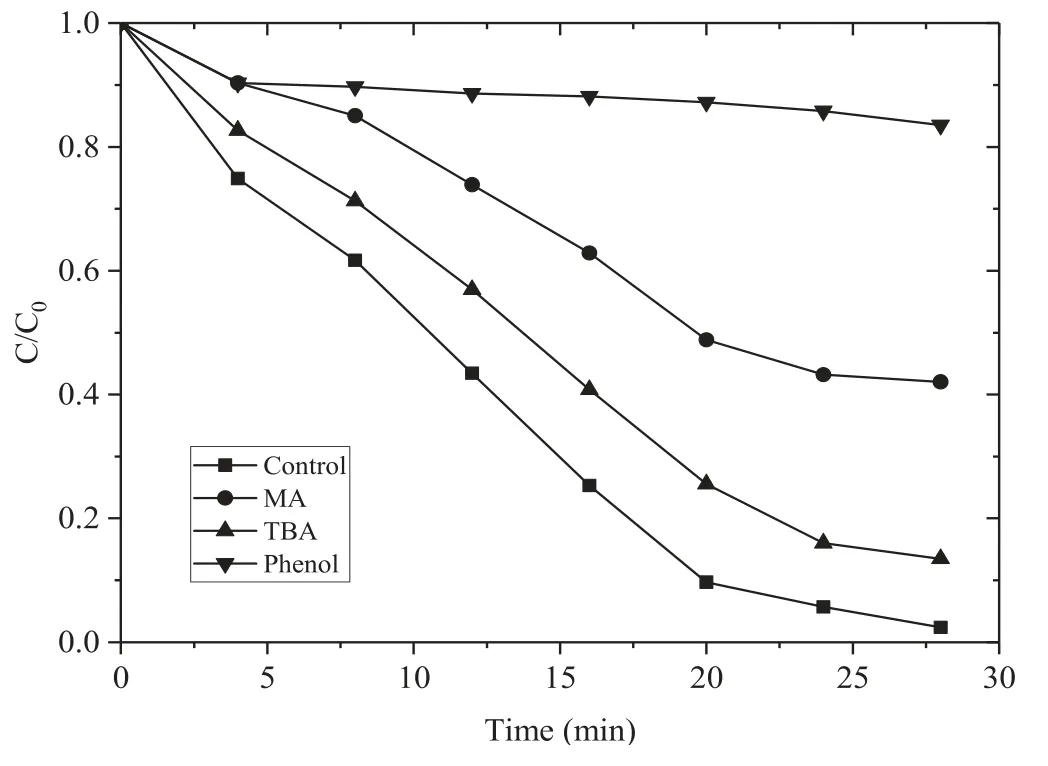
Figure 7.Effect of the different radical quenchers on the removal of AOII (HGAC 1 g l?1,PS:AOII 200:1,16 kV).
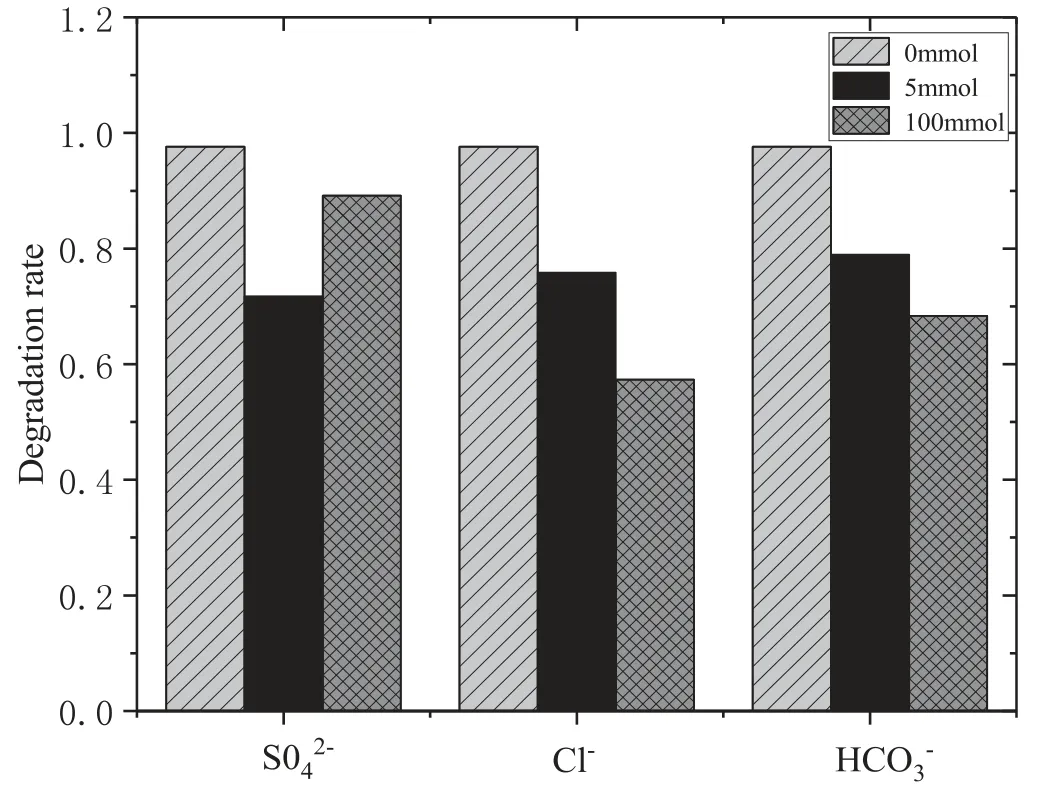
Figure 8.Effect of different inorganic anions on the removal of AOII(HGAC 1 g l?1,PS:AOII 200:1,16 kV).
