Anti-inflammatory and antinociceptive activities of Rhipicephalus microplus saliva
DF. Buccini, ?A. Nunes, GGO. Silva, ON. Silva, OL. Franco,3,4, SE. Moreno?
1Programa de Pós-Gradua??o em Biotecnologia e Biodiversidade, Universidade Federal do Mato Grosso do Sul, Campo Grande, MS, Brazil
2S-Inova Biotech Programa de Pós-Gradua??o em Biotecnologia, Universidade Católica Dom Bosco, Campo Grande, MS, Brazil
3Programa de Pós-Gradua??o em Patologia Molecular, Universidade de Brasília, Brasília, DF, Brazil
4Centro de Análises Prote?micas e Bioquímicas, Programa de Pós-Gradua??o em Ciências Gen?micas e Biotecnologia, Universidade Católica de Brasília, Brasília, DF, Brazil
1. Introduction
Inflammation is a process involved in protecting the host against injury and infection. It is characterized by redness, swelling, pain as well as tissues and organs dysfunction. Many diseases, including typeⅡ diabetes, cancer, cardiovascular disease and neurodegeneration have recently been considered as possessing a strong inflammatory component[1].
Molecular processes leading to inflammation are generally related to the activities of cells involved in restoring tissue composition and activity.When cells are exposed to immunological stimulants, proinflammatory cells such as macrophages, neutrophils, monocytes or other host cells may be recruited and initiate to synthesize several molecular mediators that start the inflammation process. Among several biological markers produced in the inflammatory process, the most outstanding are IL-1β, IL-6; IL-8;tumor necrosis factor (TNF-α); nuclear factor-κβ, intercellular adhesion molecule-1, induced cyclooxygenase-2, prostaglandin E2; lipoxygenase (5-LOX); and inducible nitric oxide synthase which stimulates the production of nitric oxide NO[2].
Inflammation is usually associated with pain as an evolution due to secretion of mediators such as bradykinin, ecoisanoids, histamine,proinflammatory cytokines (TNF, IL-1β and IFN-γ), and chemokines[3,4]. Pain is one of the first symptoms that appear in the process of inflammation; it causes intense suffering and reduces the quality of life. Currently, the therapeutic drugs in pain combat is not totally efficient in terms of efficacy, tolerability and toxicity[4]. Despite the drugs currently available, there is a lack of potential analgesics and anti-inflammatories, as a therapeutic resource for chronic pain[4].Current tools for the treatment of inflammation depend heavily on corticosteroids and AINES, which have several side effects, including osteoporosis, decreased wound healing, ulcerogenic effects and stroke[5].Thus, there is a strong interest in identifying new anti-inflammatory drugs to increase or replace current therapies[5]. Bioprospecting can be an excellent tool to identify and validate new anti-inflammatory targets[6], and ectoparasites could be model organisms to identify new molecules with biotechnological potential[7,8].
It is already known that tick saliva could be rich in molecules that have anti-inflammatory mechanisms, since such parasites inhibit the host’s inflammatory response, which obtain food successfully during a long period by remaining fixed in cattle. These mechanisms include the inhibition of proteases involved in the inflammatory response,bradykinin hydrolysis by enzymes, binding of salivary proteins to serotonin, leukotriene and histamine[9].
The large variety of species of ticks already catalogued of molecules present in the saliva of these hematophages make the saliva of these parasites an excellent source for the study of possible pharmacologically active biomolecules. The components present inRhipicephalus microplus(R. microplus) saliva inhibit the host inflammatory response,allowing the parasite to obtain food during long periods[10,11].The impressive ability of tick saliva to modulate host processes demonstrates how we can use these molecules to our advantage.Despite the wide variety of molecules present in saliva that have already been identified, no study has examined the effectsin vivoof saliva as a potential anti-inflammatory and analgesic. Therefore, this study aims to evaluate the antinociceptive and anti-inflammatory potentials as well as thein vivotoxic effects ofR. microplussaliva.
2. Materials and methods
2.1. R. microplus saliva collection
Larvae ofR. micropluswere acquired from engorged females collected in the field (20o23’16.1’’ S 54o36’25.5’’O) and incubated in biochemical oxygen demand (411-D, Nova ética, Brazil) until egg laying.A bovine (Bos taurus) was kept in a closed bay, with a cement floor and free of natural infestations. The bovine was infested with larvae.After 21 d, when the engorged tick females fell, they were collected to obtain the saliva. The experiments were approved by the Dom Bosco Catholic University Committee for Ethics in Animal Experimentation under protocol (No°005/2012). Engorged tick females were washed in sodium hypochlorite 1% and dried gauze. They were injected with 10-20 μL pilocarpine solution 0.2% (Alergan, Brazil) with the aid of a needle measuring 12.7 mm × 0.33 mm (Descarpack). Salivation started after about 10 min and continued for up to 2 h. The saliva was collected using a micropipette (Gilson) and kept on ice. The total saliva obtained was lyophilized (Scientific, VIRTUS, Brazil) and maintained at -80 ℃. The total protein concentration in the saliva was determined by means of the Bradford method[12].
