G-quadruplex-assisted enzyme strand recycling for ampli fied label-free fluorescent detection of UO22+
Pijin Zhu,Yiying Zhng,Shuxi Xu*,Xinfeng Zhng,*
a College ofMaterials and Chemistry&Chemical Engineering,Chengdu University of Technology,Chengdu 610059,China
b College of Environment and Ecology,Chengdu University of Technology,Chengdu 610059,China
Keywords:Uranyl ion DNAzyme SYBR green I Fluorescence
ABSTRACT DNAzyme that can catalytically cleave of substrate DNAhasshow n to be attractive for ampli fied detection in biosensing events.During the catalytic process,the recycling of enzyme strand of DNAzyme is critically important.In this work,a G-quadruplex-assisted enzyme strand recycling strategy was developed for ampli fied label-free fluorescent detection of uranyl ion(UO22+).The DNAzyme was activated by the target UO22+and further cleaved the substrate strand that contained the G-quadruplex sequence.The follow ing formation of G-quadruplex helps the separation between the enzyme strand and the cleaved substrate strand,thus improving the recycle use of the enzyme strand.Such strategy allowed lablel-free detection of 0.2–200 ng/m L UO22+via SYBRgreen I(SG)-based fluorescence.The detection limit(3d)is as lowas 0.06 ng/m L(about 0.2 nmol/L),comparable to those obtained by ICP-MSand labeled DNAzyme.It was applied for detection of UO22+in spiked environmental w ater samples with recoveries in the range of 96%–103%.This biosensor,with the advantages of simplicity and high sensitivity,is an appealing tool for fast detection of UO22+in environmental water samples.
Uranium,one of the actinides,is a highly radioactive element and also well-know n for its chemical toxicity.It was reported that high doses exposure of uranium could give rise to DNAdamage[1],lung disease[2],cancer[3],etc.The World Health Organization(WHO)stipulated the maximum uranium concentration in drinking w ater to be 20 ng/m L[4].The assessment of uranium contamination requires a simple and sensitive monitoring method.Current techniques for detection of uranium(commonly UO22+in w ater)mainly focuses on sophisticated instrumental methods,including atomic emission spectrometry[5],atomic absorption spectrometry[6],and inductively coupled plasma mass spectrometry(ICP-MS)[7,8].These techniques are highly accurate and sensitive with industrial standards,but they are costly,available only in large centralized laboratories and require extensive sample pretreatment.Metal sensor is a good alternative tool for detection of UO22+ow ing to its potential applications in on-site,real-time,or in-situ measurement.
Recently,DNA has been demonstrated to be ideal for construction of metal ions sensor because it can selectively bind metal ions via both the phosphate backbone and nucleobases[9].Tw o main classes of functional DNA were developed for metal sensing:aptamers[10–12]and DNAzymes[13–19].Compared to aptamer-based sensing,DNAzyme that exhibited enzyme-like activity is very appealing for sensitive detection of metal ions.As a result,a number of metal ion specific DNAzymes have been isolated and used for sensitive detection of various metal ions including Pb2+[13],Cu2+[14],Ce3+[15],Hg2+[16],Ca2+/Mg2+[17]as well as UO22+[18,19].
The typical DNAzyme-based metal ion sensing system is fabricated via fluorescence resonance energy transfer(FRET)method which requires labeling a fluorophore on one end of the substrate strand and a fluorescence quencher on the corresponding end of the enzyme strand[20].The labeling of DNA leads to complicated synthetic routes,high cost and even interference with DNAzyme cleavage.Accordingly,much effort was also paid to label-free metal ion sensors.One common w ay is via directly using DNA intercalating dyes such as SYBR green I(SG)[21],ethidium bromide(EB)[22],and picogreen(PG)[23]for signaling.These dyes exhibited strong fluorescence in the presence of DNAzymes and low fluorescence after cleavage.Although these kind of metal sensors are quite simple and also can be readily used for detection of UO22+,they are alw ays not as sensitive as the FRET-based sensors,making it difficult to detect trace UO22+in w ater samples.
