In fluence of light availability on the speci fic density, size and sinking loss of Anabaena flos- aquae and Scenedesmus obliquus*
HUANG Yingying (黃瑩瑩) , ZHANG Haichun (張海春) , GAO Rufeng (高如峰) ,HUANG Xiaochen (黃曉琛) , YU Xiaojuan (于曉娟) , CHEN Xuechu (陳雪初) , ,
Abstract Harmful algal blooms in eutrophic waters pose a serious threat to freshwater ecosystems and human health. In-situ light availability control is one of the most commonly used technologies to suppress algae in lakes and reservoirs. To develop a better understanding of the effects of light on algal growth,speci fic density, colony size and sinking loss, Anabaena flos- aquae (cyanobacteria) and Scenedesmus obliquus (green algae) were evaluated in varying light scenarios. The results showed that the speci fic density and colony size of these two species varied during growth, and there were obvious differences among the light scenarios. At the end of exponential phase, S. obliquus incubated under light-limited condition maintained a higher speci fic density and formed larger aggregates, whereas A. flos- aquae formed a longer filament length. Both species exhibited higher sinking loss rates with lower light availability. These results implied that the sinking loss rate was not always constant but should be considered as a variable response to the change of light availability, and in-situ light availability control might result in a signi ficant increase of the sinking loss of algae due to the change of size and speci fic density, thereby further affecting the algal biomass in the water column.
Keyword: speci fic density; size; light availability control; sinking loss
1 INTRODUCTION
Eutrophic lakes are often dominated by cyanobacteria, including several potentially toxic species, such as Anabaena, Microcystis and Aphanizomenon. These cyanobacteria produce gas vesicles, which leads to lower speci fic density than water. When the water column is stable, the buoyant cyanobacteria float upwards forming dense surface blooms (O’Neil et al., 2012). These cyanobacteria cause considerable degradation of water quality due to the formation of blooms and the possible production of toxins and off- flavors.
Various technologies have been developed to restore eutrophic waters with cyanobacterial blooms(Ahn et al., 2003; Dockko et al., 2015). Among them,in-situ light availability control is one of the most commonly used technologies to suppress cyanobacteria in lakes and reservoirs (Jungo et al.,2001; Maestre-Valero et al., 2013). There are two common types of in-situ light availability control,“arti ficial mixing” and “surface light-shading”.Arti ficial mixing entrains surface cyanobacteria into the aphotic layer with prolonged darkness. Due to the limited availability of light, the growth of cyanobacteria is inhibited (Jungo et al., 2001; Hudnell et al., 2010). Surface light-shading forces high-algal water to stay in a dark area for a certain period, leading to an efficient reduction of the cyanobacterial biomass(Chen et al., 2009a, 2009b). Previous researches suggested that light availability control would lead to a signi ficant decrease of phytoplankton biomass and shift the dominant species from cyanobacteria to green algae and diatoms in the water column (Visser et al., 1996; Jungo et al., 2001; Burford and O’Donohue, 2006). Existing studies showed that this phenomenon could be produced by a light-competition model, developed by Huisman and co-workers from 1990s (Huisman and Weissing, 1994, 1995; Huisman,1999; Huisman et al., 1999, 2004). This model suggests that cyanobacteria, due to its high “critical light intensity”, would be competitively excluded by other species that can thrive under low light conditions,such as green algae and diatoms.
Sinking loss is an important factor that may limit phytoplankton biomass in water column (Diehl et al.,2002; Padisák et al., 2003), and our previous study showed that sinking loss was the main mechanism for the cyanobacterial biomass reduction under lightshading (Huang et al., 2016). In the light competition model mentioned above, the speci fic sinking loss rate of phytoplankton have been considered as a constant that would not be in fluenced by light availability.Previous studies of light availability control rarely directly measured the sinking loss of phytoplankton,considering it as merely a small constant that would not signi ficantly in fluence the biomass in the column.However, this presumption is questionable because the speci fic sinking loss rate of a given taxon may vary due to the change in cell (or colony) shape and size, as well as the speci fic density, and it may further in fluence the loss process (Jezberová and Komárková,2007; Stoyneva et al., 2007; Chen et al., 2011). In particular, phytoplankton cell (or colony) size varied with the light gradient (Jezberová and Komárková,2007; Kaiblinger et al., 2007). The findings of Naselli-Flores and Barone (2007) supported this statement that there were signi ficant relationships between the daily availability of underwater light and the dominant morphology in the assemblage of a hypertrophic Mediterranean reservoir.