2.2. Experimental animals
Adult male albino mice (BALB-c) weighing 22-24 g were used in this study. The animals were obtained from the Central Laboratory for animals of the Dom Bosco Catholic University, Campo Grande, MS,Brazil. The animals were housed in standard sanitized polypropylene cages containing paddy husk as bedding and maintained under controlled conditions of temperature (22 ± 2) ℃ and light-dark cycles(12 h) with free access to standard pellet diet (Nuvilab? CR-1, Nuvital,PR, Brazil) and waterad libitum. The experiments with mice were approved by the Dom Bosco Catholic University Committee for Ethics in Animal Experimentation under protocol (No 005/2012).
2.3. Hemolytic activity
The assay for determining hemolytic activity was performed according to the methods of Parket al[13] with minor modifications. Murine erythrocytes were collected from BALB-C mice, washed with 0.9%saline and centrifuged at 580gat 4 ℃ for 2 min. For the experiment,8% blood was used, and distributed in 96-well plate wells, with the addition of different concentrations of crude saliva (300, 200, 100, 50 and 25 μg/mL). The control received Triton X-100 (Vetec) (5 μL diluted in 95 μL of Mili-Q water) and saline 0.9%, and all groups were performed in triplicate. The reading was performed at 540 nm in a microplate reader (Thermo Scientific Multiskan Britain).
2.4. Cell viability assay
Neutrophils were obtained from the mice peritoneal cavity, 6 h after intraperitoneal injection of 0.5 mg/animal of carrageenan (Sigma,USA). Animals were euthanized and 3 mL of RPMI medium was injected into the peritoneal cavity (in aseptic conditions); subsequently,exudates were collected and centrifuged at 970gat 10 ℃ for 10 min.Cells were washed twice in sterile PBS and re-suspended in incomplete RPMI medium. Then the cells were counted in a Neubauer chamber.Cell viability was determined by the Tripan Blue exclusion method,and considered adequate when greater than 90%. Cell viability was determined by using the salt dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT-Sigma, USA) colorimetric assay[14,15]. Cell suspension (2×105cells/mL) was plated in a 96-well plate (TPP, Switzerland), incubated with RPMI medium containing 2.5,5, 10, 20 and 40 μg/mL of saliva, and kept in 5% CO2, 37 ℃. Cell viability was evaluated after 24, 48 and 72 h incubation with saliva. After that, all the media were aspirated, and 10 μL of the MTT solution(5 mg/mL) was added to all plate wells and incubated for 4 h under light. After the incubation period, formazan crystals were solubilized by adding the solubilizing solution (isopropyl alcohol and hydrochloric acid-Vetec). The plates were then shaken lightly at room temperature for 5-10 min, so that all the formazan were solubilized and then read at 540 nm on a microplate reader (Thermo Scientific Multiskan Britain).Percentages of cell viability were calculated in relation to untreated cell control.
2.5. Evaluation of neutrophil migration (NM)
This assay was performed with crude saliva (10, 15, and 20 mg/kg) 6 h after the administration of the inflammatory stimulus; the groups of animals had been pre-treated or not with compounds with potential anti inflammatory effect. The mice were euthanized in a CO2chamber before starting the experiments of evaluation of NM into the peritoneal cavity.The peritoneal cavity was washed with 3 mL of saline phosphate buffer(Sigma/USA), ethylenediamine tetraacetic acid (Dinamica/Brazil) and PBS/EDTA (5%). Total and differential counts of the cells present were made from the exudate. The total count was performed with the aid of the Neubauer chamber, and the cells were expressed as number of cells×106/mL[16]. The differential counts were performed on slides of the exudate cell smear, stained with (NewProv), and were examined by optical microscope (Eclipse Microscope, Nikon, Japan). One hundred cells were counted per slide, differentiating three cell types: neutrophils,eosinophils and mononuclear cells. The amount of each cell type present in the peritoneal cavity was calculated as the percentage of those cells in counted smears and the total number of cells obtained in the total count.The results were expressed as number of neutrophils (×105/mL)[16,17].
2.6. Myeloperoxidase activity
In order to confirm the results obtained in the NM assay, the myeloperoxidase (MPO) activity of neutrophils was measured as described by ALVES-FILHOet al[18]. The peritoneal lavage of the animals treated with saline, carrageenan and 15 mg/kg saliva was subjected to MPO measurement. For this, a solution using tetramethylbenzidine (1.6 mM) and H2O2(0.5 mM) (Thermo Scientific Multiskan Britain) was added to 100 μL of the peritoneal lavage suspension of each animal group, and the change in absorbance at 450 nm was measured. The results were compared to a standard curve performed according to the aforementioned author[18], and expressed as the number of neutrophils (×105/mL).
