The Effect of Hydrolysis with Neutrase on Molecular Weight, Functional Properties, and Antioxidant Activities of Alaska Pollock Protein Isolate
LIU Chuyi, MA Xiaoming, CHE Shuai, WANG Changwei, and LI Bafang
?
The Effect of Hydrolysis with Neutrase on Molecular Weight, Functional Properties, and Antioxidant Activities of Alaska Pollock Protein Isolate
LIU Chuyi1),#, MA Xiaoming1),#, CHE Shuai2), WANG Changwei2), and LI Bafang1),*
1),,266003,2),266073,
In this study, the Alaska pollock protein isolate (APPI) was hydrolyzed by Neutrase for 20, 40, 80, 120, 160, 200, and 240min. Hydrolysates with different molecular weights were produced and they were named as H1–H7. Furthermore, the effects of hydrolysis on the average molecular weights, functional properties (solubility, oil-holding capacities, foaming activities, and emulsifying properties), and antioxidant activities (1, 1-diphenyl-2-picrylhydrazyl, superoxide, and hydroxyl free radical-scav- enging activities) were determined. It was found that when the degree of hydrolysis (DH) increased, the average molecular weights of the hydrolysates decreased significantly. The functional properties of APPI were also significantly improved. The hydrolysates of APPI exhibited better solubility, emulsifying activities, and foaming activities. Hydrolysates with low molecular weights (<1kDa) had better solubility, oil-holding capacities, and emulsifying activities, while hydrolysates with higher molecular weights (>1kDa) had better foaming activities. In addition, the hydrolysates exhibited excellent antioxidant properties, while the inhibition values of 1, 1-diphenyl-2-picryl hydroxyl (DPPH), superoxide, and hydroxyl free radical-scavenging activities, were 85.22%, 53.56%, and 75.00% respectively, when the concentration of the hydrolysates was 5.0mgmL?1. The lower the average molecular weight was, the higher was the antioxidant activity. These results indicated that hydrolysis with Neutrase is an effective method for improving the functional and antioxidant properties of APPI. The hydrolysates of APPI displayed great potentials to be used as natural antioxidants in protein-rich aqueous foods such as nutrient supplements and sports beverages.
Alaska Pollock protein isolate (APPI); solubility; enzymatic hydrolysis; functional properties
1 Introduction
Alaska pollock () belongs to the Gadidae family, and is an important commercial fish on a global scale (Kang., 2008). The Alaska pollock protein isolate (APPI) is prepared by the isoelectric solubilization/precipitation (ISP) process. Itshowsnot only high protein recovery yields (Chen and Jaczynski, 2007; Chanarat and Benjakul, 2013), but also well-balanced amino acid compositions (Sun., 2016) and high digestibility (Yang., 2011).Thus it can be used as a high-quality protein source in food processing. However, ISP process alone cannot avoid the presence of large amounts of water-insoluble proteinsand proteins with high molecular weight in APPI, which limits the functional properties and bioactivities of the Alaska pollock protein. It is necessary to explore other methods for improving the functional properties and the bioactivities of APPI.
Enzymatic hydrolysis is one of the most commonly used approaches for improving protein isolates. During the process of enzymatic hydrolysis, the fish protein is broken down into a series of smaller peptides, which can modify and even improve the functional characteristics and the bioactivities for diverse applications (Pujara., 2017). Compared with other modification methods, enzymatic hydrolysis can be conducted under a mild condition while the efficiency and safety remain satisfactory (Alu’datt., 2017). It has been reported that the functional properties of tilapia, Pacific whiting, and blue whiting proteinscould be effectively improved to varying degrees through enzymatic hydrolysis, while the functional properties include solubility, emulsifying pro- perties, foaming abilities, and surface-active properties (Geirsdottir., 2011; Fan., 2012; Garcia-Moreno., 2016; Pujara., 2017).
In addition to improving the functional properties, fish hydrolysates might alsobe used as natural antioxidants in different oxidative systems, such as 1, 1-diphenyl-2-pi- cryl hydroxyl (DPPH) free radical-scavenging, hydroxyl radical-scavenging, and superoxide radical-scavenging (Jemil., 2014; Garcia-Moreno., 2016; Nongo- nierma., 2017; Shavandi., 2017). Several efforts have been made to explore the approaches and the effects of enzymatic hydrolysis on improving the functional properties and the bioactivities of the Alaska pollock protein. The peptides hydrolyzed from the Alaska pollock skin using Protamex were found to possess antioxidant activities (Kim., 2001;Jia., 2010). The protein hydrolysates hydrolyzed from the Alaska pollock frame by pepsin and trypsin were shown to possess ACE in- hibitory activities (Je., 2004) and immunomodula- tory activities (Hou., 2012a, 2012b). Previous stud- ies have suggested that the functional properties and the bioactivities of the Alaska pollock protein hydrolysates depend on not only the substrates and reaction condi- tions but also the characteristics of the adopted enzymes. Neutrase has been widely utilized to hydrolyze proteins for improving their functional properties and bioactivi- ties.For the proteins isolated from grass carp (Xiao and Niu, 2016), salmon (Ahn., 2014), and tilapia (Fan., 2012), the hydrolysates prepared by Neutraseexhibit obvious antioxidant properties. Thus Neutrase could possibly be used for modifying the properties of the Alaska pollock protein. Although the functional proper- ties and the antioxidant activities of various fish protein hydrolysates generated by Neutrase hydrolysis have been investigated, the effect of Neutrase on APPI over different periods of hydrolysis time has not yet been evaluated.
The aims of this study were to prepare peptides from APPI by Neutrase hydrolysis with different reaction time, and to evaluate the functional properties and the antioxi- dant activities of the resulting hydrolysates with different molecular weights.
