Bioactivity of Total Flavonoids Extracted from Kunlun Chrysanthemum Flowers (Coreopsis tinctoria)
JING Siqun, ZHAI Hongyue, SU Leping, REN Zhiyan, WANG Yinna, YAN Liangjun
(1. Yingdong College of Food Science and Engineering, Shaoguan University, Shaoguan 512005, China;2. College of Life Sciences and Technology, Xinjiang University, ürümqi 830046, China; 3. Department of Pharmaceutical Sciences,UNT System College of Pharmacy, University of North Texas Health Science Center, Fort Worth 76107, USA)
Abstract: In this study, the antioxidant and antibacterial properties of total flavonoids from Kunlun chrysanthemum flower (KCTF) were evaluated together with its protective effect on liver injury induced by carbon tetrachloride in mice and anti-proliferative effect on tumor cells. The antioxidant capacity was measured by free radical scavenging capacity in vitro, as well as superoxide dismutase activity and malondialdehyde content in vivo. Moreover, the anti-proliferative activity was determined using HeLa cells and esophageal cancer Eca-109 cells. Results indicated that KCTF had significant antioxidant activity and inhibited the growth of both Gram-positive and Gram-negative bacteria.However, KCTF had no significant hepatoprotective effect. KCTF possessed potent inhibitory activity with average half-inhibitory concentration (IC50) values of (133.60 ± 0.12) and (192.00 ± 0.08) μg/mL for HeLa and Eca-109 cells,respectively. Therefore, KCTF may have potential application in functional food industries due to its antioxidant,bacteriostatic and anti-proliferative effects.
Keywords: total flavonoids from Kunlun chrysanthemum; antioxidant activity; antibacterial activity; anti-proliferative effect; protective effect on liver injury
Kunlun chrysanthemum(Coreopsis tinctoria,C. tinctoria) is an annual herbaceous plant which belongs to family Compositae, and commonly grows in the Middle East, Eastern Europe, Western and Central Asia[1]. In Xinjiang of China,C. ticntoriais commonly known as“Kunlun chrysanthemum” and “snow chrysanthemum”, and is cultivated in Karakorum Mountains of 2 600 meter above sea level. In traditional Uyghur medicine,C. tinctoriahas been used for treatment of various diseases such as hypertension,palpitation and gastrointestinal discomfort[2]. In recent years,there are more studies on the bioactive extracts from Kunlun chrysanthemum due to its antioxdation function[3]and its ability in reducing blood lipid without causing liver damage in hyperlipidemic mouse[4]. However, most research reported on biological activity ofC. tinctoriahas focused on the polysaccharide compounds[5]or its other extracts while few comprehensive studies have been performed on its flavonoids extracts.
Flavonoids are one of the major constituents ofCoreopsisgenus[6]. Flavonoids are important secondary metabolites, and widely distributed in various plants[7].Biological activities of the flavonoids have been reported in many studies, such as antioxidant, anti-tumor and hypertensive effect[8-9]. Studies using cultured cells showed that flavonoids could significantly inhibit the growth of various malignant cells, such as breast cancer[10], liver cancer[11], cervical cancer[12], and stomach cancer cells[13].As far as we know, there is a lacking of study regarding the antibacterial property and protection against liver injury and antiproliferative effects of Kunlun chrysanthemumtotal flavonoids (KCTF) on cancer cells.
In the present study, bioactivities of KCTF were evaluated from different aspects, I) the antioxidant activities of KCTF were investigated by experimentsin vitroandin vivo; II) the antibacterial property of KCTF was identified by the disc diffusion method; III) the protective effect on liver injury was studied through the acute liver injury in mice induced by CCl4model; IV) the antiproliferative effect of KCTF was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and optical microscopy. This study may provide theoretical support for potential use of scientific application of KCTF for food and drug resources.
1 Materials and methods
1.1 Materials, animals, microorganisms and reagents
DriedC. tinctoriaflowers were obtained from a local herbalist at Hetian in Xinjiang in August 2012, and were identified by Prof. Abudula Abbas from Xinjiang University, China. Kunming male mice (20 ± 2) g(2011-0003/SCXK(Xin)) were obtained from Xinjiang Laboratory Medical University Breeding Research Center and basal diet was provided by the Xinjiang Animal Center.Animals were kept under a 12 h/12 h light/dark cycle and allowed free access to food and water. The study protocols were approved by the Ethics Committee on Animal Experiment, Xinjiang University, China.
Staphylococcus aureus,Escherichia coli,Bacillus subtilis,Penicillium citri,Aspergillus niger,Saccharomyces cerevisiaewere obtained from Microorganism Laboratory of College of Life Science and Technology, Xinjiang University.The strains were subcultured for further antimicrobial test.
Human cervical carcinoma cells (HeLa), esophageal cancer cells (Eca-109) and Vero cells were obtained from Xinjiang Key Laboratory of Biological Resources and Genetic Engineering, Xinjiang University.
Standard rutin (purity 95%) Tianjin Jianfeng Biochemical Co. Ltd.; AB-8 macroporous adsorption resin Tianjin Nankai University Experimental Chemical Plant; Ascorbic acid (VC) (purity ≥ 99.8%) Shanghai Yuanye Biotechnology Co. Ltd.; Marein (purity 95%,C. tinctoriachalcone 4’-O-glycosidase Autonomous Region Pharmaceutical Research Institute; Tert-butyl hydroquinone (TBHQ) Guangzhou Taibang food additive Co. Ltd.; 1,1-diphenyl-2-picrylhydrazyl(DPPH) Tianjin The Third Chemical Reagent Factory;Malondialdehyde (MDA), superoxide dismutase (SOD),total anti-oxidant capability (T-AOC), Coomassie bright blue, lactic dehydrogenase assay kit Nanjing Institute of Biological Engineering; bovine serum albumin (BSA)Beijing Shuangxuan Microbiological Products Co. Ltd.;MTT, dimethyl sulfoxide (DMSO), Annexin V-fluorescein isothiocyante (FITC), propidium iodide (PI) America Sigma Co. Ltd.; Pancreatic enzyme Beijing Dingguo Changsheng Biotechnology Co. Ltd.; Bifendate (1.5 mg/pellet)Zhejiang Aanbang Pharmaceutical Co. Ltd.; Alanine aminotransferase (ALT), aspartate aminotransferase(AST) assay kit Zhongsheng Beikong Biotechnology Co. Ltd.; Other reagents, such as potassium iodide,azobenzene, were all domestic analytical pure.