2.7. Evaluation of analgesic activity
2.7.1. Test of abdominal contortions induced by acetic acid
The analgesic effect was evaluated according to Kosteret al[19]. The animals (BALB-Cn= 5) were pretreated with saliva (15 mg/kg) 15 min prior to pain stimulus with 0.8% acetic acid (10 mL/kgi.p.). The control group received acetylsalicylic acid (Sigma, USA) (AAS – 100 mg/kg). The animals were checked for 30 min after the stimulus and observed for contortions number.
2.7.2. Formalin test
The formalin test evaluated the analgesic activity according to the number of licks and/or bites in the paw. BALB-C mice (n= 5) were pretreated with 15 mg/kg of crude saliva, 15 min before the stimulus with 2.5% formalin (30 μL s.p.). Controls received AAS (100 mg/kg). The animals were evaluated in two phases: 1st stage in the first 5 min, taking a 10 min break, and the 2nd phase for the remaining 15 min, observing the number of licks and/or bites in the injected paw[20].
2.8. Statistical analysis
The results were expressed as mean ± standard error of the mean(SEM). Significant differences among groups were performed by ANOVA followed by Bonferroni correlation.P< 0.05 was considered to be statistically significant difference. Graphpad Prism? Software(v 5.0; Graph pad Software, USA) was used to perform the statistical analysis.
3. Results
Figure 1 demonstrated that saliva had no hemolytic activity in all concentrations tested when compared to the positive control (Triton-X 100).
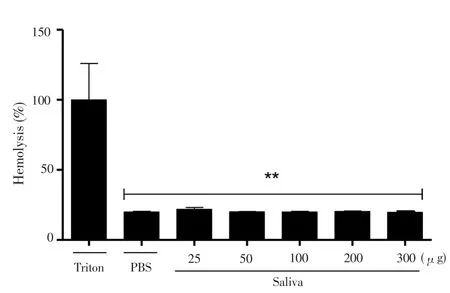
Figure 1. Evaluation of hemolytic activity of raw saliva of R. microplus ticks on murine erythrocytes.
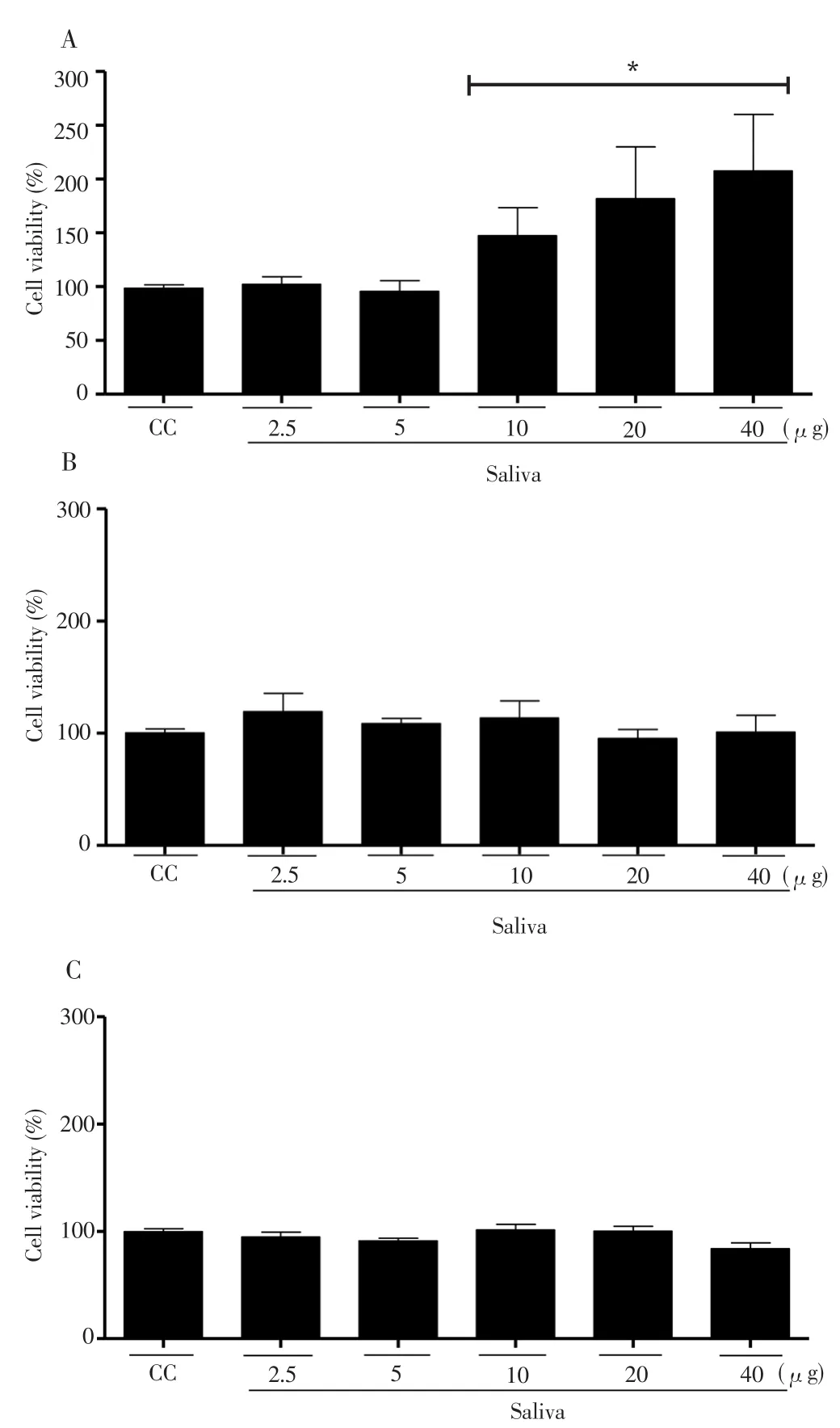
Figure 2. Determination of neutrophil viability (CC) treated with saliva of R.microplus tick for 24 (A); 48 (B) and 72 (C) h.
Also, in order to evaluate cell viability of neutrophils exposed toR. microplussaliva, the MTT assay was performed. This assay demonstrated that after 24 h of incubation crude saliva was not able to reduce neutrophil (CC) viability at any of the concentrations evaluated(Figure 2). A stimulation of the mitochondrial metabolism was observed at concentrations of 10 μg (51%), 20 μg (86%) and 40 μg(112%) when compared to untreated cells (Figure 2A). After incubation for 48 and 72 h, the saliva was not able to reduce neutrophil viability in any of the concentrations evaluated (Figure 2B and 2C). These results demonstrated thatR. micropluscrude saliva did not present a cytotoxic effect capable of decreasing neutrophil viability. The data suggested that NM decrease into mice peritoneal cavity was not due to the cytotoxic saliva effect over these cells (Figure 3A).