2 Materials and Methods
2.1 Materials
The Alaska pollock was provided by Yantai New Era Health Industry Daily Chemical Co., Ltd. (China). The amino acid standards lysozyme, aprotinin, vitamin B12, l-glutathione, and 1, 1-diphenyl-2-picryl-hydrazyl (DPPH)were obtained from Sigma-Aldrich (St. Louis, MO, USA). Neutrase was a food-grade protease obtained from Pang- bo Biological Engineering Co., Ltd. (Guangxi Nanning, China). The hydroxyl free radical assay kit and the assay kit for evaluating the inhibition and production of superoxide anionwere obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other che- micals were analytical-grade reagents obtained from Si- nopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2 Preparation and Extraction of APPI
Frozen Alaska pollock mince was kept at ?20℃ before protein isolation. The protein was isolated using the pH-shift method as previously described (Speroni., 2010). In brief, the frozen Alaska pollock mince was thawed at 4℃ for 24h, mixed with nine parts of cold (4℃) distilled water, and then homogenized by vibration at 12000. The pH of the protein homogenate was ad- justed to 11.5 using 2molL?1NaOH. The solubilization reaction was maintained at pH 11.5 for 10min, followed by centrifugation at 10000for 10min at 4℃. The su- pernatant was collected, and 2molL?1HCl was added to adjust the pH to 5.5 for isoelectric protein precipitation. The solution was centrifuged as described above to col- lect the precipitated protein, which was further dispersed in distilled water with pH 7.0. Finally, the protein disper- sion was freeze-dried. The composition of the freeze- dried APPI was as follows: dry matter 96.40%, protein 89.34%, salt 6.63%, and fat <0.60%. The same batch of APPI was used for the entire study. APPI was used as the control. With the pH-shift method, the protein content increased from 18.81% in the mince to 89.34% in the APPI.
2.3 Preparation of Protein Hydrolysates
Neutrase has been widely used to produce hydrolys- ates exhibiting functional and bioactive properties (Kam- moun., 2003; Ou., 2010). In our previous study, we had tested the degree of hydrolysis (DH) and the solubility of six food-grade proteases under the optimal hydrolysis conditions.It was found that Neutrase was the most efficient enzyme to improve the solubility and other related functional properties of the proteases (data not shown). Protein hydrolysates were prepared from the extracted proteins using Neutrase (protease activity was 2.01×105U) under the optimal reaction conditions ac- cording to the procedure described by Shavandi. (2017). APPI, the substrate for protease digestion, was homogenized and diluted to 20mgmL?1using cold dis- tilled water. The pH was adjusted to 8.0 by the addition of a known amount of 4molL?1NaOH or 4molL?1HCl. The enzymatic hydrolyses were performed under the optimized condition (50℃) for 20, 40, 80, 120, 160, 200, and 240min, and the products were numbered as H1–H7, respectively. The reaction mixtures were then centrifuged at 4000for 20min at 4℃.Then the hydrolysates were freeze-dried and collected. APPI was used as a control for examining the effect of enzymatic hydrolysis.
2.4 Determination of DH
The DHs were determined by the ninhydrin colori- metric method as described previously (Hou., 2012b).l-glycine (2–20mgmL?1) was used to prepare the stan- dards. Each determination was performed in triplicate. The DH value was defined as follows:
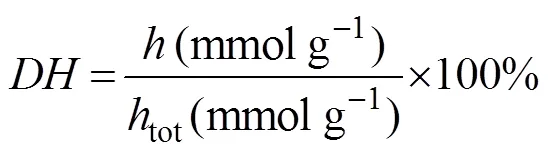
whereandtotare the number of broken peptide bonds and the total number of bonds per unit weight, respec- tively. In this study,totwas equal to 9.3mmolg?1proteins.
2.5 Molecular Mass Distribution of the Hydrolysates
The molecular mass distribution of the hydrolysates were investigated by a high-performance liquid chroma- tography (HPLC) system (Agilent 1100, USA) equipped with a TSK gel 3000 PWXL column (Tosoh, Tokyo, Ja- pan) as described by Hou. (2011). Lysozyme (14300Da), aprotinin (6511Da), vitamin B12 (1355Da), l-glu- tathione (307Da), and uracil (112Da) were used as themolecular mass standards. The molecular weight dis- tributions were determined by the Agilent GPC Data Analysis software. The relative molecular mass of the treated and untreated APPI samples was calculated ac- cording to the relative molecular mass calibration curve equations.
2.6 SDS-PAGE Analysis
Sodium dodecyl sulfate polyacrylamide gel electro- phoresis (SDS-PAGE) was performed to analyze the mo- lecular weights of the hydrolysates and the APPI using an AE6400 Dual Mini Slab electrophoresis apparatus (Atto Corporation, Tokyo, Japan). Aliquots of protein samples were boiled for 5min before being resolved on acryla- mide gels consisting of 8%–15% separating gel and 4% stacking gel. The gels were electrophoresed at a constant current of 30mA for 2–3h and analyzed by staining with Coomassie Brilliant Blue R-250. Protein molecular weight markers (TianGen Biotech Co. Ltd., Beijing, China) with molecular weights of 15, 25, 35, 45, 65, 75, 100, and 135kDa were used as references.
2.7 Functional Properties
2.7.1 Solubility
The solubility in water was determined in triplicate according to dos Santos. (2011) with some modi- fications. The freeze-dried samples (100mg) were dis- persed in 10mL of distilled water at 4000for 30s. All the samples were kept at 25℃ for 60min and then cen- trifuged for 15min at 10000. The total protein and the soluble protein in the water phase were measured using the Kjeldahl method (N×6.25) (AOAC International. 1995). The protein solubility was calculated as the per- centage ratio of the supernatant protein content to the total protein content.