The objectives of this study were to investigate the variation trend of the speci fic density and the cell (or colony) size of two typical species, Anabaena flosaquae and Scenedesmus obliquus, under different light incubation conditions and to discuss the constant of sinking loss rate in the light competition model. To simulate the sedimentation process, a mixing-static water column experimental apparatus was designed for this study. The sinking performance of two species was compared at the end of the experiment to investigate whether variations in the cell (or colony)size and speci fic density would further in fluence the sinking loss.
2 MATERIAL AND METHOD
2.1 Experimental design
Anabaena flos- aquae (FACHB 245) and S. obliquus(FACHB 416) were provided by the Freshwater Algae Culture Collection of the Institute of Hydrobiology,the Chinese Academy of Sciences (Wuhan, China).Scenedesmus obliquus normally forms four-celled colonies, known as coenobia, and A. flos- aquae forms filaments of 8–50 cells. The cells were maintained in a liquid axenic culture using BG11 medium.Illumination was provided using cool-white fluorescent light at a continuous intensity of 50 μmol photons/(m2·s) at the water surface. Then, the phytoplankton was cultured in 500 mL flasks containing 300 mL BG11 medium, with a 14 h:10 h light/dark cycle, at 25±1°C. The initial concentration was (5×104)–(1×105) cells/mL. Adjustments of shade cloth were used to obtain different light incubation conditions, including 20, 60, 100, 200 and 300 μmol photons/(m2·s) for S. obliquus and 2, 10, 20, 60 and 100 μmol photons/(m2·s) for A. flos- aquae. Each treatment had three replicates. Samples of approximately 8 mL were taken every 4 d for measurements of Chlorophyll a (Chl a). The speci fic densities and colony sizes of the two species were measured during the experiment. The experiment lasted 22 d for S. obliquus and 35 d for A. flos- aquae,respectively, but the average speci fic growth rate ( μ)was calculated according to the shortest exponential phase under different light intensity, as 10 d for S.obliquus and 31 d for A. flos- aquae, respectively. The average speci fic growth rate ( μ) was calculated as:

where N is the final concentration of Chl a (μg/L) at the end of the exponential phase, N0is the initial concentration of Chl a (μg/L), and t (d) is the experiment period.
According to the average speci fic growth rate, the light incubation conditions were divided into light-limited condition, light-sufficient condition and lightinhibited condition.
At the end of the experiment, the sedimentation characteristics of suspended phytoplankton cultures were studied. A mixing-static water column experimental apparatus was designed for the simulation of sedimentation processes. The apparatus was a vertical glass cylinder (diameter, 5 cm; height,50 cm) consisting of two chambers: (1) an upper mixing chamber (30 cm high), and (2) a lower settling chamber (15 cm high), which contained a honeycomb(Fig.1).
The whole cylinder was filled with the phytoplankton culture to a height of 45 cm. The upper 30 cm of the water column, which was located in the mixing chamber, was continuously aerated with an airline at a flow rate of 0.5 L/min. The portion of the water column in the settling chamber remained undisturbed, preventing re-suspension of any material that had settled out of the mixing chamber. Eight milliliter of water samples were taken from the mixing chamber before and at the end of the 2-h experimental period, for Chl a measurements. Accordingly, the sinking loss rate over a 2-h period was calculated as:
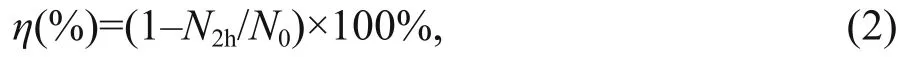
where N2his the final concentration of Chl a (μg/L)and N0is the initial concentration of Chl a (μg/L).
2.2 Analyses
The phytoplankton biomass, expressed as the concentration of Chl a, was determined using pulseamplitude modulated fluorometry (PHYTO-PAM,WALZ, Germany, Phytowin v1.32).
The speci fic density of phytoplankton was measured using the Percoll density gradient centrifugation method (Pertoft, 2000). The Percoll solution was made by mixing 2.7 parts of Percoll with one part of the growth media (BG11 medium).Samples of approximately 2 mL were added to the mixture. The mixture was centrifuged at 14 000 r/min for 30 min. Next, a stable density gradient was formed, with the cells or colonies banded at their buoyant density during the centrifugation (Wolff,1975). The speci fic density of each visible phytoplankton band was measured with a kerosene-CCl4column of a known density gradient (Condie and Bormans, 1997).