1.2 Instruments and equipments
SHB-III circulating water vacuum pump Zhengzhou Great Wall Science and Trade Co. Ltd.; RE-52AA rotary evaporator Shanghai Yarong Biochemical Instrument Factory; Spectrumlab 53 ultraviolet-visible spectrophotometer Shanghai Lengguang Technology Co. Ltd.; H-1 micromixer Shanghai Kanghe Photoelectric Instrument Co. Ltd.; High speed freezing centrifuge Germany Sigma Electrical Instrument Co. Ltd.; SW-CJ-2FD bechtop Shanghai Boxun Industrial Co. Ltd.; Rotary evaporator Guangzhou Instrument Laboratory Technology Co. Ltd.; V-1100D visible spectrophotometer Shanghai Meibuda Instrument Co. Ltd.;LC-10AT high performance liquid chromatograph (HPLC)with UV-Vis detector Japan Shimazu Co. Ltd.; SABA18 automatic biochemical analyzer Italian Medical Analytical Instruments Group; GMSX-280 otoscope Beijing Yongguang Medical Instrument Factory, Beijing; TDL80-2B table centrifuge Shanghai Anting Scientific Instrument Factory; Model 3423 CO2incubator American Thermo Fisher Scientific Inc.; XD-101 inverted biological microscope Nanjing Jiangnan Yongxin Optics Co. Ltd.; Benchmark enzyme standard instrument American Bio-Rad Company; 985-370 homogenizer American Biospec Company; EX20 biological microscope Ningbo Shunyu Instrument Co. Ltd..
1.3 Methods
1.3.1 Preparation of KCTF
The optimum conditions of KCTF by ultra high pressure(UHP) extraction[14]were as following: extraction temperature 25 ℃, ratio of solid to liquid 1:16 (m/V), pressure 340 MPa,UHP time 3 min, material particles 80 meshes, and under this condition, the yield of KCTF was up to 12.35%. Then, the KCTF was further purified by AB-8 macroporous adsorption resin and with rutin being used as the standard, the content of total flavonoids could reach 52.43% after purification.
1.3.2 HPLC analysis of KCTF
In order to investigate the main chemical composition of KCTF, HPLC analysis was adopted[15]and commercially available marein were used as reference substance.The chromatographic separation was performed on a Phenomenex Gemini C18column (250 mm × 4.6 mm,5 μm) with the mobile phase, acetonitrile: 0.5% formic acid solution. The percentage of acetonitrile was changed from 5% to 20% for 60 min. The flow rate, column temperature and detection wavelength were 1.0 mL/min, 35 ℃ and 378 nm, respectively.
1.3.3 Determination ofin vitroantioxidant activities of KCTF
Antioxidant activity of KCTF was determined by reducing power, DPPH radical scavenging, and hydroxyl radical scavenging tests. A series concentrations of KCTF(0.1, 0.3, 0.5, 0.7 and 0.9 mg/mL) were prepared with the same concentrations of VC and TBHQ as positive control.While the mixture of equal volume distilled water and anhydrous ethanol was blank. All tests were carried out in triplicate.
1.3.3.1 Assay of reducing power
The reducing power of KCTF was determined according to the method of Jing Siqun et al[16]with slight modifications.Briefly, various concentrations of KCTF (0.1–0.9 mg/mL)in 1.0 mL methanol were mixed with 2.5 mL of phosphate buffered saline (PBS) (0.2 mol/L, pH 6.6) and 2.5 mL of 1%potassium ferricyanide in 10 test tubes. The mixtures were incubated at 50 ℃ for 20 min. At the end of the incubation,2.5 mL of 10% trichloroacetic acid was added to the mixtures and then centrifuged at 5 000 ×gfor 10 min. The upper layer (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride, and the absorbance was measured at 700 nm with a UV-Vis spectrophotometer.
1.3.3.2 Assay of DPPH radical scavenging activity
Generally, radical scavenging activities of antioxidants in the plant extracts were evaluated using DPPH radicals,which was measured by the method of Ullah et al[17]. Briefly,2 mL of 2 × 10-4mol/L DPPH radicals-ethanol solution was added to 2 mL various concentrations (0.1–0.9 mg/mL) of KCTF solution. The reaction mixture was incubated for 30 min at room temperature in the dark. The absorbance was measured at 514 nm with aUV-Vis spectrophotometer. The DPPH radical scavenging activity was calculated as formula (1).
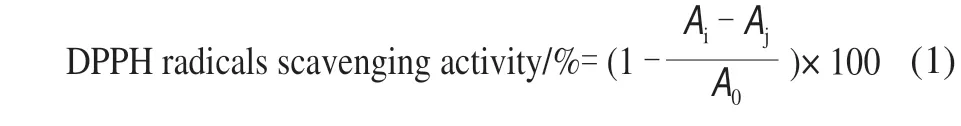
whereA0is the absorbance of the control;Aiis the absorbance of the sample andAjis the absorbance of the blank.
1.3.3.3 Assay of hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity was determined according to reported phenanthroline-Fe2+oxidation method with minor modifications[18]. Briefly, 4.0 mL PBS (pH 7.4)was mixed with 1.5 mL of 5 mmol/L phenanthroline solution in a test tube. Then, 1.0 mL FeSO4solution(7.5 mmol/L) and 1.0 mL different concentrations(0.1–0.9 mg/mL) of KCTF solution were added. Finally,1.5 mL double distilled water and 1.0 mL 1% H2O2solution were added. The absorbance of the final solutions was measured at 536 nm with a UV-Vis spectrophotometer after incubation at 37 ℃ for 60 min. The hydroxyl radical scavenging activity was calculated as formula (2).
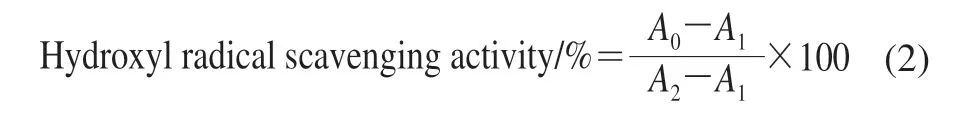
whereA0is the absorbance after adding the sample and H2O2;A1is the absorbance after adding H2O2without sample;andA2is the absorbance without sample and H2O2.