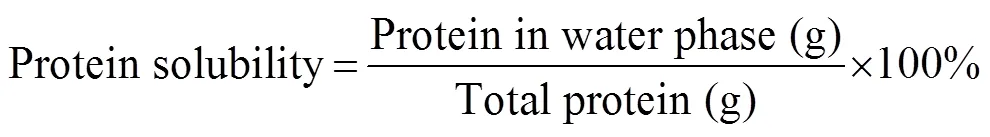
2.7.2 Oil-holding capacity (OHC) analysis
The OHC was determined using the method described by Garcia-Moreno. (2017) with some modifications. The H1–H7 samples (2g) were mixed into 100mL of oil for 30min and then centrifuged at 10000for 10min, and the supernatant was removed. The total weight of the centrifuge tube and the samples were determined before centrifugation and after the removal of the supernatant. The OHC was expressed as the ratio of sample weight after centrifugation to the initial sample weight. Each measurement was performed in triplicate.
2.7.3 Emulsifying properties
The emulsifying activities (EA) and the emulsion sta- bility indexes (ESI) were measured based on the method described by Pacheco-Aguilar. (2008). A freeze- dried APPI sample was dissolved in 0.02molL?1phos- phate buffer (pH 7.0), and the final concentration was adjusted to 10mgmL?1. A mixture consisting of 25mL of vegetable oil and 75mL of protein solution was homogenized for 2min at 10000. Then, 50μL of the emulsion was immediately diluted with 10mL of 0.1% sodium dodecyl sulfate (SDS) solution. The absorbance of the diluted sample was measured at 500nm at 0 (0) and 10min (10). The EA and the ESI were calculated as fol- lows:
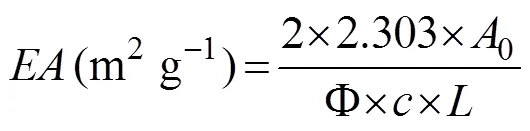
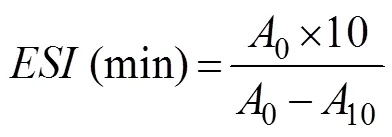
where0is the absorbance at 500nm measured at 0min;10is the absorbance at 500nm measured at 10min; Φ is the volume fraction of the oil phase, 0.25;is the con- centration of the hydrolysate in the aqueous solution be- fore the emulsion was prepared (mgmL?1); andis the path length of the cuvette.
2.7.4 Foaming properties
The foaming capacities (FC) were determined using the method proposed by Suppavorasatit. (2011) with minor modifications. Protein dispersion (10mL, 0.3% w/v) was mixed with 0.1molL?1phosphate buffer at pH 7.0 and then homogenized at 10000for 1min. The FC were recorded as the percentage increases in volume upon mixing. Foam stability (FS) was calculated as the percentages of remaining foam after 30min of standing at room temperature.
2.8 Antioxidant Activity
2.8.1 Hydroxyl radical-scavenging activity
The hydroxyl radical-scavenging activities of APPI and H1–H7 were measured at 5.0mgmL?1using the hy- droxyl free radical assay kit (Nanjing Jiancheng Bio- engineering Institute, Nanjing, China) according to the method described by Fan. (2012) and Sampath Ku- mar. (2011).
2.8.2 Superoxide anion-scavenging activity
The superoxide anion-scavenging activities of APPI and H1–H7 were measured at 5.0mgmL?1using the in- hibition and production of superoxide anion assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as described by Chi. (2015).
2.8.3 DPPH radical-scavenging activity
The DPPH radical-scavenging activity was measured according to Zheng. (2015). Briefly, a 50-μL sample of APPI or ascorbic acid as a positive control was mixed with 150μL of 150mmolL?1DPPH or 150μL of ethanol as the blank, and the mixture was kept in the dark for 30min. The absorbance was measured at 517nm using a microplate reader (Spectra Max, CA, USA).
2.9 Statistical Analysis
Statistical analysis was performed using the SPSS 11.5 software. Data are presented as the mean±standard de- viation (SD). Significant differences between the mean values were identified using Duncan’s multiple range test (<0.05).
3 Results and Discussion
3.1 Degree of Hydrolysis (DH)
In the present study, we investigated the differences in the functional properties and the biological activities of the hydrolysates obtained by enzymatic hydrolysis using Neutrase for different lengths of time under optimized conditions. First, the DH was estimated to determine the extent of protein degradation by the proteolytic enzymes. As shown in Fig.1, the DH of the APPI increased with increasing reaction time. However, the specific rate at which DH increased did gradually decrease. In addition, the following DH values were found: 10% for H1, 13% for H2, 18% for H3, 23% for H4, and 25% for H5–H7. When the reaction time was 160 min, the DH reached equilibrium. This trend is consistent with the results ob-tained by Choi. (2009) in their preparation of pro- tein hydrolysates from the protein isolates of frozen small croaker muscle using Neutrase. The increase in the DH values indicated that the number of free amino groups in the protein gradually increased, the peptide bonds of the protein were interrupted by enzymatic hydrolysis, and the molecular weight of the protein changed. Thus, the aver- age molecular weight and the molecular weight distribu- tion of the hydrolysates H1–H7 after different reaction times must be investigated.
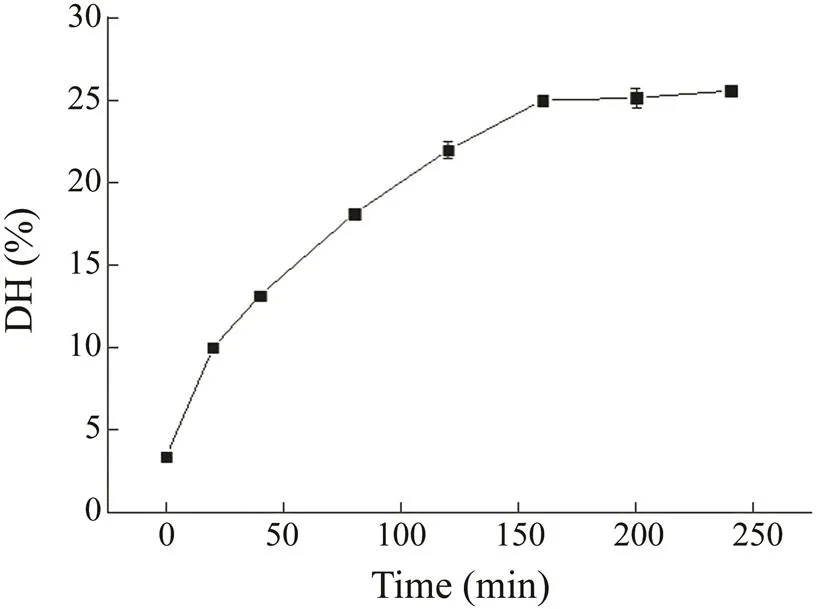
Fig.1 Degree of hydrolysis of hydrolysates at different reaction time.