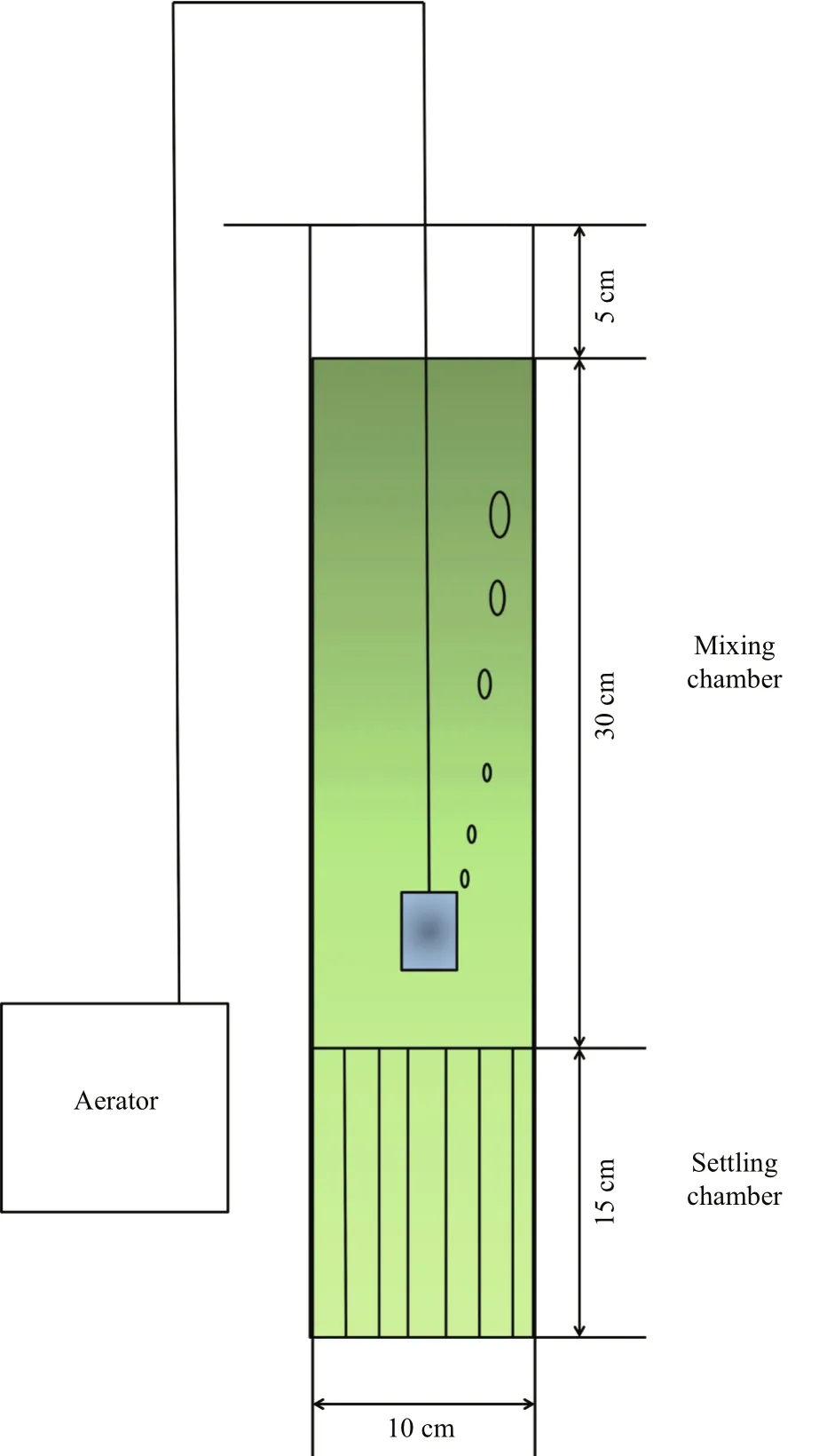
Fig.1 The mixing-static water column experimental system for simulation of sedimentation process
The colony sizes of A. flos- aqua and S. obliquus were expressed as the filament length and the aggregation size (cell number within aggregation),respectively. The colony sizes were observed and recorded with a fluorescence microscope (Eclipse E400 DS-Fi; Nikon, Melville, NY, USA). More than 50 photographs were taken for each sample to generate sufficient data for statistical analysis. The filament length of A. flos- aquae and the aggregation size of S. obliquus cells could be extracted from the photographs using the NIS elements microscopic photograph software.
Speci fic growth rate and speci fic density of the two species under different light incubation conditions were compared using an ANOVA, followed by an S-N-K (s) test. Correlations among speci fic density,filament length or aggregation size, incubation light,and sinking loss at the end of experiment were analyzed by Pearson correlation. P-values less than 0.05 and 0.01 were considered as signi ficant and extremely signi ficantly, respectively.