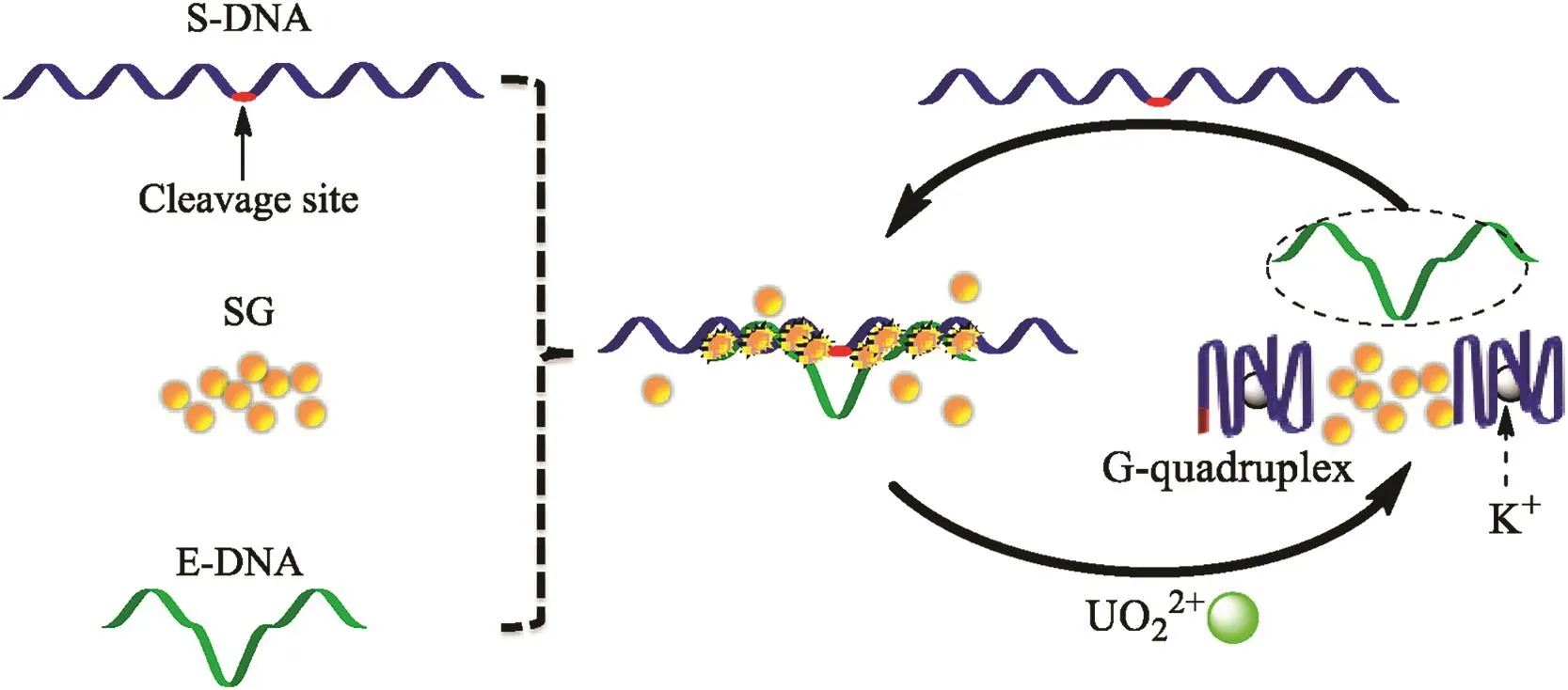
Fig.1.The scheme of G-quadruplex-assisted enzyme strand recycling strategy for label-free detection of UO22+.