3.2 Molecular Weight Characterization
The molecular weight distribution and the average molecular weight of APPI and the seven hydrolysates were analyzed by SDS-PAGE and HPLC, respectively. SDS-PAGE was performed to investigate the effect of enzymatic hydrolysis on the protein breakdown of APPI, and the results are displayed in Fig.2B. The bands of APPI were distributed in the range of 15–135kDa on the electrophoresis profile. When the enzymatic reaction start- ed, the H1 macromolecules were rapidly decomposed, leaving only a small amount of protein weighing >25kDa. The enzymatic hydrolysates H2–H7 showed complete de- gradation of the polypeptides weighing >15kDa, and the average molecular weights of H2–H7 were <10kDa. The molecular size profile of APPH based on HPLC is pre- sented in Fig.2A. The molecular weights of the proteins can be reduced by proteolytic hydrolysis, and the proteins were broken down into peptides of varying sizes. The relative average molecular weight of APPH was analyzed using the GPC software. The average molecular weights of the hydrolysates are shown in Fig.2C; the average molecular weight of H1 was >3kDa; the average mo- lecular weights of H2 and H3 were between 1 and 3kDa, respectively; and the average molecular weights of H4– H7 were between 1kDa and 500Da.
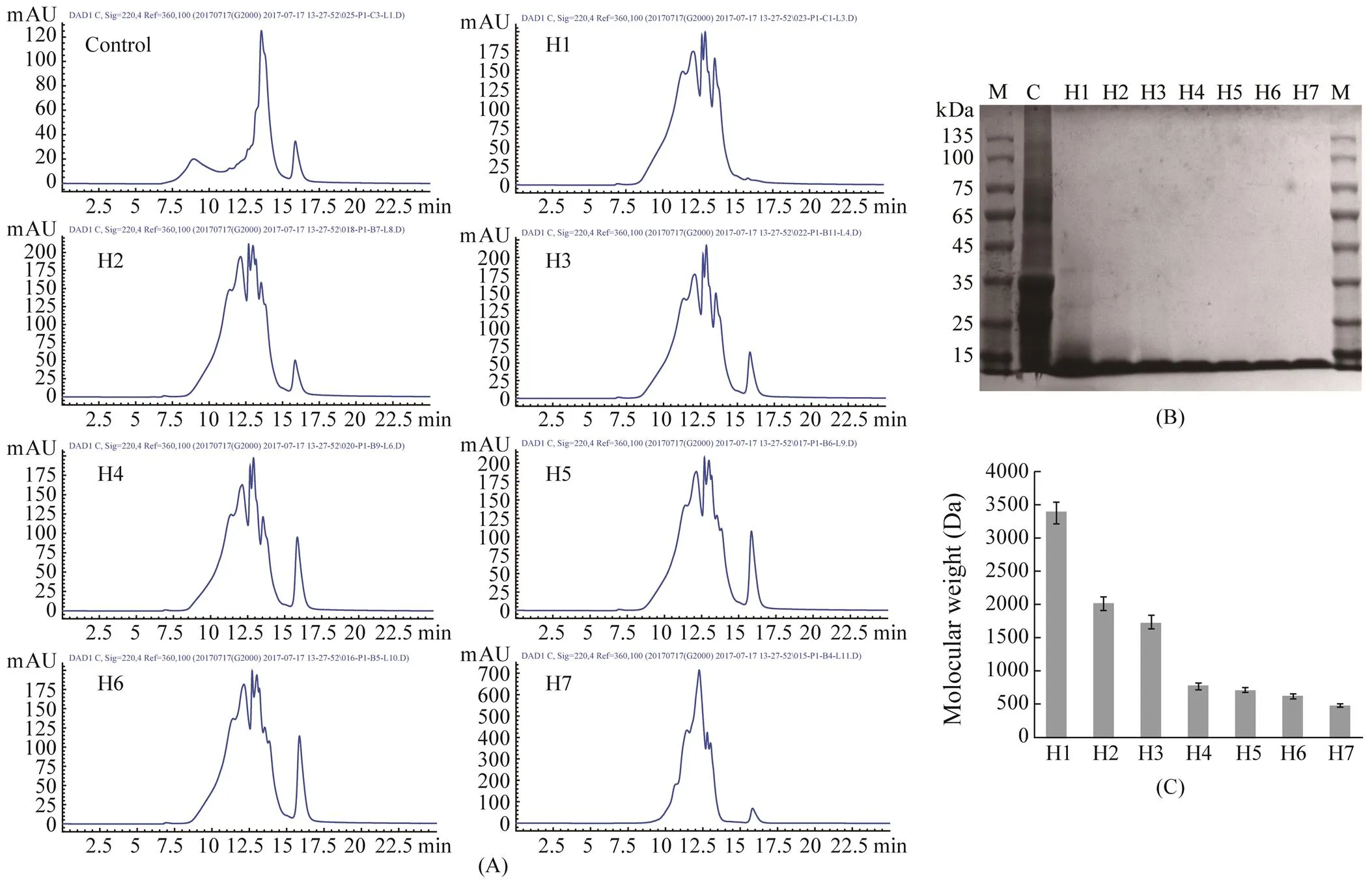
Fig.2 HPLC chromatograms and SDS-PAGE molecular weight distribution profiles of APPH (H1–H7). (A) HPLC chromatograms of APPH; (B) Molecular weight distribution of APPH; (C) Average molecular weight of APPH.