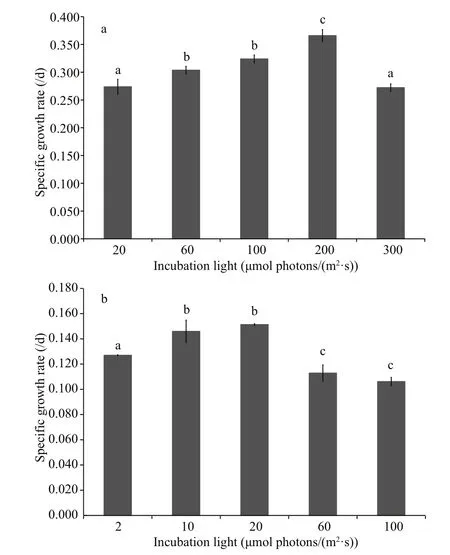
Fig.2 The speci fic growth rate of Scenedesmus obliquus (a)and Anabaena flos- aquae (b) under different light incubation conditions
3 RESULT
3.1 Growth of two species under different light incubation conditions
For S. obliquus (Fig.2a), the speci fic growth rate increased from 20 μmol photons/(m2·s) to 200 μmol photons/(m2·s). When the incubation light was as high as 300 μmol photons/(m2·s), the rate decreased obviously ( P <0.05), suggesting the growth of S. obliquus was inhibited due to excessive light.Accordingly, it could be inferred that S. obliquus experienced light-limited and light-inhibited conditions under 20 μmol photons/(m2·s) and 300 μmol photons/(m2·s), respectively, whereas incubation light of 200 μmol photons/(m2·s) supplied sufficient light for the growth of S. obliquus. Similarly,for A. flos- aquae (Fig.2b), with increasing amounts of light, the speci fic growth rate first increased gradually and then decreased sharply after peaking; however,there were no signi ficant differences between the speci fic growth rates under 10 μmol photons/(m2·s)and 20 μmol photons/(m2·s) ( P >0.05), and between the speci fic growth rates under 60 μmol photons/(m2·s) and 100 μmol photons/(m2·s) ( P >0.05). The light-limited, light-sufficient and light-inhibited conditions were approximately 2 μmol photons/(m2·s), 20 μmol photons/(m2·s) and 100 μmol photons/(m2·s), respectively.
3.2 Variation of speci fic density under different light incubation conditions
For S. obliquus (Fig.3a), the speci fic density was in fluenced by both incubation time and light availability. Under light-limited condition (20 μmol photons/(m2·s)), the speci fic density gradually increased for approximately 14 d, until it achieved the maximal value of 1.070. Under light-sufficient condition (200 μmol photons/(m2·s)), a similar variation trend of the speci fic density with incubation time was observed. However, the speci fic density increased more rapidly during the first 3 d but achieved a lower maximal value of 1.045 after 8 d of incubation. Under the light-inhibited condition(300 μmol photons/(m2·s)), the speci fic density continually increased during the experiment, and the final speci fic density value was approximately 1.039.The maximal values of the speci fic density under three light incubation conditions were signi ficantly different ( P <0.05). At the end of the experiment, the speci fic density under the light-limited condition was obviously higher than that under the light-sufficient condition and the light-inhibited condition ( P <0.05),but there was no difference between the speci fic densities under the latter two conditions ( P >0.05).
For A. flos- aquae (Fig.3b), the variation trend of speci fic density was more complex. Under lightlimited condition (2 μmol photons/(m2·s)), the speci fic density decreased obviously during the first two d( P <0.05) and then fluctuated from 1.091 to 1.097. At the end of the experiment, it achieved the maximal value of 1.116. Under light-sufficient condition(20 μmol photons/(m2·s)), the speci fic density also decreased obviously during the first two d ( P <0.05)and then increased until it achieved the maximal value of 1.116. After that, the variation of speci fic density was not obvious ( P >0.05). Under light-inhibited condition (100 μmol photons/(m2·s)), the speci fic density increased rapidly to the maximal value of 1.171 during the first 10 d, which was signi ficantly higher than the maximal values under light-limited condition and light-sufficient condition ( P <0.05), and then it decreased sharply. Unlike S. obliquus, the speci fic density of A. flos- aquae showed no signi ficant differences among the three different light incubation conditions at the end of the experiment ( P >0.05).