When we evaluated theR. microplussaliva anti-inflammatory effects over NM to the peritoneal mice cavity, saliva was capable of inhibiting the NM at doses of 15 and 20 mg/kg when compared to the untreated group (carrageenan) (Figure 3A). In order to confirm these data, we evaluated the MPO enzyme as an indicator of neutrophil number. The MPO assay was in agreement with the NM inhibition demonstrated before (Figure 3B). MPO could be used as a local inflammation marker, correlating the enzyme amount to the number of neutrophils present in the tissue. Corroborating with NM data, the group treated with carrageenan presented a higher MPO amount when compared to the groups treated with saliva at the concentration of 15 mg/kg.
Because pain was a secondary process usually associated with inflammation, we also examined the analgesicR. microplussaliva potential. Figure 4A showed that 15 mg/kg of saliva of theR. microplustick was able to reduce the number of abdominal contortions. The inhibition was 61.62% in the positive control (AAS, 100 mg/kg) and 69.96% in the saliva treated group, when compared to the untreated control; suggesting the remarkable analgesic action of saliva. It was worth noting that the saliva was administered in a concentration almost seven times lower than the AAS and yet it presented a marked analgesic effect.
Figure 4B demonstrated the evaluation of the analgesic effect ofR.microplussaliva. In this test, two distinct periods of licking activity could be identified, an early phase lasting the first 5 min and a late phase lasting 15 min. The results demonstrated that none of the concentrations used were able to reduce nociception at stage 1,suggesting the absence of central analgesic effects of saliva. However,in phase 2, 15 mg/kg of saliva was able to reduce the nociceptive response triggered by formalin, by 84.41%, and the ASS control was able to inhibit by 76.62% when compared to the untreated group (formalin).Thus, the results showed the analgesic effects ofR. microplussaliva.
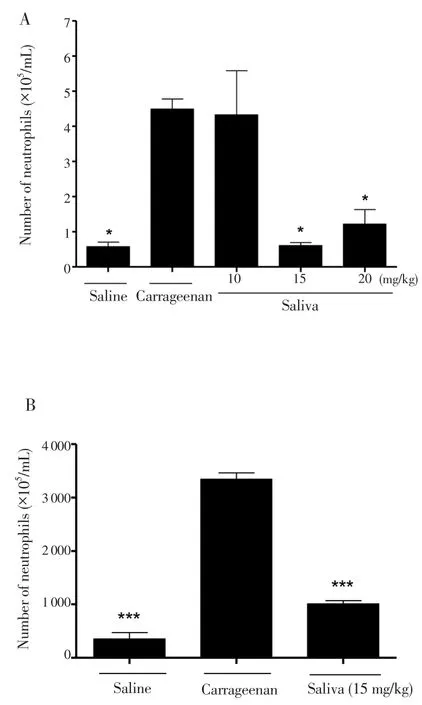
Figure 3. Evaluation of the effect of R. microplus tick saliva on the migration of neutrophils into the peritoneal cavity of mice (A); MPO dosage (B).