3.7.Effect of inorganic anions
To investigate the effects of different types of inorganic anions on the AOII removal in the DBD/HGAC/PDS system,5 mmol l?1and 100 mmol l?1of SO42?,Cl?,and HCO3?were added into the solutions.As shown in figure 8,the removal rate was 71.7%at a concentration of 5 mmol l?1SO42?after 28 min of reaction,while it was 89.1% at a concentration of 100 mmol l?1SO42?.It has been found that SO42?could inhibit contaminant degradation in the activated PS process for reducing the semi-reaction reduction potential of SO4?· [36].However,studies have shown that the presence of SO42?can promote the adsorption of activated carbon [36,37].With increasing dosage of SO42?,the extent of adsorption of dye molecules onto carbon catalysts is promoted,which is favorable for pollutant degradation.That is the reason why the decrease of removal rate with 100 mmol l?1SO42?addition is lower than that with 5 mmol l?1SO42?addition.
The removal rates were 75.9% and 78.9% at a concentration of 5 mmol l?1Cl?and HCO3?after 28 min of reaction.The removal rates were 57.3% and 68.4% at a concentration of 100 mmol l?1Cl?and HCO3?.As the concentration ofthe two inorganic anions increased,the removalrate gradually decreased.The Cl?in the solution will affect the degradation effect because Cl?will react with OH· generated by DBD and SO4?·generated by the synergistic system activation [38],thereby producing a weak oxidation activity of Cl·.The reaction formula is shown in reactions (18)–(21).The experimental phenomenon after adding Cl?is the same as that in Ren et al [39],where DBD plasma and O3were combined to synergistically degrade trans-ferulic acid.Accordingly,the addition of Cl?inhibited the removal effect
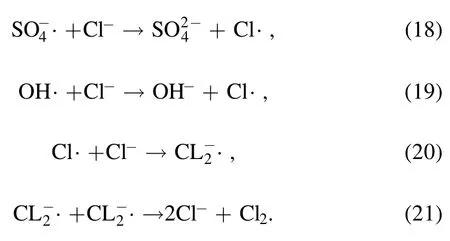
The effect of HCO3?in the solution is mainly caused by the hydrolysis reaction occurring in the aqueous solution.The formation of a substantial amount of OH?makes the solution alkaline.Such a solution is not conducive to the activation of PDS by carboxyl groups and acidic oxygen-containing functional groups on HGAC.Moreover,OH?will react with SO4?·following reaction(9).HCO3?can also react with SO42?to generate weak carbonate radicals and bicarbonate radicals.This result is consistent with that of Shi et al [40],who studied the degradation efficiency and mechanism of PS activated by different GACs.The removal rate of AOII significantly decreased when different concentrations of HCO3?were added.
4.Conclusions
The DBD/HGAC/PDS synergistic system is more conducive to the efficient removal of AOII than sole system.When the AOII dye wastewater concentration was 20 mg l?1,DBD applied voltage was 16 kV,HGAC dosage was 1 g l?1,PS and AOII molar ratio was 200:1 and initial pH value was 5.4,the removal rate of the dye AOII reached 97.6%.The increase in HGAC dosage is beneficial to the removal of AOII.The increase of PDS dosage in a certain concentration can increase the removal rate of AOII,but an excessively high concentration of PDS is not conducive to AOII removal.The removal rate in acid and neutral conditions is better than that in the alkaline condition.SO4?· and OH· radicals play an important role in the removal of AOII in the DBD/HGAC/PDS system and the inorganic anion has an inhibitory effect on the removal of AOII.
Acknowledgments
This work is supported by National Natural Science Foundation Youth Project of China (No.51707093).
 Plasma Science and Technology2020年3期
Plasma Science and Technology2020年3期
- Plasma Science and Technology的其它文章
- DBD coupled with MnOx/γ-Al2O3 catalysts for the degradation of chlorobenzene
- Investigation on pulsed discharge mode in SF6-C2H6 mixtures
- Temporal evolution of atmospheric cascade glow discharge with pulsed discharge and radio frequency discharge
- How bead shapes affect the plasma streamer characteristics in packed-bed dielectric barrier discharges:a kinetic modeling study
- Comparative analysis of the arc characteristics inside the convergingdiverging and cylindrical plasma torches
- 1,2,4-trichlorobenzene decomposition using non-thermal plasma technology