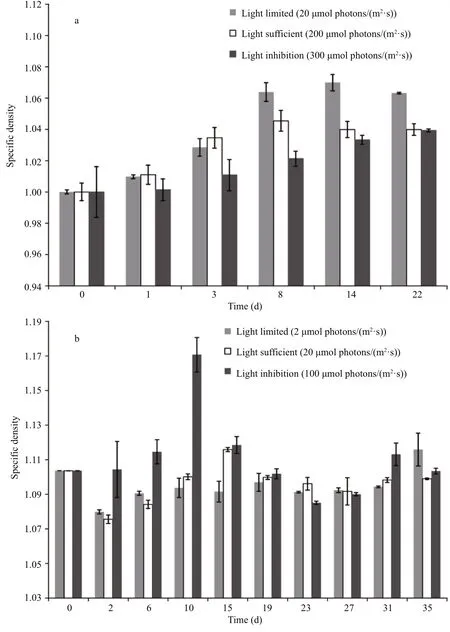
Fig.3 Variation of speci fic density of Scenedesmus obliquus (a) and Anabaena flos- aqua (b) under different light incubation conditions
3.3 Variation of colony size under different light incubation conditions
For S. obliquus, there was no observed change in shape during the exponential phase of growth under different light incubation conditions. However, a phenomenon of cell aggregation was observed at the end of the exponential phase. As shown in Fig.4, the size of the aggregation decreased with the increase of light availability. Furthermore, the cell numbers were counted within observed aggregations under different light incubation conditions. It appeared that S. obliquus formed smaller aggregations under higher light availability conditions.
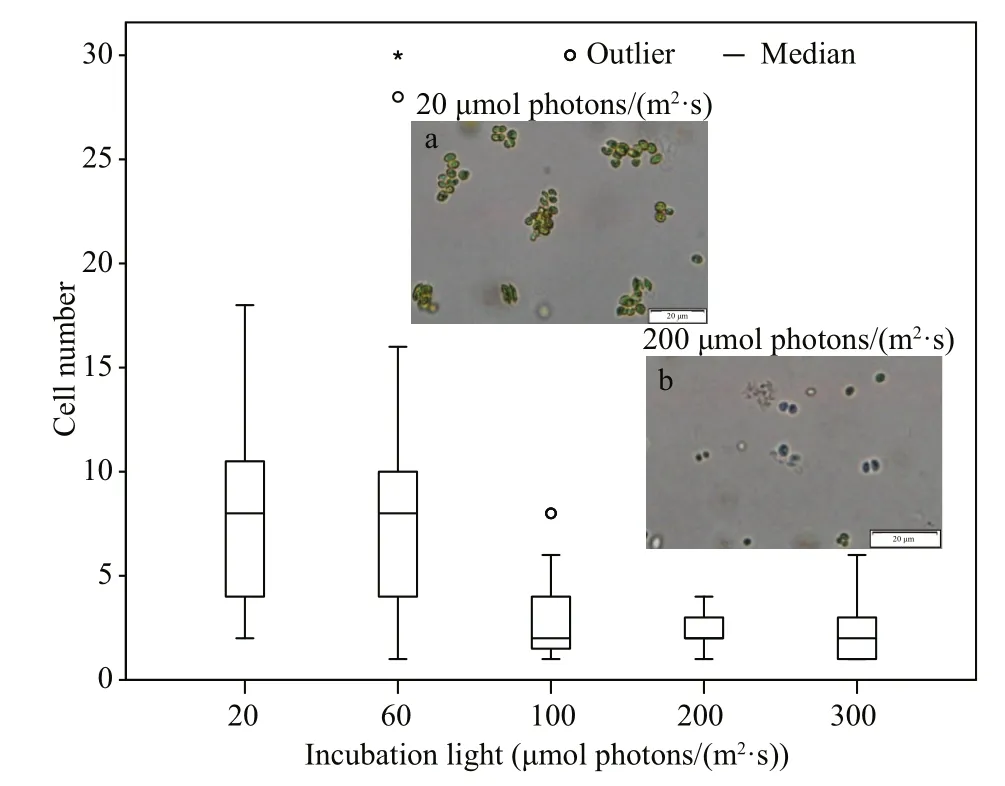
Fig.4 Frequency distribution of cell number within aggregate and microscopic photos of Scenedesmus obliquus under different light incubation conditions
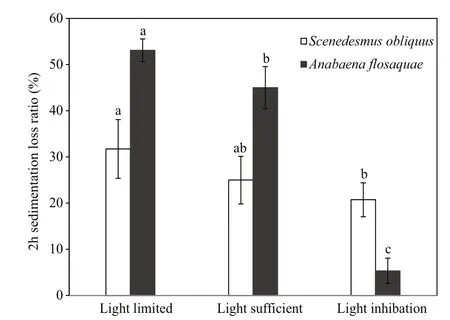
Fig.6 Two-hour sinking loss rate of Scenedesmus obliquusand Anabaena flos- aqua under different lightincubation conditions
For A. flos- aquae, a notable morphological characteristic is filament length. Figure 5 shows the variation in filament length ( n ≥50) under the three light conditions. Under light-limited condition, the median filament length showed an increasing trend during the experiment, from an initial value of 118 μm to a final value of 490 μm, indicating that the filament length increased with incubation time when the light was limited. Under light-sufficient and light-inhibited conditions, the variation trend of filament length was similar. Both filament lengths increased from the beginning of experiment until they achieved maximal values of 495 μm and 284 μm, respectively, and thereafter decreased to 59 μm and 47 μm, respectively.In addition, it seemed that higher light availability could induce A. flos- aquae to achieve its maximal filament length more quickly.