1.3.4 Determination ofin vivoantioxidant activities of KCTF
Antioxidant activityin vivoof KCTF was carried out by the method based on JingSiqun et al[19]with a few modifications. The concentrations of KCTF used were based on data reported in the reference [19] in conjunction with purity and dosage used in mice. After a week acclimation to the laboratory, 50 mice were randomly divided into five groups: normal control group (NC), low-dose group (LD,30 mg/(kgmb·d)), middle-dose group (MD, 100 mg/(kgmb·d)),high-dose group (HD, 300 mg/(kgmb·d)) and positive control group (NC+, VC 100 mg/(kgmb·d)). The NC group of mice was given 0.2 mL physiological saline while low, middle and high dose groups were fed KCTF once a day for 28 days, and positive group was fed VC. The body mass of mice was measured once a week. After the final intragastric administration, the mice were fasted for 12 h and blood were then taken by pricking the eyeball. The mice serum was separated at 3 500 ×gfor 15 min and stored at 4 ℃. The organs of mice were removed out and weighed immediately after they were sacrificed. Then 10% concentration of liver homogenate was made with physiological saline at 4 ℃and the supernatant was removed and refrigerated after centrifugation at 3 500 ×gfor 5 min. The activity of SOD,the content of MDA in serum and liver homogenate was determined by commercial reagent kits according to the instruction manuals.
1.3.5 Analysis of antibacterial activity of KCTF
The antibacterial activity of KCTF was measured by the disc diffusion method shown by Wang Hongbin[20]with a little modification. The activated strain of bacteria,mold spores, and yeast were picked and transferred with loop into 9 mL sterile water respectively, and then shaken well to make spores and cell suspensions which contained 1 × 108–2 × 108cells/mL of bacteria, 1 × 106–8 × 106cells/mL of mold spores, and 1 × 106–7 × 106cells/mL of yeast. Under aseptic conditions, 200 μL of each bacteria suspension was added to the prepared medium plates and coated evenly. Sterile paper discs (9 mm in diameter) were impregnated with 5.00 mg/mL extracts solution, 0.03 mg/g potassium sorbate solution, 95%ethyl alcohol and sterile water saline solution overnight and then placed on the bacteria plate. Bacterial plates were incubated at(36 ± 1) ℃ for 18–24 h, while mold plates were incubated at (28 ± 1) ℃ for 48 h and yeast plates were incubated at(30 ± 1) ℃ for 24 h. The inhibition of bacterial and fungal growth were recorded by measuring the diameter (mm) of the clear zones surrounding the disc which indicate the presence of antimicrobial activity[21]. All data of antimicrobial assays are the average of triplicate analyses.
The minimum inhibitory concentration (MIC) were determined as described by Swenson et al[22]previously. The MIC was defined as the lowest concentration of a substance preventing visible growth of the test organisms. Sterile water alone was used as control.
1.3.6 Protective effect of KCTF on CCl4-induced acute liver injury in mice
The protective effect of KCTF on liver injury was observed by establishing a CCl4-induced acute liver injury model in mice described by Cai Hao et al[23]with a slight modification. Male Kunming mice (body mass: 18–22 g)were housed in standard cages at a constant temperature of (22 ± 1) ℃ and relative humidity of (55 ± 5) % with 12 h light-dark cycle (8:00 to 20:00) for at least 1 week before experiment.
For liver protection experiments[24-25], blank and model groups of mice were intragastrically administrated with 0.2 mL/10 g distilled water as the non-therapeutic control.The positive control group was given bifendate (0.4 g/kgmb)orally for 6 consecutive days. The KCTF groups of mice were orally administered KCTF (LD, 0.10g/kgmb, MD,0.20 g/kgmband HD, 0.40 g/kgmb) for 6 consecutive days.Both bifendate and KCTF were administered at the same time. One hour after the administration of the experimental drugs on Day 5, intraperitoneal injection of 0.1% CCl4olive oil (0.2 mL/10 g) was carried out except the mice in blank group. The mice were fasted overnight for 24 h with CCl4injection. Then the mice were sacrificed under anesthesia and the blood was taken by picking the mice’s eyeball and centrifuged (3 500 ×gfor 10 min) for supernatant analysis.The activities of the ALT and AST were measured. The liver coefficient was defined as liver mass/body mass.
1.3.7 Antiproliferative activity against tumor cells
1.3.7.1 Determination of cell proliferation by MTT assay
In vitroantiproliferative activity of KCTF against two tumor cells (HeLa and Eca-109) was used, together with Vero cells (normal cells) as control. The proliferation of cells mentioned above was conducted by MTT assay with some slight modifications[26-27]. Briefly, logarithmically growing cells were seeded in 96-well culture plates (5 × 104cells/well)for 24 h at 37 ℃ with 5% CO2in the atmosphere. The cultures were washed and treated with a serial concentration(12.5, 25, 50, 100, 200, 400 and 800 μg/mL) of 200 μL KCTF solution. 20 μL MTT solution (5 mg/mL) was then added after 12, 24, 48 h, or 72 h. After 4 h incubation at 37 ℃,at the end of the treatment, the incubation medium was discarded, and the formed crystals were dissolved in 100 μL DMSO. MTT reduction was quantified by measuring the light absorbance of each well at 570 nm using a Universal Microplate Reader to evaluate the proliferation of cancer cells. All experiments were performed in triplicate and cell survival was expressed as a percentage of the control, which was considered to be 100%. The half-inhibitory concentration(IC50) was calculated as the sample concentration that caused a 50% inhibition of cell proliferation.
1.3.7.2 Phase contrast inverted microscope observation of morphological changes of cells
HeLa cells, Eca-109 cells, and Vero cells (5 × 104cells/well)were incubated for 24 h in 96-well plates respectively. After incubation, the cells were untreated or treated with KCTF at different concentrations (12.5, 100 and 800 μg/mL) for 12–72 h[28]. Then the medium was removed and cells in wells were washed twice with PBS. This was followed by examination under phase contrast inverted microscope at 200 × magnification.