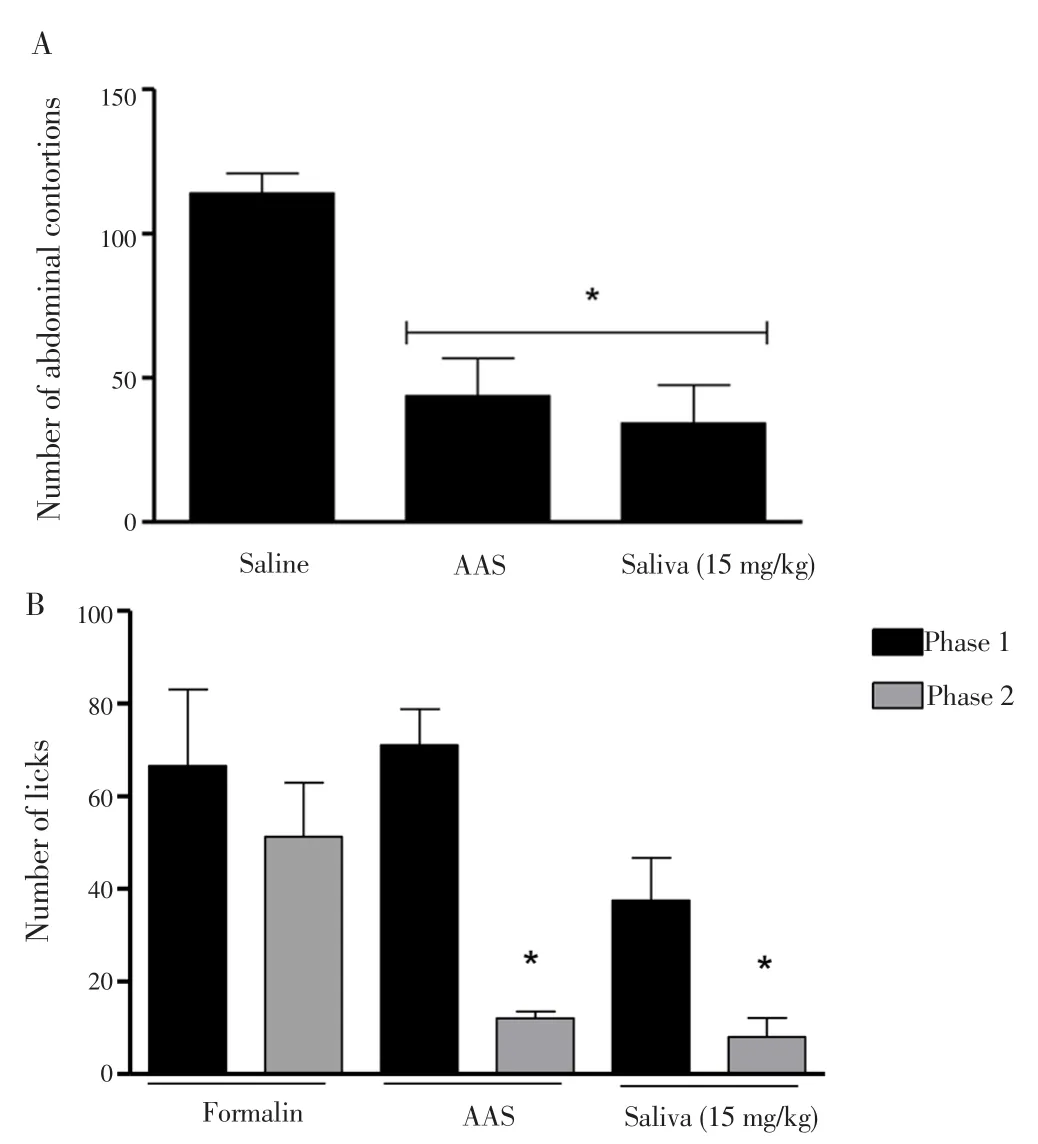
Figure 4. Evaluation of the antinociceptive effect of the saliva of the R.microplus tick by means of the abdominal writhing test (A) and formalininduced lithium test (B) in mice.