As shown in Figs.1 and 2C, the higher the DH was, the smaller was the average molecular weight of the hydro- lysates. When the DH was <10%, the average molecular weight was >3kDa; when the DH was 10%–20%, the molecular weight was between 1 and 3kDa; and the av- erage molecular weight was <1kDa when the DH was >20%. The enzymatic hydrolysis of a fish protein pro- duced a series of smaller polypeptides that had different functional properties and bioactivities (Geirsdottir., 2011). Thus, changing the molecular weight of the APPH might affect not only the functional properties, including solubility, emulsifying properties, foaming properties, and OHC (Jemil., 2014), but also their antioxidant activities (Li., 2013).
3.3 Functional Properties
3.3.1 Solubility
The functional properties of H1–H7 and APPI, includ- ing solubility, OHC, EA, and foaming ability, were evaluated and compared. Solubility is a critically impor- tant functional characteristic of a protein and is closely related with other major functional properties, such as emulsifying and foaming capacities (Pujara., 2017). Proteins with high solubility can be widely applied in protein-rich aqueous foods and protein-based formula- tions such as emulsions and foams (Natarajan., 2005). Fig.3A shows the effect of Neutrase treatment on the solubility of APPI. As shown in the figure, all the hydrolysates were significantly (<0.05) more soluble than the control (28.3%). The solubility of the hydrolys- ates positively correlated with the DH. The primary trend was that the solubility increased with increasing reaction time and decreasing average molecular weight; however, the rate at which the solubility increased did gradually decrease. The maximum solubility of the APPH was ob- served in H6 (90.0%). The results of this study demon- strated the potential for APPH to be used in protein-rich aqueous foods such as nutrient supplements and sports beverages.
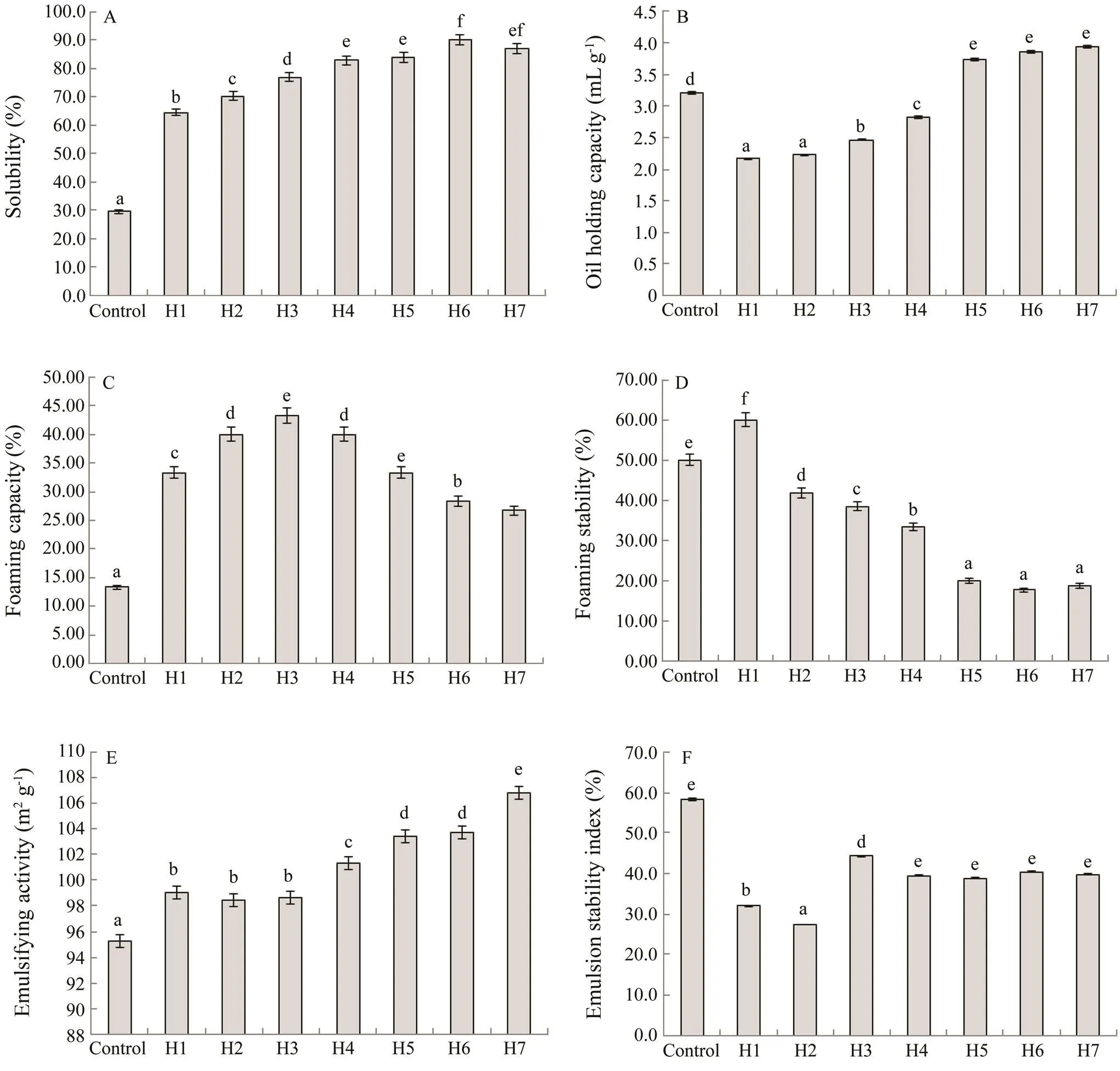
Fig.3 Functional properties of APPI and hydrolysates H1–H7.(A) Solubility; (B) Oil-holding capacity; (C) Foaming capacity; (D) Foaming stability; (E) Emulsifying activity; (F) Emulsion stability index. Values are reported as the mean±SD of three determinations. a–h: The values with different subscripts indicate significant differences (P<0.05).