1.3.7.3 Fluorescence microscopy observation of HeLa cells
The cells, plated onto glass cover slips in 6-well plates and treated with 0–400 μg/mL of the KCTF solution for 48 h,were washed twice with PBS and stained with Hoechst 33258 for 15 min at 37 ℃. After washing by PBS twice,cover slips were mounted onto microscope slide and nuclear morphology was observed under a fluorescence microscope at 200 × magnifications.
1.3.7.4 Determination of LDH activity of HeLa cells
According to the reported method[29], the cells in logarithmic growth phase were seeded in 24-well culture plate, where each well having 5 × 104cells/mL cell suspension. After incubation for 12 h, the old medium was gently sucked out. Then 1.0 mL KCTF (0, 800 μg/mL medium) were added in each sample at three replicates and cultured for 72 h. Medium was centrifuged at 2 000 ×gfor 5 min according to reference [29] methods with minor modifications. The activity of lactate dehydrogenase (LDH)in the supernatant (as LDHa) was measured as an index of cell necrosis, the activity of LDH in suspended cells (as LDHn)was measured as an apoptotic index, the activity of LDH of a culture bottle adherent cell was (as LDHv) measured as the level of intracellular LDH. Apoptosis ratio and necrosis ratio were calculated as the following formula (3), (4).
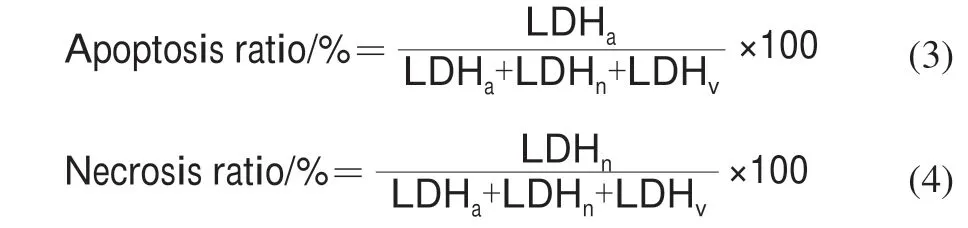
1.4 Statically analysis
Data were expressed as means ± standard deviations of three determinations. Statistical analysis was performed usingt-test and One-way analysis of variance. A value ofP< 0.05 was considered significantly different. All computations were made with SPSS 19.0 software.
2 Results and analysis
2.1 HPLC chromatograms of KCTF
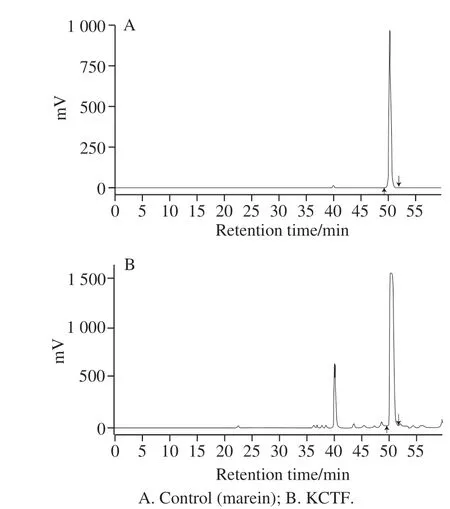
Fig. 1 HPLC chromatogram of KCTF
The result of HPLC analysis of KCTF showed that under such chromatographic conditions, KCTF mainly contained 2 characteristic components and one of the higher content was found to be marein (Kunlun chrysanthemum chalcone 4’-O-β-glucoside) after comparing with the spectrum of marein standard. Nonetheless, the area of another peak was too small to be analyzed (Fig. 1).
2.2 In vitro antioxidant activity of KCTF
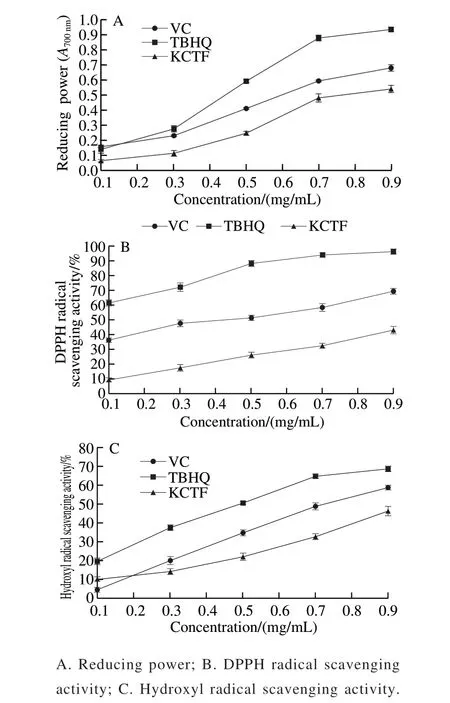
Fig. 2 In vitro antioxidant activity of KCTF
Reducing power may serve as an important indicator of a compound’s potential antioxidant activity. The antioxidants like VC could interrupt or inhibit the chain reaction by capturing and removing free radicals, and TBHQ terminate the chain reaction by providing a hydrogen atom[30-31]. As shown in Fig. 2A, the reducing power of samples were enhanced with the increasing concentration of KCTF. However, the growing trend becoming slower at the concentration of 0.7 mg/mL. The range of reducing power of the three tested samples from strong to weak was as following: TBHQ > VC > KCTF. This result indicated that KCTF has antioxidant capacity.
DPPH radical is stable, which has been widely used as a substrate to estimate antioxidative activity of antioxidants. The results showed that KCTF for DPPH radical scavenging ability has concentration-dependence(Fig. 2B). The scavenging activity of KCTF on DPPH radicals was 32.24% at the concentration of 0.9 mg/mL which is weaker than that of VC and TBHQ (68.12% and 97.23%, respectively). In general, the KCTF has antioxidant activity (IC50= (1.369 ± 0.052) mg/mL) but was lower than that of VC (IC50= (0.336 ± 0.089) mg/mL) and TBHQ(IC50= (0.074 ± 0.233) mg/mL). The DPPH radical scavenging activities could be attributed to their hydrogen donating abilities. Hence, the mechanism may be due to the supply of hydrogen by KCTF, which combines with radicals and forms a stable radical to terminate the radical chain reaction[32].