4. Discussion
R. microplusis one of the bovine parasites of major economic importance, affecting livestock production worldwide and further causing weight loss, reduced milk production, leather quality losses, toxicoses,skin lesions that favor the occurrence of anemia and transmission of pathogens[21]. For the success of parasitism, hematophagous animals need to block host defenses by producing substances such as potent pharmacologic molecules with vasoactive, antihemostatic, antiinflammatory, and immunomodulatory action[10,11] that will be injected together with saliva. The characterization of these substances has revealed a wide variety of compounds with diverse functions, and they are potential sources of pharmacological compounds. Despite many studies, the composition of saliva is not fully understood, nor are its pharmacological effects[9,11].
According to several studies found in the literature, tick saliva has a number of molecules with varied activities, such as anti-inflammatory and immunosuppressive activity[22], this activity may be due to the lipocalin complex which is determined by the presence of the prostaglandins[23,24], apirases[25] and the lipocalin binding proteins[26].According to Ramachandra and Wikel[27],Dermacentor andersonitick saliva was able to decrease the production of IL-1 and TNF-α by macrophages, IL-2, IFN-γ and by T lymphocytes[27]. Tianet al[28]found in anin vitroexperiment that Amblyomma variegatum saliva inhibited the production of TNF-α, IL-1, CXCL8 and IFN-γ[28].Oliveiraet al[29], demonstrated that the saliva of the tickRhipicephalus sanguineuswas able to inhibit the production of proinflammatory cytokines IL-12 and TNF-α and stimulate IL-10 production by murine dendritic cells,in vitro[29].
Studies by Tirloneet al[11] have demonstrated thatR. microplussaliva can present a variety of lipocalins. These substances are involved with the immunomodulatory activity of tick saliva[30]. As discussed by Kovar[31], some lipocalins have been characterized as histamine binding proteins, exhibiting anti-inflammatory action[31,32], and presenting as a functional characteristic the ability to act as binders of a large variety of biomolecules, such as nucleotides, bioactive amines (histamine and serotonin), anti-coagulant agents, thromboxanes, leukotrienes,complement system inhibitors and immunoglobulins[22,33].
Tirloneet al[34,35] also showed that most of the serpinins play crucial roles in managing endopeptidases involved in blood coagulation,fibrinolysis, inflammation and complementary activation[34,35]. It is assumed that the serpin secreted by ticks influences the homeostatic balance of the host to facilitate parasitism[36]. The potential effects of these proteins on host systems have been supported by several studies,showing hematophagous parasite serpins acting as anti-coagulant and anti-inflammatory agents which are essential for successful ectoparasite feeding[30].
Ribeiro[37] performedin vitrotests with the saliva of theIxodes damminitick, which belongs to the same family asR. microplus[37].Tick saliva contains immunomodulatory compounds that prevent host inflammatory reactions from interfering with the feeding process,creating an environment that allows blood flow without inducing pain[38]. Therefore,R. microplussaliva does have a potential antiinflammatory activity.
According to the literature, histamine and serotonin secreted by the host at the tick feeding site induce cutaneous inflammation, and ticks must overcome this host response to be successful in feeding[39]. It is suggested thatR. micropluspossesses a blocking agent or neutralizing for histamine and serotonin by decreasing or inhibiting the host’s local immune response[40,41]. The high lipocalin content inR.microplussaliva may also be related to the level required to block the concentration of prostaglandins that accumulate at the feeding site[34,42]. Ticks have been selected during the coevolution process,according to their capacity to disable the host defense responses, with sophisticated mechanisms[10,23].
In this work it was demonstrated that theR. microplussaliva showed significantin vivoanti-inflammatory and antinociceptive activities. Such activities could be deemed the key elements for its successful parasitic life-style. However, we can take advantage of its biotechnological potential to develop novel anti-inflammatory and analgesic drugs bioinspired by saliva.
Despite the large number of such drugs available, their side effects and the inefficacy of some drugs under some conditions require a continuous search for new drugs. The data presented here support the development of further studies to elucidate the active principles ofR. microplussaliva and their respective mechanisms of action, and in future to develop novel anti-inflammatory and analgesic drugs.
Conflict of interest statement
The authors declare that there is no conflict of interest,
Acknowledgments
We thank Souza JVF and Barboza AP for their help in obtaining the saliva and in thein vitroandin vivotests.
The authors are grateful to FUNDECT (Foundation for Support for the Development of Education, Science and Technology of the State of Mato Grosso do Sul), Coordination for the Improvement of Higher Level Education Personnel (CAPES), National Council for Scientific and Technological Development (CNPq) and Foundation for Research Support of the Federal District (FAPDF) for financial support.
[1] Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs.Oncotarget2018;9(6): 7204-7218.
[2] Levine TB, Levine AB. Metabolic syndrome and cardiovascular disease.Am Heart J2012; 142(6): 1108-1116.