An effective approach for enhancing the sensitivity of DNAzyme-based metal sensor is to improve catalytic turnovers[20].Herein,we developed a G-quadruplex-assisted enzyme strand recycling strategy for increasing the catalytic turnovers and further used it for detection of nanomolar UO22+.As show n in Fig.1,the substrate strand was designed with a G-rich sequence at the both ends;after UO22+-induced cleavage,the tw o cleaved fragments were folded into stable G-quadruplex structure,helping the separation of the complex between enzyme strand and fragment,and also improving the recycle use of enzyme strand.Hence,sensitive and label-free detection of UO22+can be readily achieved by this G-quadruplex-assisted enzyme strand recycling strategy.
Initially,the conventional substrate sequence that was unable to form G-quadruplex after cleavage was used for construction of label-free UO22+sensor.For the design with short hybridization length(only 4 bps),the complex of enzyme strand and substrate strand is quite unstable and easily dissociated automatically,thus resulting only a small SG fluorescent signal and also a tiny decrease after UO22+-induced cleavage(Fig.2A).The longer hybridization length(8 bps)leads to much stronger SG fluorescent signal,but the fluorescence decrease of SGdecreased only about 20%(Fig.2B).The low fluorescent drop of SG here is probably attributed to incomplete separation between enzyme strand and the cleaved fragments.When the dsDNA interacting dye,namely SG,was incubated with the enzyme strand and split conventional substrate fragments,we found a remarkably increased fluorescent signal,indicating the easy formation of dsDNA between the enzyme strand and split conventional substrate fragment(Fig.S1 in Supporting information).This result also means the difficult separation between enzyme strand and the cleaved fragments.Therefore,G-quadruplex was subsequently used for assisting the separation of enzyme strand with the cleaved fragments and further improving its recycle use.
It has been demonstrated that the G-quadruplex structure can even lead to the separation of the dsDNA formed by G-quadruplex sequence and its complementary strand[24].In the G-quadruplexassisted enzyme strand recycling strategy,the substrate strand contained G-quadruplex sequence at the both ends;after cleavage,the tw o fragments were folded into G-quadruplex structure in the presence of K+,leading to the complete release of enzyme strand.The result in Fig.S1 also provesthat the formation of G-quadruplex w ill contribute to the separation of enzyme strand and cleaved substrate fragments.As expected,the strategy lead to an obvious SG fluorescence drop(>58%)in the presence of 100 ng/m L UO22+(Fig.2C).Therefore,sensitive and label-free quanti fi cation of UO22+can be achieved.
G-quadruplex was reported to be more stable in the presence of K+[25].The experiments showed small fluorescent decrease in the absence of K+(Fig.3A);upon addition of KCl solution,the fluorescent intensity has a noticeable decrease.The result implied the possible formation of G-quadruplex after cleavage.Subsequently,hemin that was know n for its catalytic activity after embedding into the G-quadruplex was used for con fi rmation of G-quadruplex formation[26].Fig.3B displayed that the absorbance of 3,3',5,5'-tetramethylbenzidine(TMB)signal enhanced significantly upon addition of UO22+,implying that formation of G-quadruplex which catalyze the oxidation of TMB by H2O2.To further identify the formation of G-quadruplex,we used thio fl avin T(Th T)as a probe,since ThT has been con fi rmed as highly responsive for G-quadruplexes as compared with duplex DNA[27,28].As show n in Fig.3C,after cleavage by UO22+,the fluorescent signal of Th Tgreatly enhanced,revealing the formation of G-quadruplex.It was notew orthy that one G-quadruplex interacts with only one Th T molecule,w hereas several SG molecules could be embedded into one dsDNA strand.So,the dissociation of the complex between enzyme strand and cleaved substrate strand can lead to release several SGmolecules,resulting in higher sensitivity.

Fig.2.The UO22+-triggered fluorescence decrease using different substrate strand:(A)hybridizing 4 bps with enzyme strand,(B)hybridizing 8 bps with enzyme strand,and(C)containing G-quadruplex sequence and hybridizing 8 bps with E-DNA.Experimental conditions:substrate strand and enzyme strand concentration,100 nmol/L;UO22+concentration,100 ng/m L;SG concentration,2?;DNAzyme hybridization time,30 min;UO22+cleavage time,1 h.