Among the oxygen radicals, hydroxyl radical is the most active and toxic free radical, and induces severe damage to adjacent biomolecules. Therefore, hydroxyl radical scavenging ability can be accepted as an illustrator of antioxidant activity. Fig. 2C shows that the scavenging activity of KCTF on hydroxyl radical was in a concentration-dependent manner and inhibition efficiency on hydroxyl radical is 44.28% at the concentration of 0.9 mg/mL while the scavenging activity of VC and TBHQ at the same concentration (0.9 mg/mL) were 57.62% and 69.43%,respectively. Moreover, the IC50of KCTF, VC and TBHQ for scavenging hydroxyl radical were (1.126 ± 0.315),(0.736 ± 0.080) mg/mL, and (0.441 ± 0.104) mg/mL, respectively.
2.3 In vivo antioxidant activity of KCTF
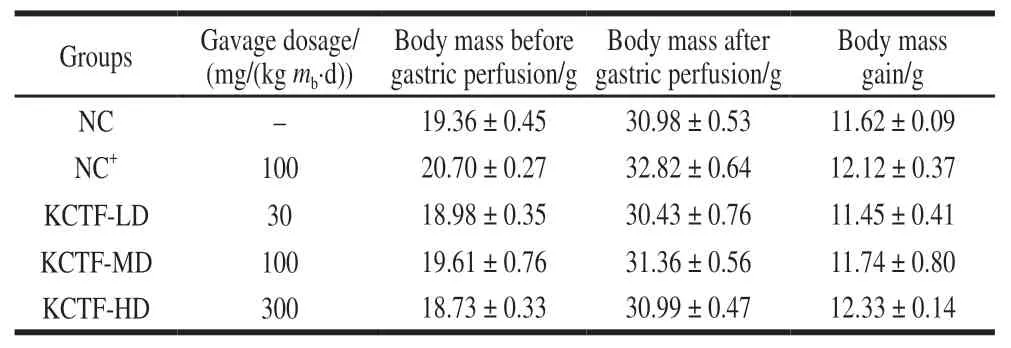
Table 1 Body mass change of mice before and after gastric perfusion with KCTF (n= 10)
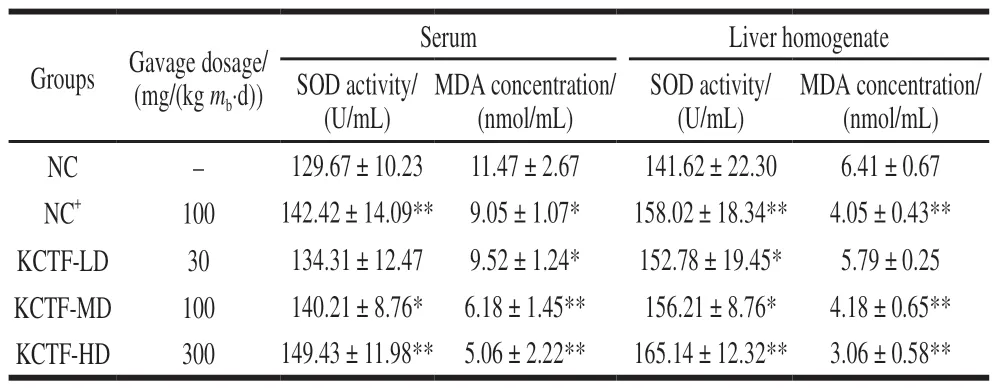
Table 2 Effect of KCTF on MDA concentration and SOD activity in serum and liver of mice (n= 10)
The influence of KCTF on mice body mass was shown in Table 1. There was no significant difference (P> 0.05)of mice final body mass among all the groups. The results implied that the concentration of KCTF had little effect on the mass of mice and its mass gain. SOD is the main antioxidant enzyme of removing free radicals that are generated during metabolic processes. MDA is metabolic parameters of lipid peroxidation, which leads to destruction of cell function[33]. As shown in Table 2, the serum SOD activity in KCTF-MD and KCTF-HD groups was significantly (P< 0.05) or extremely significantly (P< 0.01) higher than that of NC group, which had no significant difference with KCTF-LD group. However,all three different doses of KCTF could significantly improve liver homogenate SOD activities (P< 0.01 andP< 0.05), and the effect of the KCTF-HD group was equivalent to that of positive control group (NC+). Thus, our results showed that KCTF could improve SOD activity in mice and reflected an obvious dose-dependent effect. Various dose groups of KCTF all significantly decreased MDA concentration both in serum and in liver homogenate. Particularly, both KCTF-MD and KCTF-HD group could extremely significantly decrease the MDA concentration compared with NC group (P< 0.01). The observations indicated that KCTF could decrease the MDA concentration in mice in a dose-dependent manner.
In summary, the above results of experimentsin vitroandin vivoproved that KCTF had antioxidant activity.Oxidative stress plays an important role in various diseases.When our body suffered from oxidative damage, excessive free radicals were produced and beyond the capacity of antioxidant systems which may trigger numerous molecular and cellular damage. Therefore, KCTF could help to strengthen the free radical scavenging ability and could be used as a potential antioxidant agent.
2.4 Antibacterial activity of KCTF
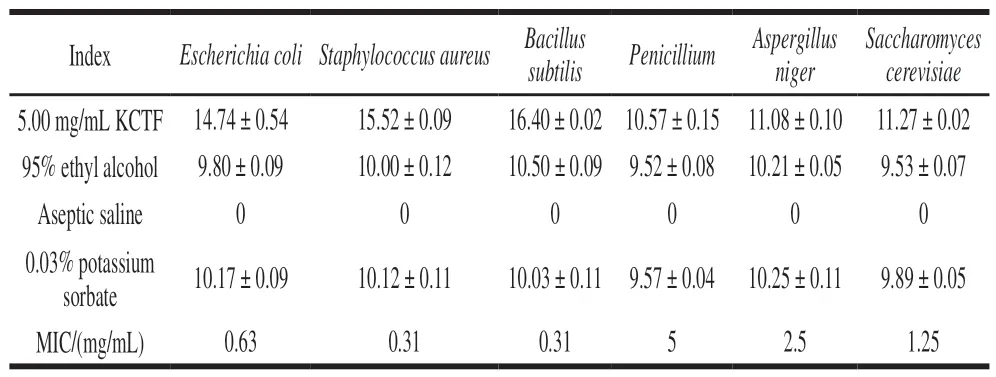
Table 3 Diameters of inhibition zones of test strains when exposed to KCTF and various antimicrobials and MIC of KCTFmm
Table 3 showed that the KCTF had good inhibitory effect on bacteria, especially on the Gram positive bacteria, which is the same as a previous study carried onArtemisia rupestrisL.flavonoids by Fang Meizhu et al[34].Results showed that the diameters of inhibition zone of KCTF(5.00 mg/mL) againstBacillus subtilisandStaphylococcus aureuswere (16.40 ± 0.02) mm and (15.52 ± 0.09) mm,respectively, while the MIC of themwas at the same of 0.31 mg/mL. Furthermore, yeasts and molds are less susceptible than bacteria to treatment by KCTF. The above results clearly showed that KCTF is a potential source of broad spectrum of antibacterial agent. Therefore, the mechanisms involved in antibacterial activity of KCTF are worthy of further investigation.