[3] Choi JH, Cha DS, Jeon H. Anti-inflammatory and anti-nociceptive properties ofPrunus padus.J Ethnopharmacol2012; 144(2): 379-386.
[4] Oliveira Júnior JO, Portella Junior CSA, Cohen CP. Inflammatory mediators of neuropathic pain.Revista Dor2016; 17(Suppl 1): S35-42.
[5] Tamrat Y, Nedi T, Assefa S, Teklehaymanot T, Shibeshi W. Antiinflammatory and analgesic activities of solvent fractions of the leaves ofMoringa stenopetalaBak. (Moringaceae) in mice models.BMC Complement Altern Med2017; 17(1): 473.
[6] Srilekha V, Krishna G, Seshasrinivas V, Charya MAS. Antibacterial and antiinflammatory activities of marineBrevibacteriumsp.Res Pharm Sci2017;12(4): 283-289.
[7] Vizioli J, Bulet P, Hoffmann JA, Kafatos FC, Muller HM, Dimopoulos G. Gambicin: A novel immune responsive antimicrobial peptide from the malaria vectorAnopheles gambiae.Proc Natl Acad Sci U S A2001; 98(22):12630-12635.
[8] Porto WF, Fensterseifer GM, Franco OL.In silicoidentification, structural characterization, and phylogenetic analysis of MdesDEF-2: A novel defensin from the Hessian fly,Mayetiola destructor.J Mol Model2014; 20(7): 2339.
[9] Esteves E, Maruyama SR, Kawahara R, Fujita A, Martins LA, Righi AA, et al. Analysis of the salivary gland transcriptome of unfed and partially fedAmblyomma sculptumticks and descriptive proteome of the saliva.Front Cell Infect Microbiol2017; 7: 476.
[10] Ribeiro JM. Role of saliva in blood-feeding by arthropods.AnnuRev Entomol1987; 32: 463-478.
[11] Tirloni L, Kim TK, Coutinho ML, Ali A, Seixas A, Termignoni C, et al. The putative role ofRhipicephalus microplussalivary serpins in the tick-host relationship.Insect Biochem Mol Biol2016; 71: 12-28.
[12] Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal Biochem1976; 72: 248-254.
[13] Park Y, Kim HN, Park SN, Jang SH, Choi CH, Lim HT, et al. Design of novel analogues with potent antibiotic activity based on the antimicrobial peptide, HP(2-9)-ME(1-12).Biotechnol Lett2004; 26(6): 493-498.
[14] Takeuchi M, Kobata A. Structures and functional roles of the sugar chains of human erythropoietins.Glycobiology1991; 1(4): 337-346.
[15] Sieuwerts AM, Klijn JG, Peters HA, Foekens JA. The MTT tetrazolium salt assay scrutinized: How to use this assay reliably to measure metabolic activity of cell culturesin vitrofor the assessment of growth characteristics,IC50-values and cell survival.Eur J Clin Chem Clin Biochem1995; 33(11):813-823.
[16] Moreno SE, Alves-Filho JC, Alfaya TM, da Silva JS, Ferreira SH, Liew FY.IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture.J Immunol2006; 177(5): 3218-3224.
[17] Machado RJ, Monteiro NK, Migliolo L, Silva ON, Pinto MF, Oliveira AS, et al. Characterization and pharmacological properties of a novel multifunctional Kunitz inhibitor fromErythrina velutinaseeds.PLoS One2013; 8(5): e63571.
[18] Alves-Filho JC, de Freitas A, Russo M, Cunha FQ. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis.Crit Care Med2006; 34(2): 461-470.
[19] Koster R, Anderson M, De-Beer EJ. Acetic acid for analgesic screening.Fed Pro1959; 18(1): 412-418.
[20] Rosland JH, Hunskaar S, Hole K. The effect of diazepam on nociception in mice.Pharmacol Toxicol1987; 61(2): 111-115.
[21] Figueiredo A, Fantatto RR, Agnolon IC, Lopes LG, de Oliveira PR, Mathias MIC, et al.In vivostudy of a homeopathic medicine againstRhipicephalus(Boophilus)microplusin dairy cow.Rev Bras Farmacogn2018. Doi:org/10.1016/j.bjp.2018.01.008.
[22] Anatriello E, Oliveira CJF, Oliveira NB, Fisch A, Milanezi CM, Silva JS, et al. Interaction between saliva’s adenosine and tick parasitism: Effects on feeding and reproduction.Parasite Vector2017; 10(1): 326.
[23] Ribeiro JM, Makoul GT, Levine J, Robinson DR, Spielman A.Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick,Ixodes dammini.J Exp Med1985; 161(2): 332-344.
[24] Ribeiro JM, Makoul GT, Robinson DR.Ixodes dammini: Evidence for salivary prostacyclin secretion.J Parasitol1988; 74(6): 1068-1069.