3.4 Sedimentation performance of two species
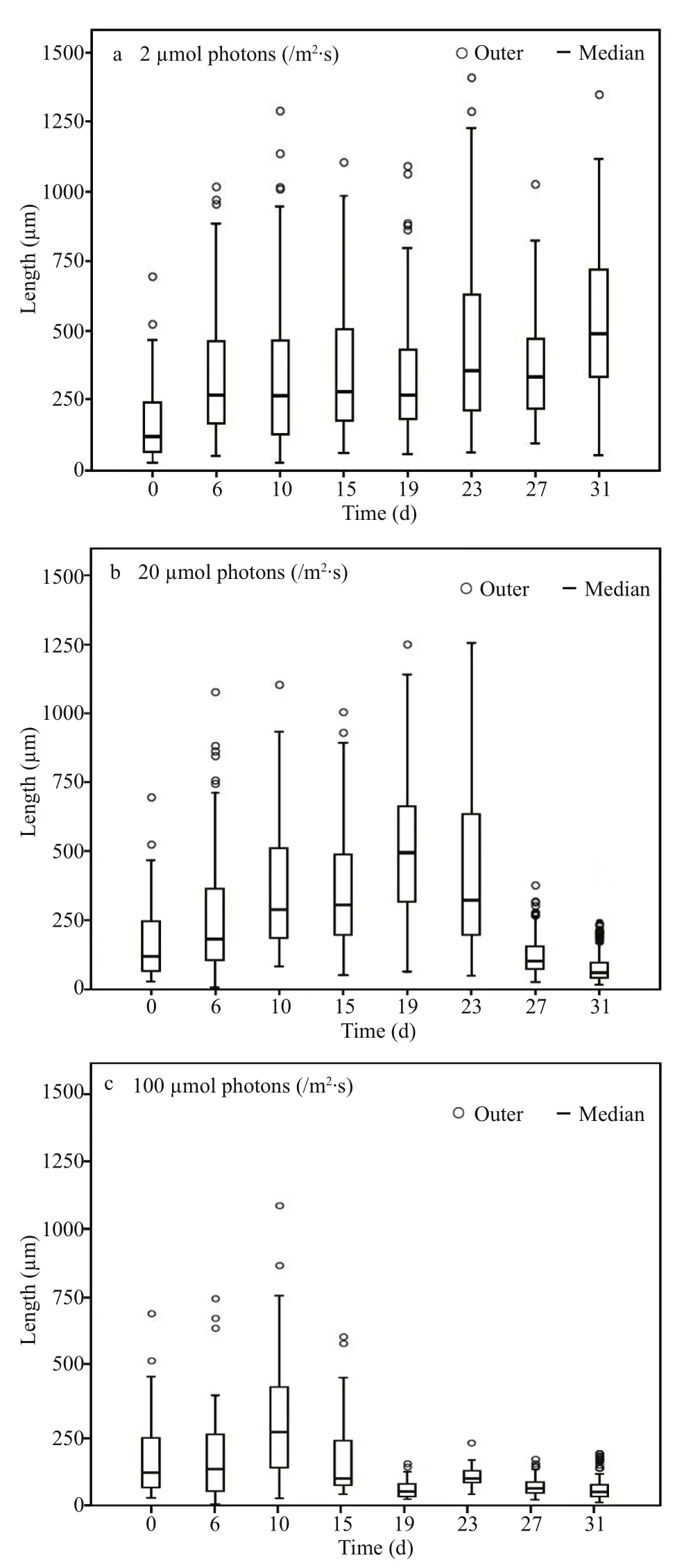
Fig.5 Frequency distribution of chain length of Anabaenaflos- aquae under different light incubation conditions
At the end of the experiment, the sinking loss of S. obliquus and A. flos- aquae was studied through a mixing-static column experiment. This experiment indicated that the sinking loss rates of both species tended to decrease with higher light availability. As shown in Fig.6, the maximal 2-h sinking loss rates of both taxa were observed under the light-limitedcondition, having values as high as 31.75% and 53.09%, respectively. Under the light-sufficient condition, the 2-h sinking loss rates of the two taxa decreased to 25.00% and 45.04%, respectively. Under the light-inhibited condition, the 2-h sinking loss rate was the lowest, showing the values of 20.73% and 5.36%, respectively. Interestingly, A. flos- aquae showed a better sinking performance than S. obliquus under light-limited and light-sufficient conditions,whereas S. obliquus showed a better sedimentation performance under the light-inhibited condition.