2.5 Effect of KCTF on CCl4-induced acute liver injury in mice
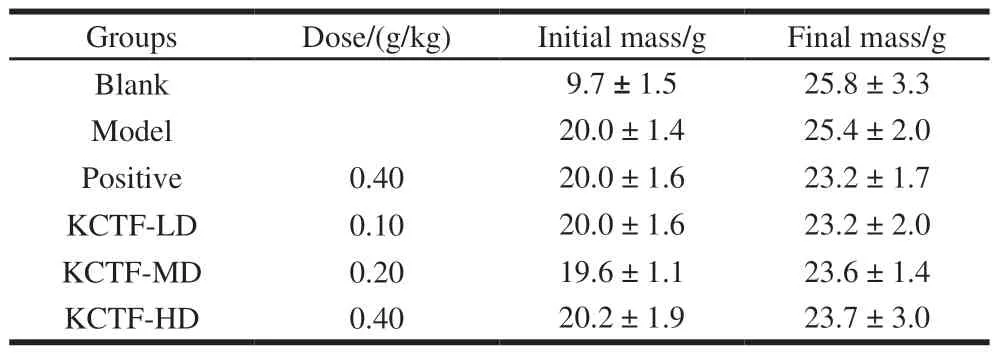
Table 4 Effect of KCTF on body mass of mice with acute liver damage (n= 10)
The effect of KCTF on mass of mice and the effect of KCTF on the acute liver injury induced by CCl4in mice was given in Table 4 and 5, respectively. It was indicated in Table 4 that the final body mass of mice in all the other groups had no significant differences compared with blank group (P> 0.05). The results indicated that KCTF had small impact on the mass of mice and their mass gain. As is shown in Table 5, ALT and AST activities in model group showed extremely significantly different (P< 0.01)when compared with blank group, indicating that the model was successfully made. However, the results showed that there was no obvious protective effect of different doses of KCTF on acute liver injury induced by CCl4. Furthermore,after comparison of the effect on liver protection of total flavonoids, proanthocyanidins and polysaccharides extracted from Kunlun chrysanthemum, we find that only high does group of Kunlun chrysanthemum proanthocyanidins revealed significant protective effect on acute liver injury induced by CCl4(unpublished).
2.6 Antiproliferative activity of KCTF
2.6.1 Effect of KCTF on proliferation of HeLa cells,Eca-109, and Vero cells
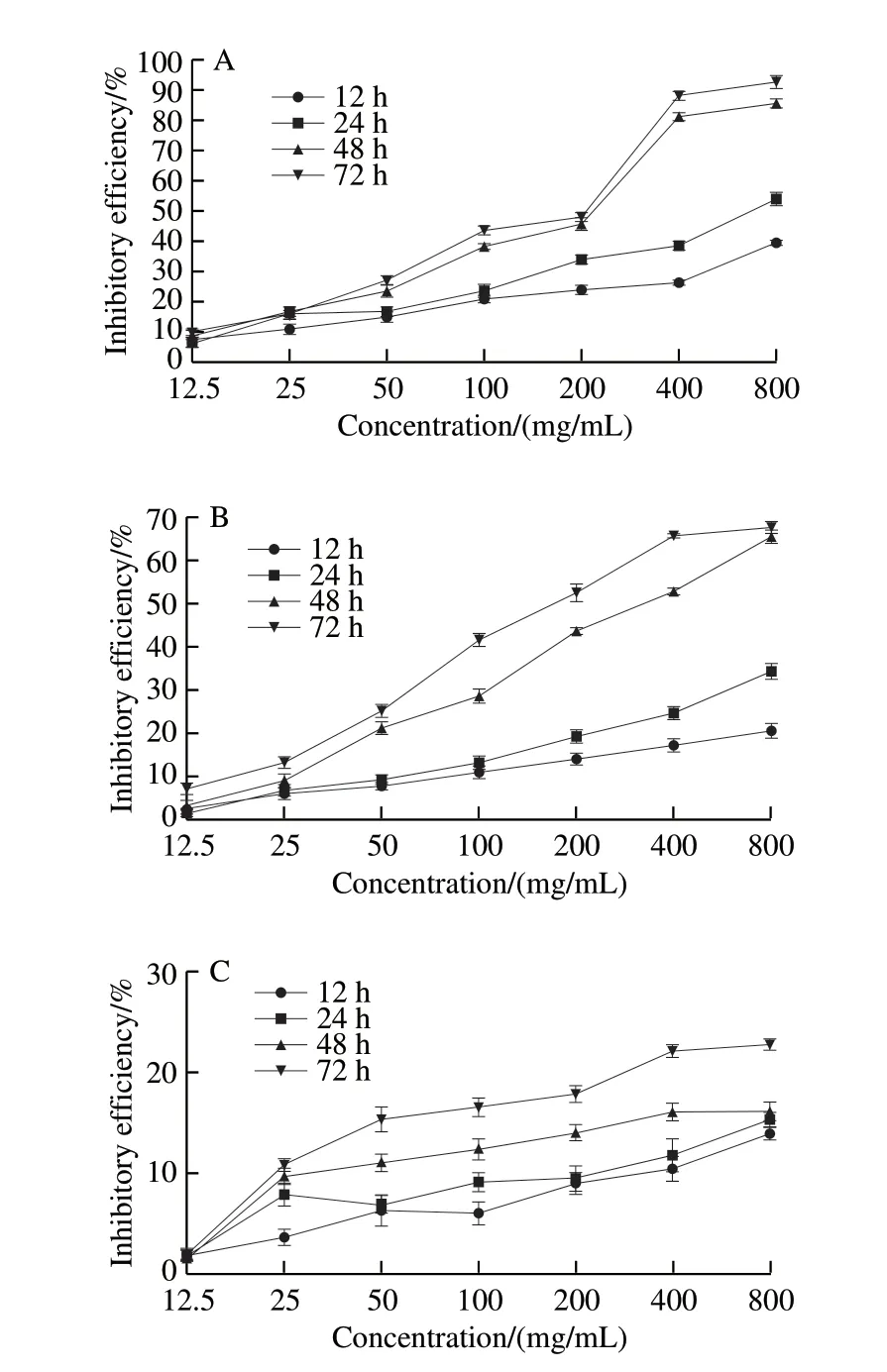
Fig. 3 Anti-proliferative effect of KCTF on HeLa (A), Eca-109 (B) and Vero cells (C)
HeLa cells, Eca-109 cells and Vero cells were incubated with different concentrations (12.5, 25, 50, 100, 200, 400 and 800 μg/mL) of KCTF for a certain time (12, 24, 48, and 72 h),then were measured by MTT assay (Fig. 3). KCTF could inhibit the proliferation of HeLa cells and Eca-109 cells with average IC50of (133.60 ± 0.19), (192.0 ± 0.08) μg/mL,respectively. The inhibitory effect of KCTF on HeLa cells and Eca-109 cells showed a significant increasing trend with the increase of the KCTF concentration and treatment time while the inhibitory effect on Vero cells was weak. The inhibitory efficiency on Vero cells was only 21.68% after incubation for 72 h at a concentration of 400 μg/mL. Compared with untreated control cells, KCTF