Fig.3.Verifying the formation of G-quadruplex via the effect of K+(A),hemin(B)and ThT(C).The experimental conditions are the same as Fig.2.

Fig.4.The effect of hybridization length between substrate strand and enzyme strand concentration(A),K+concentration(B),the molar ratio between S-DNAand E-DNA(C)and solution p H(D)on the detection of UO22+.
Sensing conditions have profound influence on the performance of the sensor.Thus several important conditions were inspected.The effect of hybridization length between enzyme strand and substrate strand was examined fi rstly by fi xing the enzyme strand sequence.When the hybridization length is short(4 bps),the dsDNA between enzyme strand and substrate strand is not stable and easily dissociated,so the signal decline of SGis tiny in the presence of UO22+(Fig.4A and Fig.S2 in Supporting information);however,the long hybridization length(12 bps)leads to difficult separation between the cleaved substrate strand and enzyme strand.The optimum hybridization length between enzyme strand and substrate strand is 8 bps.
The effect of K+concentration was showed in Fig.4Band Fig.S3 in Supporting information.With the increasing amount of K+solution,the(F0?F)/F0values went up.When 50 mmol/L of K+solution was added,the(F0?F)/F0reached its plateau.Afterwards,little change was observed upon addition of 100–200 mmol/LK+.Therefore,50 mmol/LK+was chosen to ensure a high(F0?F)/F0.
The cleavage reaction was also performed with different molar ratio between substrate strand and enzyme strand.As show n in Fig.4Cand Fig.S4 in Supporting information,the(F0?F)/F0was greatly dependent on the ration of substrate strand and enzyme strand,and a maximum(F0?F)/F0value was attained at molar ratio of 1:1.A higher concentration of substrate strand induced more cycles of DNAzyme cleavage[29],providing higher cleavage ef fi ciency.Inadequate substrate strand w ill lead to the incomplete hybridization of enzyme strand as well as decreased the(F0?F)/F0.Thereby,a molar ratio of 1:1 for substrate strand and enzyme strand was considered to be optimum.
The effect of p H was investigated in the range of p H 3.0–5.5.Fig.4D showed that the blank fluorescence signal increased as p H increased,probably because the dsDNA is more stable at low acidity.However,the complex between cleaved fragment and enzyme strand also becomes more difficult to be dissociated under low acidic conditions.Therefore,a maximum(F0?F)/F0reached at p H 3.5 as the p H increased from 3.0 to 5.5(show n in Fig.S5 in Supporting information).Therefore,the p H 3.5 was chosen as the optimum condition for the detection of UO22+in this work.
It was reported that ethanol could influence the DNA hybridization[30].Therefore,the effect of anhydrous ethanol was investigated from 0 to 20%.As show n in Fig.S6(Supporting information),the(F0?F)/F0value increased with the concentration of anhydrous ethanol ranging from 0 to 10%,and then decreased at the concentration higher than 10%.The complex between enzyme strand and substrate strand fragment is stable at low concentrations of ethanol and become labile at high concentration of ethanol,benefiting to the release of SG molecule.However,too high concentration of ethanol leads to low background fluorescence(Fig.S7 in Supporting information).Hence,10%of ethanol was chosen for further work.

Fig.5.(A)The fluorescence spectra of SGin the presence of UO22+from 0.2 ng/m Lto 200 ng/m L(inset:the calibration curve obtained by the proposed sensing platform),(B)The fluorescence decline of the sensing system in the presence of 100 ng/m L UO22+and 5 m g/m Lother metal ions.Experimental conditions:substrate strand and enzyme strand concentration,100 nmol/L;UO22+concentration,100 ng/m L;SG concentration,2?;hybridization time of the tw o strands,30 min;UO22+cleavage time,1 h.

Table 1 Comparison of different methods for UO22+determination.