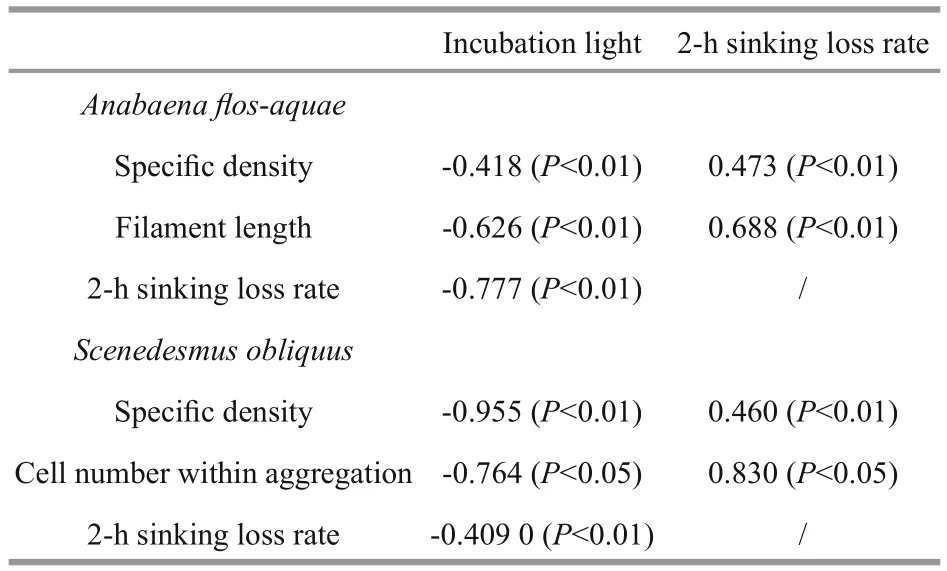
Table 1 Correlations among speci fic density, filament length or aggregation size, incubation light, and sinking loss according to values observed at the end of experiment
4 DISCUSSION
4.1 Speci fic density, colony size and sinking loss in response to light availability
Sinking loss of phytoplankton is an important factor that limits the phytoplankton biomass in a water column. To obtain enough light for survival,phytoplankton populations dwell in the wellilluminated upper regions of the water column.However, most phytoplankton species have speci fic densities somewhat higher than the surrounding medium and, consequently, move downwards on average even in turbulent waters (Bright and Walsby,1999; Ptacnik et al., 2003). The speci fic sinking loss of a given taxon depends strongly on its sinking velocity. The sinking velocity ( ws) of a small phytoplankton that satis fies the condition of laminar flow, without frictional drag ( R e <0.1), can be predicted by the modi fied Stokes equation (Reynolds,2006). According to this equation, sinking velocities vary among different species, depending mainly on cell (or colony) size, cell shape and speci fic density(Padisák et al., 2003; Naselli-Flores et al., 2007; Kruk et al., 2011). Nevertheless, most models describing the dynamics of phytoplankton populations in water columns implicitly assume that the changes in these three parameters are very slight and would not in fluence the sinking velocity and speci fic sinking loss of particular species.
Actually, a few of studies have shown changes in cell (or colony) size, cell shape and speci fic density in response to the environment (Naselli-Flores and Barone, 2007; Yamamoto and Nakahara, 2009), and cell (or colony) size would affect speci fic density and further in fluent their sinking performance (Li et al.,2016). Similarly, the result of our experiment indicated that colony size and speci fic density might vary signi ficantly during the exponential growth phase. This experiment also suggested that light availability could affect the colony size and speci fic density. The variation trend of these parameters showed signi ficant differences under the different light incubation conditions (Figs.4, 5). In particular,when the growth of phytoplankton was ceased at the end of the experiments, S. obliquus maintained a higher speci fic density and formed larger aggregates under the light-limited condition, whereas A. flosaquae showed a longer filament length. Similarly, Li et al. (2015) also found that unicellular forms of S. obliquus increased when light availability increased up to 40 μmol photons/(m2·s). To further evaluate the in fluence of light availability, the correlations among speci fic density, filament length or aggregation size,incubation light, and sinking loss were calculated based on the values observed at the end of the experiment. As shown in Table 1, for both species,incubation light showed a negative correlation with all the other parameters, and there were signi ficant positive correlations between the sinking loss rate and the speci fic density and between the sinking loss rate and either filament length or aggregate size. These results indicated that the changes in speci fic density and size responses to light availability could further affect the sinking loss of a certain species.