3.3.2 Oil-holding capacity
The OHC of peptides is especially important in the fish and meat industries because it can influence the taste of the food product. The OHC of the proteins was found to correlate with the hydrophobicity of the surface and the protein bulk density. As shown in Fig.3B, the OHCs of APPH were significantly changed by enzymatic hy- drolysis (<0.05). With the increase in the DH, the OHC of the hydrolysates gradually increased. The OHC of the APPI was 3.25mLg?1. When the average molecular weight of the hydrolysate was >1kDa, the OHC of the hydrolys- ate was lower than that of the control. When the average molecular weight was <1kDa, the OHC was higher than that of the control. The samples showed increased OHCs as the peptide molecular weights decreased. This result was consistent with the conclusion of dos Santos. (2011). Thus, if the molecular weight of the hydrolysate is <1kDa, the DH will be >20% and the OHC of the APPH will be significantly improved.
3.3.3 Foaming property
Foam is a type of colloidal system with a continuous aqueous phase and a dispersed gas phase. Foaming prop- erties are affected by the tension of the proteins at the air–water interface. The foaming properties of proteins are also affected by the molecular weight of the peptides. As shown in Fig.3C, the FC of all the hydrolysate sam- ples (H1–H7) were significantly improved (<0.05) relative to the control. The nonhydrolyzed protein dis- played the lowest initial foaming capacity. The hydrolys- ate with the highest foaming capacity was H3. When the average molecular weight was <1kDa (H4–H7), both the foaming capacity and the foaming stability decreased with decreasing average molecular weight (Fig.3D). This may be because low molecular weight peptides (<1kDa) were unable to maintain a well-ordered interface due to the orientations of the molecules.
3.3.4 Emulsion property
The emulsifying activity (EA) represents the adsorp- tion capacity of the protein at the oil–water interface. The EA values for the samples that were enzymatically hy- drolyzed from the APPI with Neutrase are shown in Fig.3E. The EA values of all the hydrolysate samples were significantly improved (<0.05) compared with the control. The EA of the hydrolysates gradually increased as the DH increased. In addition, when the molecular weight of the hydrolysates was >1kDa (H1–H3), the emulsion activity of the sample was low, and there were no significant differences among them (>0.05). When the molecular weight of the hydrolysates was <1kDa, the emulsion stability of the proteolytic product did not change significantly (>0.05) with further changes in the molecular weight (Fig.3F), and the emulsifying ac- tivities of the samples gradually increased. The sample with the highest emulsion capacity was H7. This result was in agreement with the findings of several previous studies (Li., 2013; Pacheco-Aguilar., 2008). This result may be due to the newly exposed amino acids and the surface stability residues in the original protein uncovered during the enzymatic hydrolysis.
The results indicated that compared with APPI, the hydrolysates of APPI displayed better solubility, EA, and foaming activities. The hydrolysates with low molecular weights (<1kDa) had better solubility, OHCs, and EA, and those with higher molecular weights (>1kDa) had better foaming activities.
3.4 Antioxidant Activity
3.4.1 Hydroxyl radical-scavenging activity
The hydroxyl radical is one of the most reactive radi- cals, and can damage almost any compound in contact with living cells. Some bioactive peptides from the hy- drolysates of fish have been proven to be good natural antioxidants (Mendis., 2005; Nazeer., 2012). Fig.4A shows the values of the hydroxyl radical-scav- enging activity of APPI and each hydrolysate sample at a concentration of 5gL?1. As shown in the figure, the hy- droxyl radical-scavenging rates of all the hydrolysates were significantly (<0.05) greater than that of the con- trol. The hydroxyl radical-scavenging rates of the hydro- lyzed samples positively correlated with the DH. The radical-scavenging abilities of the hydrolysates with si- milar DH (H5, H6, and H7) were different while the dif- ference wasn’t significant (>0.05). The peptides with molecular weights between 500 and 800Da (H5–H7) were found to have the highest radical-scavenging activities.
3.4.2 Superoxide anion-scavenging activity
The superoxide anion-scavenging abilities of the hy- drolysates from APPI are shown in Fig.4B. Similar to the trend of hydroxyl radical-scavenging activities, the su- peroxide anion-scavenging activities of the hydrolysates increased with decreasing average molecular weights. Few differences were observed in the superoxide anion- scavenging activities and the hydroxyl radical-scavenging activities of the samples; H5 and H6 exhibited greater DPPH free radical-scavenging activities than H7. Thus, hydrolysates with low molecular weights (<1kDa) had better superoxide anion-scavenging activities.

Fig.4 Antioxidant activities of APPI and hydrolysates H1– H7.(a) Hydroxyl radical-scavenging activity; (b) Super- oxide anion-scavenging activity; (c) DPPH radical-sca- venging activity. Values are reported as the mean±SD of three determinations. a–h: The values with different sub- scripts are significantly different (P<0.05).
3.4.3 DPPH radical-scavenging activity
The DPPH radical-scavenging assay is a commonly used reactive oxygen-scavenging test for determining the ability of a certain substrate to scavenge free radicals (Zhang., 2012). Fig.4C shows the DPPH radical- scavenging activities of APPI and each hydrolysate at a concentration of 5gL?1. The experimental DPPH radi- cal-scavenging activities ranged from 48.92% to 85.22%, indicating that peptides of all molecular sizes can scav- enge free radicals. The DPPH radical-scavenging activi- ties of the hydrolysates gradually increased as the average molecular weights decreased. The DPPH radical-scav- enging activities of H1 (>3kDa) and the control were different but the difference wasn’t significant (>0.05). The DPPH radical-scavenging abilities of the hydrolysates with molecular weights between 3kDa and 800Da (H2, H3, and H4) were different but the difference wasn’t sig- nificant (>0.05). Furthermore, the hydrolysate H7 showed a significantly (<0.05) higher DPPH radical-scavenging activity than the other samples.