To test the performance of the sensor,the fluorescence decrease of SG in the presence of varied UO22+concentrations was monitored(Fig.5A).As show n in Fig.5A,upon the addition of UO22+,the fluorescence intensity gradually decreased.The decreased fluorescence showed a good linear correlation with the concentration ranging from 0.2 ng/m L to 200 ng/m L.The detection of limit(LOD,3d)for UO22+was calculated to be 0.06 ng/m L(about 0.2 nmol/L),which is over 25-fold lower thanthat of the reported DNAzyme catalytic system without G-quadruplex structure and even comparable to those obtained by labeled DNAzymes and ICP-MS(Table 1).The sensitivity of our method meets the requirement of fast detection of UO22+in drinking w aters,w hose maximum allowable level is 20 ng/m L de fined by the WHO.Additionally,the sensing system is quite simple and easy-to-operate because it avoids the labeling process,the signal am pli fi cation procedure as well as the use of nanomaterials.
In order to investigate the specificity of the system,twelve potentially co-existing metal ions including Na+,Cd2+,Co2+,Mg2+,Mn2+,Ni2+,Zn2+,Ca2+,Hg2+,Fe3+,Pb2+and Al3+were also tested with our sensing system.As show n in Fig.5B,obvious fluorescence drop was found for 100 ng/m LUO22+;other metallic ions which are 50-fold higher in concentration induced negligible fluorescence changes.This indicated that only UO22+can trigger the cleavage of the substrate strand and the release of SG.Therefore,the proposed sensing system had a high selectivity to UO22+over other metal ions.
To demonstrate the potential application in environmental analysis,four w ater samples were analyzed,i.e.,one lake w ater sample,one river w ater sample and tw o mining w ater samples.UO22+was not found in these w ater samples using our sensing system as well as ICP-MS.Therefore,20 ng/m L of UO22+were spiked to the w ater samples,and obvious fluorescent drops were detected.The recoveries of the four w ater samples ranged from 96 to 103%(Table 2),suggesting that the system can be applied for fast sensing of UO22+in environmental w ater samples.
In conclusion,IFF]a G-quadruplex-assisted enzyme strand recycling strategy was successfully developed and further applied forlabel-free detection of UO22+via SG fluorescence signalling.The cleavage of the substrate by the DNAzyme in the presence of UO22+caused its conformation change from dsDNA to the energetically favored G-quadruplex,leading to release SG and decreased fluorescent signal.Such a simple strategy provides a rapid sensing platform for detection of UO22+with a LOD as lowas 0.06 ng/m L.Therefore,this sensing system exhibited the advantages of simplicity,high sensitivity as well as low cost.Using different metal ion specific DNAzymes,this strategy w ill become a promising alternative tool for the rapid and on-site detection for metal ions in environmental w aters.

Table 2 Analytical results of UO22+in spiked w ater samples.
Acknow ledgm ents
The authors gratefully acknow ledge the fi nancial support from the National Natural Science Foundation of China(Nos.21475013 and 21305009),and China Postdoctoral Science Foundation(Nos.2015M 572453 and 2016T90839).
Appendix A.Supplem entary data
Supplementary data associated with thisarticle can be found,in the online version,at https://doi.org/10.1016/j.cclet.2018.02.003.
 Chinese Chemical Letters2019年1期
Chinese Chemical Letters2019年1期
- Chinese Chemical Letters的其它文章
- Information for authors
- A one-pot protocol for copper-mediated azide–alkyne cycloaddition using alkenyl tri fl ate precursors
- One-pot synthesis of tetrahydroindoles via a copper catalyzed N-alkynation/[4+2]cycloaddition cascade
- Gram-scale preparation of dialkylideneacetones through Ca(OH)2-catalyzed Claisen-Schmidt condensation in dilute aqueous EtOH
- Tetra-phthalimide end-fused bi fl uorenylidene:Synthesis and characterization
- Water bridges are essential to neonicotinoids:Insights from synthesis,bioassay and molecular modelling studies