had no significant immediate effect on cell viability when applied at low concentrations (12.5–100 μg/mL) to HeLa cells. KCTF could specifically inhibit the growth of HeLa cells from 100 μg/mL to 800 μg/mL (P<0.01) and the inhibitory efficiency of 800 μg/mL was up to 91.27% at 72 h. At the concentration of 800 μg/mL at 72 h, the inhibitory efficiency of Eca-109 cells was 66.38%. KCTF exhibited significant proliferation inhibition effect on HeLa cells and Eca-109 cells.
2.6.2 Influence of KCTF on HeLa cells, Eca-109 cells and Vero cells morphology
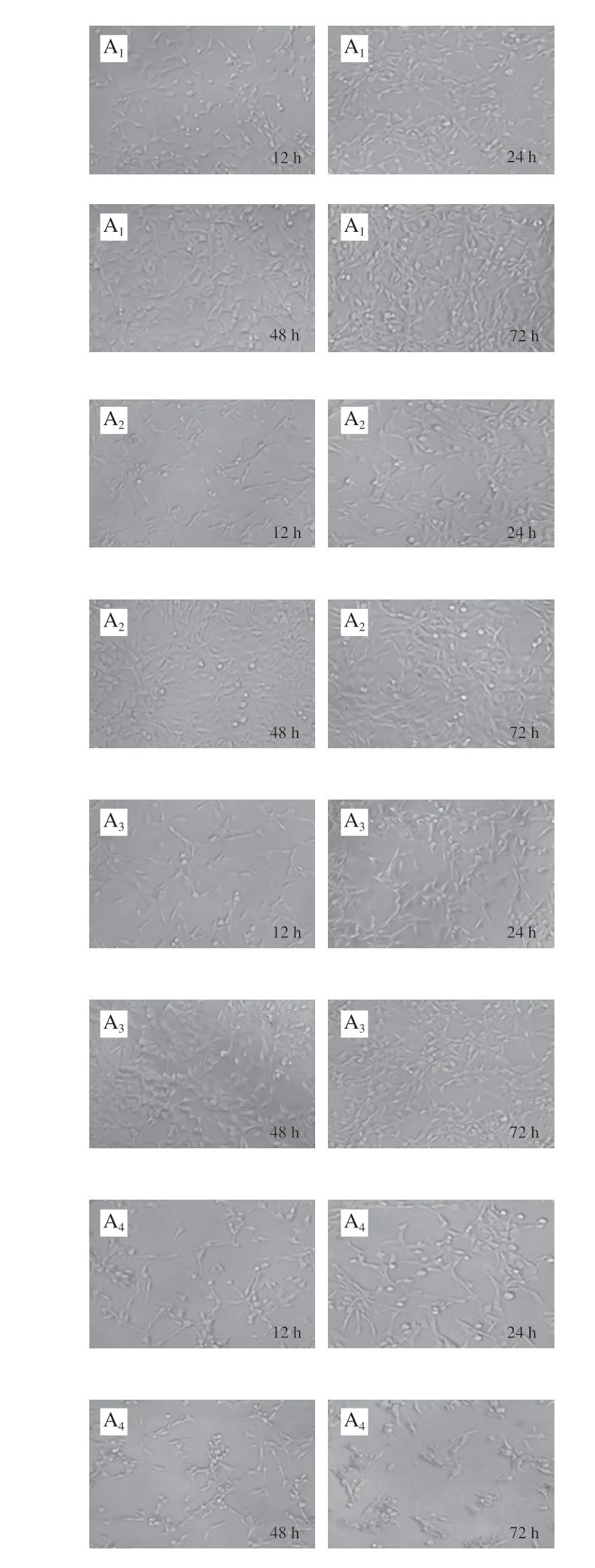
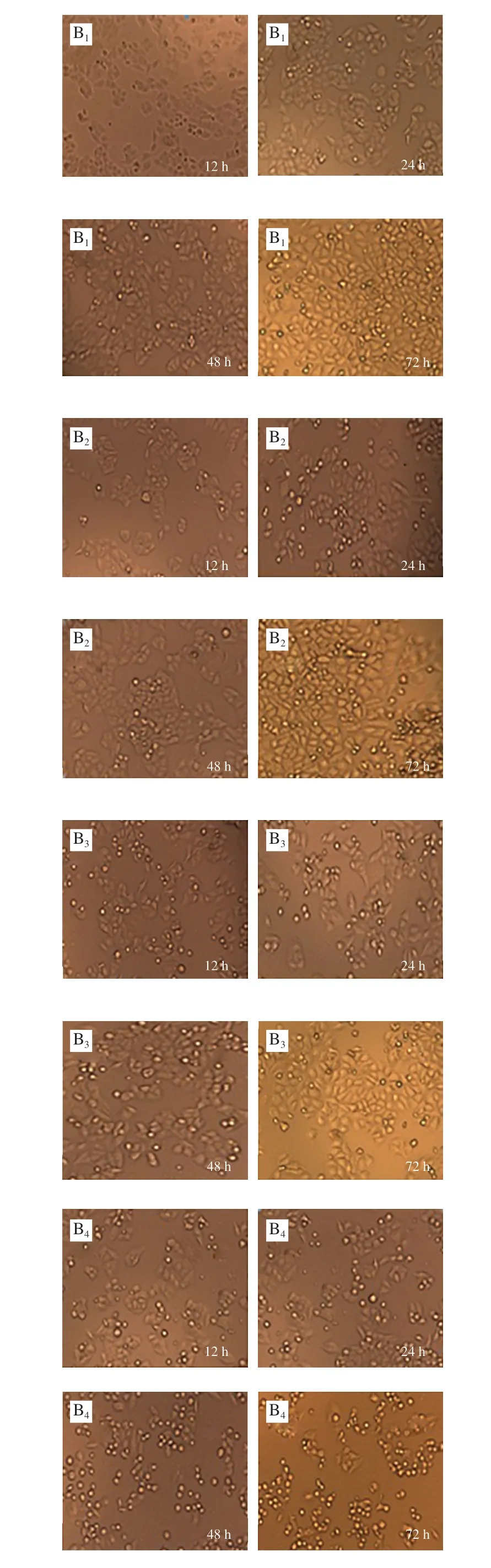
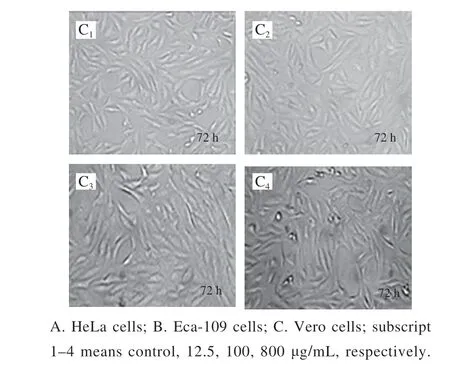
Fig. 4 Effect of KCTF on cell morphology (× 200)
The morphology changes of HeLa cells, Eca-109 cells and Vero cells treated with KCTF were observed by phase contrast inverted microscope (Fig. 4). The control groups(untreated with KCTF) of HeLa cells and Eca-109 cells grew against the wall of flask and formed a monolayer with regular polygons morphology. But after incubation with KCTF at different concentration, the number of the cells significantly decreased and morphology changed. Cells are relatively loose and intercellular connections are reduced, resulting in round shrinkage and shedding. However, KCTF has little effect on cell morphology and the number of Vero cells after treatment with KCTF for 72 h. The Vero cells yielded a slight change,and a small portion of which became round cells. Results illustrated that KCTF had antiproliferative effect against HeLa cells and Eca-109 cells with little cytotoxic effect on normal Vero cells.
Taken together, KCTF had antiproliferative activity,which could inhibit the growth of HeLa cells and Eca-109 cells but had little cytotoxic effect on Vero cells.
2.6.3 Effect of KCTF on HeLa cell apoptosis
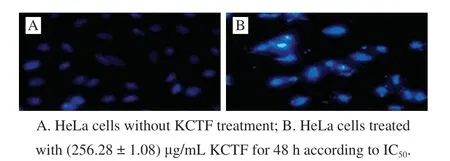
Fig. 5 Fluorescence microscopic image of HeLa cells (× 200)