These findings demonstrate the hydroxyl radical- scavenging rate, the superoxide anion-scavenging activity, and the DPPH free radical-scavenging rate of the APPI were significantly improved by the hydrolysis using Neu- trase. The hydrolysates of APPI produced by Neutrase showed better antioxidant activities. In addition, the lower the average molecular weight was, the higher was the antioxidant activity. With a concentration of 5.0mgmL?1, the highest values of DPPH, superoxide, and hydroxyl free radical-scavenging activities were 85.22%, 53.56%, and 75.00%, respectively.
4 Conclusions
This study demonstrated that Neutrase could be used to improve the functional properties and the antioxidant ac- tivities of the APPI. During the hydrolysis process, the DH of the hydrolysates increased as the average mole- cular weight of the hydrolysates decreased slightly. The functional properties of APPI were also significantly improved. The hydrolysates of APPI exhibited better solubility, EA, and foaming activities. The hydrolysates with low molecular weights (<1kDa) had better solubility, OHCs, and EA, while those with higher molecular weights (>1kDa) had better foaming activities. In addition, the hydrolysates exhibited excellent antioxidant properties including DPPH, superoxide, and hydroxyl free radical- scavenging activities. The lower the average molecular weight was, the higher was the antioxidant activity. The results indicated that the hydrolysis using Neutrase was an effective method for improving the functional pro- perties and the antioxidant properties of the APPI. The hydrolysates of APPI have the potential to be widely used as natural antioxidants and sources of dietary nutrients in the food industry.
Acknowledgements
This work was supported by grants from the China Postdoctoral Science Foundation to Dr. Chuyi Liu (No. 2016M592251).
Ahn, C. B., Kim, J. G., and Je, J. Y., 2014. Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion.-,147:78-83.
Alu’datt, M. H., Rababah, T., Alhamad, M. N., Alodat, M., Al-Mahasneh, M. A., Gammoh, S., Ereifej, K., Almajwal, A., and Kubow, S., 2017. Molecular characterization and bio-functional property determination using SDS-PAGE and RP-HPLC of protein fractions from two Nigella species.,230:125-134.
Chanarat, S., and Benjakul, S., 2013. Impact of microbial transglutaminase on gelling properties of Indian mackerel fish protein isolates., 136: 929-937.
Chen, Y. C., and Jaczynski, J., 2007 Gelation of protein re- covered from whole Antarctic krill () by isoelectric solubilization/precipitation as affected by func- tional additives., 55: 1814-1822.
Chi, C. F., Hu, F. Y., Wang, B., Li, Z. R., and Luo, H. Y., 2015. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna () dark muscle., 13: 2580-2601.
Choi, Y. J., Hur, S., Choi, B. D., Konno, K., and Park, J. W., 2009. Enzymatic hydrolysis of recovered protein from frozen small croaker and functional properties of its hydrolysates., 74: C17-24.
dos Santos, S. D., Martins, V. G., Salas-Mellado, M., and Pren- tice, C., 2011. Evaluation of functional properties in protein hydrolysates from bluewing searobin () obtained with different microbial enzymes., 4: 1399-1406.
Fan, J., He, J., Zhuang, Y., and Sun, L., 2012. Purification and identification of antioxidant peptides from enzymatic hydro- lysates of tilapia () frame protein., 17: 12836-12850.
Garcia-Moreno, P. J., Perez-Galvez, R., Espejo-Carpio, F. J., Ruiz-Quesada, C, Perez-Morilla, A. I., Martinez-Agustin, O., Guadix, A., and Guadix, E. M., 2017. Functional, bioactive and antigenicity properties of blue whiting protein hydrolys- ates: Effect of enzymatic treatment and degree of hydrolysis., 97: 299-308.
Garcia-Moreno, P. J., Perez-Galvez, R., Javier, E. F., Ruiz-Que- sada, C., Perez-Morilla, A. I., Martinez-Agustin, O., Guadix, A., and Guadix, E.M., 2016. Functional, bioactive and anti- genicity properties of blue whiting protein hydrolysates: Ef- fect of enzymatic treatment and degree of hydrolysis., 94: 234-242.
Geirsdottir, M., Sigurgisladottir, S., Hamaguchi, P. Y., Thor- kelsson, G., Johannsson, R., Kristinsson, H. G., and Krist- jansson, M. M., 2011. Enzymatic hydrolysis of blue whiting (); functional and bioactive proper- ties., 76: C14-20.
Hou, H., Fan, Y., Li, B., Xue, C., and Yu, G., 2012a. Preparation of immunomodulatory hydrolysates from Alaska pollock frame., 92: 3029-3038.
Hou, H., Fan, Y., Li, B., Xue, C., Yu, G., Zhang, Z., and Zhao, X., 2012b. Purification and identification of immunomodu- lating peptides from enzymatic hydrolysates of Alaska pollock frame., 134: 821-828.
Hou, H., Li, B. F., Zhao, X., Zhang, Z. H., and Li, P. L., 2011. Optimization of enzymatic hydrolysis of Alaska pollock frame for preparing protein hydrolysates with low-bitterness., 44: 421-428.
Je, J. Y., Park, P. J., Kwon, J. Y., and Kim, S. K., 2004. A novel angiotensin I converting enzyme inhibitory peptide from Alaska pollack () frame protein hy- drolysate., 52: 7842-7845.
Jemil, I., Jridi, M., Nasri, R., Ktari, N., Salem, R. B., Mehiri, M., Hajji, M., and Nasri, M., 2014. Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented byA26., 49: 963-972.
Jia, J., Zhou, Y., Lu, J., Chen, A., Li, Y., and Zheng, G., 2010. Enzymatic hydrolysis of Alaska pollack (-) skin and antioxidant activity of the resulting hy- drolysate., 90: 635-640.