[25] Mans BJ, Gaspar AR, Louw AI, Neitz AW. Apyrase activity and platelet aggregation inhibitors in the tickOrnithodoros savignyi(Acari: Argasidae).Exp Appl Acarol1998; 22(6): 353-366.
[26] Paesen GC, Adams PL, Harlos K, Nuttall PA, Stuart DI. Tick histaminebinding proteins: Isolation, cloning, and three-dimensional structure.Mol Cell1999; 3(5): 661-671.
[27] Ramachandra RN, Wikel SK. Modulation of host-immune responses by ticks (Acari: Ixodidae): Effect of salivary gland extracts on host macrophages and lymphocyte cytokine production.J Med Entomol1992; 29(5): 818-826.
[28] Tian Y, Chen W, Mo G, Chen R, Fang M, Yedid G, et al. An immunosuppressant peptide from the hard tickAmblyomma variegatum.Toxins (Basel)2016; 8(5): 133.
[29] Oliveira CJ, Cavassani KA, More DD, Garlet GP, Aliberti JC, Silva JS, et al.Tick saliva inhibits the chemotactic function of MIP-1alpha and selectively impairs chemotaxis of immature dendritic cells by down-regulating cellsurface CCR5.Int J Parasitol2008; 38(6): 705-716.
[30] Blisnick AA, Foulon T, Bonnet SI. Serine protease inhibitors in ticks: An overview of their role in tick biology and tick-borne pathogen transmission.Front Cell Infect Microbiol2017; 7: 199.
[31] Kovar L. Tick saliva in anti-tick immunity and pathogen transmission.Folia Microbiol (Praha)2004; 49(3): 327-336.
[32] Paesen GC, Adams PL, Nuttall PA, Stuart DL. Tick histamine-binding proteins: Lipocalins with a second binding cavity.Biochim Biophys Acta2000; 1482(1-2): 92-101.
[33] Schlehuber S, Skerra A. Lipocalins in drug discovery: From natural ligandbinding proteins to “anticalins”.Drug Discov Today2005; 10(1): 23-33.
[34] Tirloni L, Reck J, Terra RM, Martins JR, Mulenga A, Sherman NE, et al.Proteomic analysis of cattle tickRhipicephalus (Boophilus) microplussaliva:A comparison between partially and fully engorged females.PLoS One2014;9(4): e94831.
[35] Tirloni E, Bernardi C, Colombo F, Stella S. Microbiological shelf life at different temperatures and fate ofListeria monocytogenesandEscherichia coliinoculated in unflavored and strawberry yogurts.J Dairy Sci2015;98(7): 4318-4327.
[36] Syrovets T, Tippler B, Rieks M, Simmet T. Plasmin is a potent and specific chemoattractant for human peripheral monocytes acting via a cyclic guanosine monophosphate-dependent pathway.Blood1997; 89(12): 4574-4583.
[37] Ribeiro JM. Blood-feeding arthropods: Live syringes or invertebrate pharmacologists?Infect Agents Dis1995; 4(3): 143-152.
[38] Rodrigues V, Fernandez B, Vercoutere A, Chamayou L, Andersen A, Vigy O. Immunomodulatory effects ofAmblyomma variegatumsaliva on bovine cells: Characterization of cellular responses and identification of molecular determinants.Front Cell Infect Microbiol2017; 7: 521.
[39] Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding:Sialome and post-sialome perspectives.Annu Rev Entomol2003; 48: 73-88.
[40] Kemp DH, Bourne A.Boophilus microplus: The effect of histamine on the attachment of cattle-tick larvae--studiesin vivoandin vitro.Parasitology1980; 80(3): 487-496.
[41] Wikel SK. Host immunity to ticks.Annu Rev Entomol1996; 41: 1-22.
[42] Tatchell RJ, Bennett GF.Boophilus microplus: Antihistaminic and tranquillizing drugs and cattle resistance.Exp Parasitol1969; 26(3): 369-377.
 Asian Pacific Journal of Tropical Biomedicine2018年4期
Asian Pacific Journal of Tropical Biomedicine2018年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Proteomic approach in human health and disease: Preventive and cure studies
- Efficacies of four plant essential oils as larvicide, pupicide and oviposition deterrent agents against dengue fever mosquito, Aedes aegypti Linn. (Diptera: Culicidae)
- Phenological stage effect on phenolic composition and repellent potential of Mentha pulegium against Tribolium castaneum and Lasioderma serricorne
- Physicochemical properties, antioxidant and anti-inflammatory activities of coumarin-carbonodithioate hybrids
- Comparison of antioxidant capacity and α-glucosidase inhibitory activity between bitter melon (Momordica charanti) fruit and leaf extract