Apoptosis, also known as programmed cell death,is a vital physiological process that removes cells at the appropriate time in order to better control the number of cells in development throughout the life of an organism[35].It is a strict regulatory pathway responsible for the order to remove the superfluous, elderly, and damaged cells[36]. The relationship between apoptosis and cancer has been stressed,and more evidence indicate that the relative processes of tumor transformation, progression and metastasis involve the changes in normal apoptosis pathways[37]. It was found that many tumor chemotherapeutic drugs play an anticancer effect on malignant cells by inducing apoptosis[38]. The apoptosis inducing activities of KCTF on HeLa cells were investigated through Hoechst 33258 staining assay. Apoptotic cells had bright blue nuclei because of karyopyknosis and chromatin condensation while the nuclei of dead cells could not be stained. HeLa cells treated with KCTF at(256.28 ± 1.08) μg/mL for 48 h were stained with Hoechst 33258. Compared with the normal blue of control group(0 g/mL), the nuclei of HeLa cells treated with KCTF appeared to be highly condensed and the granular fluorescence intensity was high, indicating that KCTF could induce apoptosis in HeLa cells (Fig. 5).
2.6.4 Effect of KCTF on LDH activity of HeLa cells
LDH can catalyze lactate acid to pyruvic acid which can be detected by kit. As seen from Fig. 6, under the dosage of 800 μg/mL KCTF, the activity of LDH in necrotic cells is more than those in apoptotic cells of HeLa cells at 0–12 h,and apoptotic cells apparently increased at 24 h. The results showed that KCTF could induce apoptosis on HeLa cells in a suitable time.

Fig. 6 Apoptosis and necrosis ratio assessed by LDH activity
3 Conclusions
In this study, KCTF extracted by UHP method was verified to have remarkable antioxidant capacity and antibacterial prosperity which could be used as natural agents for food preservation instead of chemical additives in food industry. In addition, KCTF may find a wide utilization in human health system due to its antioxidant effect which will inhibit oxidative damage involved in the pathogenesis of many diseases. In conclusion, the present study suggests that KCTF may be a potential natural health-promoting bioactive constituent in food and medical fields. Further investigations in terms of structure of KCTF and mechanisms of antiproliferative activity of tumor cells are in progress.