Kammoun, R., Bejar, S., and Ellouz, R., 2003. Protein size distribution and inhibitory effect of wheat hydrolysates on Neutrase., 90: 249-254.
Kang, E. J., Hunt, A. L., and Park, J. W., 2008. Effects of salin- ity on physicochemical properties of Alaska pollock surimi after repeated freeze-thaw cycles., 73: C347-355.
Kim, S. K., Kim, Y. T., Byun, H. G., Nam, K. S., Joo, D. S., and Shahidi, F., 2001. Isolation and characterization of antioxi- dative peptides from gelatin hydrolysate of Alaska pollack skin., 49: 1984-1989.
Li, Z. R., Wang, B., Chi, C. F., Gong, Y. D., Luo, H. Y., and Ding, G. F., 2013. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from,and., 51: 283-293.
Mendis, E., Rajapakse, N., and Kim, S. K., 2005. Antioxidant properties of a radical-scavenging peptide purified from en- zymatically prepared fish skin gelatin hydrolysate., 53: 581-587.
Natarajan, S., Xu, C., Caperna, T. J., and Garrett, W. M., 2005. Comparison of protein solubilization methods suitable for proteomic analysis of soybean seed proteins., 342: 214-220.
Nazeer, R. A., Kumar, N. S., and Jai, G. R., 2012.andstudies on the antioxidant activity of fish peptide iso- lated from the croaker () muscle protein hy- drolysate., 35: 261-268.
Nongonierma, A. B., Maux, S. L., Esteveny, C., and FitzGerald, R. J., 2017. Response surface methodology applied to the generation of casein hydrolysates with antioxidant and di- peptidyl peptidase IV inhibitory properties., 97: 1093-1101.
Ou, K., Liu, Y., Zhang, L., Yang, X., Huang, Z., Nout, M. J., and Liang, J., 2010. Effect of neutrase, alcalase, and papain hydrolysis of whey protein concentrates on iron uptake by Caco-2 cells., 58: 4894-4900.
Pacheco-Aguilar, R., Mazorra-Manzano, M. A., and Ramirez-Suarez, J. C., 2008. Functional properties of fish protein hy- drolysates from Pacific whiting() mus- cle produced by a commercial protease., 109: 782-789.
Pujara, N., Jambhrunkar, S., Wong, K. Y., McGuckin, M., and Popat, A., 2017. Enhanced colloidal stability, solubility and rapid dissolution of resveratrol by nanocomplexation with soy protein isolate., 488: 303-308.
Sampath-Kumar, N. S., Nazeer, R. A., and Jaiganesh, R., 2011. Purification and biochemical characterization of antioxidant peptide from horse mackerel () viscera protein., 32: 1496-1501.
Shavandi, A., Hu, Z., Teh, S., Zhao, J., Carne, A., Bekhit, A., and Bekhit, A. E., 2017. Antioxidant and functional proper- ties of protein hydrolysates obtained from squid pen chitosan extraction effluent., 227: 194-201.
Speroni, F., Jung, S., and de Lamballerie, M., 2010. Effects of calcium and pressure treatment on thermal gelation of soybean protein., 75: E30-38.
Sun, L., Chang, W., Ma, Q., and Zhuang, Y., 2016. Purification of antioxidant peptides by high resolution mass spectro- metry from simulated gastrointestinal digestion hydrolysates of Alaska pollock () skin collagen., 14: 2212-2231.
Suppavorasatit, I., DeMejia, E. G., and Cadwallader, K. R., 2011. Optimization of the enzymatic deamidation of soy protein by protein-glutaminase and its effect on the functional properties of the protein., 59: 11621-11628.
Xiao, J. H., and Niu, L. Y., 2016. Antilisterial and antioxidant activities of neutrase-treated grass carp proteins and their ef- fects on the storage and quality properties of fresh noodle.
, 40: 1421-1428.
Xu, J., Zhao, Q., Qu, Y., and Ye, F., 2015. Antioxidant activity and anti-exercise-fatigue effect of highly denatured soybean meal hydrolysate prepared using neutrase., 52: 1982-1992.
Yang, Z. H., Miyahara, H., Takeo, J., Hatanaka, A., and Kata- yama, M., 2011. Pollock oil supplementation modulates hy- perlipidemia and ameliorates hepatic steatosis in mice fed a high-fat diet., 10: 189.
Zhang, Y., Duan, X., and Zhuang, Y., 2012. Purification and characterization of novel antioxidant peptides from enzy- matic hydrolysates of tilapia () skin gelatin., 38: 13-21.
Zheng, L., Lin, L., Su, G., Zhao, Q., and Zhao, M., 2015. Pit- falls of using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay to assess the radical scavenging activity of peptides: Its suscep- tibility to interference and low reactivity towards peptides., 76: 359-365.
September 1, 2017;
December 18, 2017;
July 7, 2018
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
# These authors contributed equally to this work.
. E-mail: bfli@ouc.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2018年6期
Journal of Ocean University of China2018年6期
- Journal of Ocean University of China的其它文章
- Comparative Evaluation of Toleration to Heating and Hypoxia of Three Kinds of Salmonids
- Optimization of Hydrolysis Conditions for the Isolation of Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides from Rhopilema hispidum
- Trophic Interaction in a Portunus rituberculatus Polyculture Ecosystem Based on Carbon and Nitrogen Stable Isotope Analysis
- An Evaluation on the Ratio of Plant to Animal Protein in the Diet of Juvenile Sea Cucumber (Apostichopus japonicus): Growth, Nutrient Digestibility and Nonspecific Immunity
- Weighted Correlation Network Analysis (WGCNA) of Japanese Flounder (Paralichthys olivaceus) Embryo Transcriptome Provides Crucial Gene Sets for Understanding Haploid Syndrome and Rescue by Diploidization
- Lead Induces Different Responses of Two Subpopulations of Phagocytes in the Holothurian Eupentacta fraudatrix