4.2 Implication for in-situ light availability control
It has been observed that in-situ light availability control would lead to a decrease of cyanobacterial biomass, shifting the dominant species from buoyant cyanobacteria to green algae and diatoms in water column (Visser et al., 1996; Jungo et al., 2001;Burford and O’Donohue, 2006). The existing theory shows the phenomenon is attributed to the occurrence of light-limited condition, and the growth process of certain algae will be inhibited due to the lack of available light (Diehl et al., 2002; Huisman et al.,2004). The light competition model suggests that the population dynamics in well-mixed waters depends on the “critical light intensities” of the species. Under light-limited condition, the species with lowest critical light intensity should competitively exclude all other species (Diehl et al., 2002; Huisman et al.,2004). However, the results of our experiments indicated another possible mechanism that might regulate the community structure. For instance,A. flos- aquae showed a better sinking performance than S. obliquus under the same light incubation condition of 20 μmol photons/(m2·s). Moreover, there was a signi ficant positive correlation between the sinking loss rate and speci fic density, as well as filament length or aggregation size. Arti ficial aeration in reservoirs or deep lakes and light-shading at more than 30% of the water surface can create light-limited conditions for algae (Kojima, 2000; Kim et al., 2007).Our results indicated that in-situ light availability control might result in an increase of sinking loss of cyanobacteria due to the change of size and speci fic density, which would further promote the decrease in cyanobacterial biomass in the column. However,further studies should be conducted to con firm this hypothesis.
On the other side, the well-known light competition model predicts that the increase of phytoplankton population in column will absorb more light and thus make the overall light condition become less sufficient for growth, and negatively affects the speci fic growth rate (Huisman and Weissing, 1994, 1995; Huisman,1999). Accordingly, during the period of light-limited growth, the phytoplankton experience variable but prolonged light-limited condition. Our experiments suggested that under prolonged light-limited condition, the size and speci fic density of S. obliquus and A. flos- aquae would vary gradually, and leaded to a signi ficant increase of sinking loss compare to the ones grew under light-sufficient condition. As shown in Table 1, speci fic density of both species was negatively related to light availability ( P <0.01). For S. obliquus, lower speci fic density under higher light availability might be related to smaller size of cell and cell number within aggregation under higher light availability conditions (Fig.4). For Anabaena flosaqua, the mechanism for lower speci fic density under higher light availability might involve increased cell turgor pressures at higher light intensities which resulted in collapse of gas vacuoles (Spencer and King, 1989). These results implied that the sinking loss rate was not always stable as expected, but should be consider as a variable response to the change of light availability. Therefore, to a certain extent, the light competition model should be revised considering the in fluence of light availability on the morphology and speci fic density of certain phytoplankton.However, further studies should be conducted to con firm this hypothesis.
5 CONCLUSIO N
1) With increasing amounts of light, the speci fic growth rates of these two species first increased gradually and then decreased sharply after peaking.The speci fic density was in fluenced by incubation time and light availability. However, the speci fic density of A. flos- aquae showed no signi ficant differences among the three light incubation conditions at the end of the experiment.
2) Under light-limited condition, the median filament length of A. flos- aquae showed an increasing trend during the experiment. Under light-sufficient and light-inhibited conditions, the median filament length increased, and thereafter decreased to very short lengths. For S. obliquus, a phenomenon of aggregation was observed at the end of the exponential phase. The size of the aggregation decreased with the increase of light availability.
3) The sinking losses of S. obliquus and A. flosaquae were not always stable but tended to decrease with higher light availability. Their sinking loss should be considered as a variable response to the change of light availability.
6 DATA AVAILABILITY STATEMENT
The authors declare that all data supporting the findings of this study are available within the article.
 Journal of Oceanology and Limnology2018年4期
Journal of Oceanology and Limnology2018年4期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Effects of seawater acidi fication on the early development of sea urchin Glyptocidaris crenularis*
- Dietary effects of A zolla pinnata combined with exogenous digestive enzyme (Digestin?) on growth and nutrients utilization of freshwater prawn, Macrobrachium rosenbergii(de Man 1879)
- Preliminarily study on the maximum handling size, prey size and species selectivity of growth hormone transgenic and non-transgenic common carp Cyprinus carpio when foraging on gastropods*
- Hydrodynamic characteristics of the double-winged otter board in the deep waters of the Mauritanian Sea*
- De novo transcriptome sequencing reveals candidate genes involved in orange shell coloration of bay scallop Argopecten irradians*
