Effect of heat flux and inlet temperature on the fouling characteristics of nanoparticles☆
Jingtao Wang,Zhiming Xu*,Zhimin Han,Yu Zhao
College of Energy and Power Engineering,Northeast Electric Power University,Jilin 132012,China
1.Introduction
Fouling is the deposition of undesirable material on a heat transfer surface,which may diminish heat transfer performance and increase the pressure drop in heat exchangers.Due to fouling,the costs of operation and maintenance increase significantly.Particulate fouling is defined as the deposition of fine particles on a heat transfer surface and is considered one of the most common fouling occurrences when cooling water,and the formation process and mechanism of particulate fouling have been well studied by many researchers.Previous experimental studies in particulate fouling have focused on the effect of chemical composition on the fouling rate during isothermal laminar[1]and turbulent flows[2].Karabelas et al.[3]experimentally studied particles with a mean size of 5 μm in plate heatexchangers.The experimental results suggested that the flow velocity and the flow passage geometry significantly affect fouling resistance.Turner et al.[4]experimentally studied the fouling characteristics of colloidal magnetite particles at an alkaline pH under single-phase flow and flow boiling.Basset et al.[5]used chemical and radiotracing techniques to investigate the deposition of magnetite particles from suspension in water at90°C,and the results showed that mechanisms based on diffusion and thermophoresis controlled deposition,while removal was found to negligible with non-boiling conditions.Henry et al.[6]found that particulate fouling generally arises from the continuous deposition of colloidal particles on initially clean surfaces,and this process can even lead to a complete blockage of a fluid cross-section.They also proposed numerical models to describe later stages in the fouling process,and they developed a new Lagrangian stochastic approach to understand clogging in industrial case.Grandgeorge et al.[7]investigated the liquid-phase particulate fouling in stainless steel corrugated-plate heat exchangers,with deionized water containing TiO2particles being chosen as the foulant fluid.The results showed that there is an obvious asymptotic value of fouling resistance,and the influence of physic-chemical conditions on fouling behavior.Zhang et al.[8]investigated water-side fouling inside four corrugated-plate heat exchangers by experimental and theoretical methods.The experiments were primarily focused on the effects of concentration and average velocity,and observing the microscopic fouling structures by scanning electron microscope(SEM).Yiantsions et al.[9]experimentally studied micron-sized particle deposition on a flat surface,aimed at delineating the effects of hydrodynamic and physicochemical interactions on particle transport and attachment mechanisms.Particle stickiness significantly affected the process of fouling.Lipp et al.[10]conducted a membrane test with both artificial and natural water,the results showed that,the membrane permeability decreased over time during the filtration experiments,indicating that the nanoparticles blocked the membrane pores.Under these the experimental conditions,the concentration of nanoparticles seemed to have no obvious in fluence on membrane fouling.
There are many factors that affect fouling formation on heat exchangers,with temperature being one of the most influential factors.When the temperature of the heat transfer surface exceeds the saturation temperature,the flow state transits to the subcooled- flow boiling regime.Fouling on the heat transfer surfaces during single-phase flow and subcooled- flow boiling is an important challenge in thermal engineering and process industries.Hasan et al.[11]experimentally studied crystallization fouling in a channel for a cross flow of hot saturated sodium sulfate(Na2SO4)solution over a pipe of cold water.The results showed that the temperature of the salt solution significantly affected the fouling rate.The fouling rate of crystallization fouling decreases with the increase of the pipe surface temperature.P??kk?nen et al.[12]investigated the surface crystallization of CaCO3and its effect on the fouling rate for a heated surface.The results showed that the crystallization fouling rate can be controlled by the wall temperature,and it was noted that the flow velocity affects the fouling rate especially at high wall temperature.Al-Otaibi et al.[13]investigated the fouling characteristics of coated carbon steel and titanium tubes.The experiments were carried out in various brackish waters under different velocities and different tube surface temperatures.The results showed that the asymptotic value of fouling resistance increases with the increase of tube wall temperature,and increasing the flow velocity could mitigate the formation of fouling.Abd-Elhady et al.[14]investigated the influence of gas-side temperature on particulate fouling layers in a gas cooler.Their findings showed that sintering significantly affects the particulate fouling rate in the gas cooler,explaining why the growth rate of particulate fouling decreases with gas-side temperature.Mwaba et al.[15]conducted experiments to investigate the rate of deposition at different positions.The results indicated that the growth of fouling layers increases increasing surface temperature and decreases with the increase of flow velocity.The rate of fouling growth as a function of position increases with the initial wall temperature distribution.Peyghambarzadeh et al.[16,17]experimentally studied the effect of operating conditions such as fluid velocity and bubble generation on fouling formation.The results revealed that achieving the boiling condition has an opposite influence on crystallization and particulate fouling.Furthermore,a model for predicting fouling resistance is applied for mixed salts and particulate fouling.Zhao et al.[18]experimentally studied the effect of temperature on structure and morphology of the crystallization fouling.The results revealed that the changes of temperature not only affect the type of crystallization fouling,but also the lattice parameter and particle size of the precipitated phase.
Ourprevious works[19–22]suggested that the operating conditions could affect fouling characteristics more or less,such as the temperature,the inlet velocity and the concentration.Temperature significantly affects crystallization fouling and microbial fouling.According to the literatures,most of the previous studies have focused on the effect of temperature on crystallization fouling and microbial fouling,with little attention to the effect of heat flux and inlet temperature on the particulate fouling.In this work,the γ-Al2O3/water suspension was chosen as the research object.The fouling characteristics of the γ-Al2O3/water suspension on a surface of stainless steel have been experimentally studied by changing the heat flux and the inlet temperature under single-phase flow and subcooled- flow boiling conditions,with mechanism analysis by a visible spectrophotometer.
2.Experiment
2.1.Experimental apparatus
A schematic of the experimental setup is shown in Fig.1,consisting of working fluid circulation,temperature control,and data acquisition systems.(1)The working fluid circulation system stores the working fluid in a constant temperature water tank with a volume of 27 L(THD-0530-L),and the working fluid flows into the loop through isolated pipes and where it is continuously circulated by a circulating water pump.A mass flow meter is installed at the inlet of the loop(manufactured by Beijing Sincerity Automatic Equipment Co.).The working fluid is heated in the annular loop,which employs heating rods operating with a voltage rating of 110 V.This heating is supplied by a DC power supply(manufactured by Shanghai GOODENG Electric Co.)with a voltage range from 0 to 120 V,a current range from 0 to 30 A,and a maximum power of 3600 W.(2)The temperature control system draws heat away from the circulating working fluid ensure it remains at a set temperature.The heated working fluid flow is primarily cooled before entering the experimental section,where it then flows into the constant temperature water tank.The control system of the constant temperature water tank can be operated over the temperature range from?5°C to 95°C.Its precision is±0.05°C for good control to achieve a constant inlet temperature.(3)The data acquisition system measures the wall temperatures through four Pt100 resistance thermometers with diameter of 2 mm,which have been installed close to the heat transfer surface.The thermocouple position is in the middle of the heating rod.The inlet and the outlet temperatures are also measured by Pt100 resistance thermometers with a diameter of 3 mm.All of the measurement signals are acquired by the data acquisition system(manufactured by ICP DAS Co.)and are stored and processed by an industrial-grade personal computer.The systemhas a high level of autonomy and can monitor the system for 24-h with online connectivity.
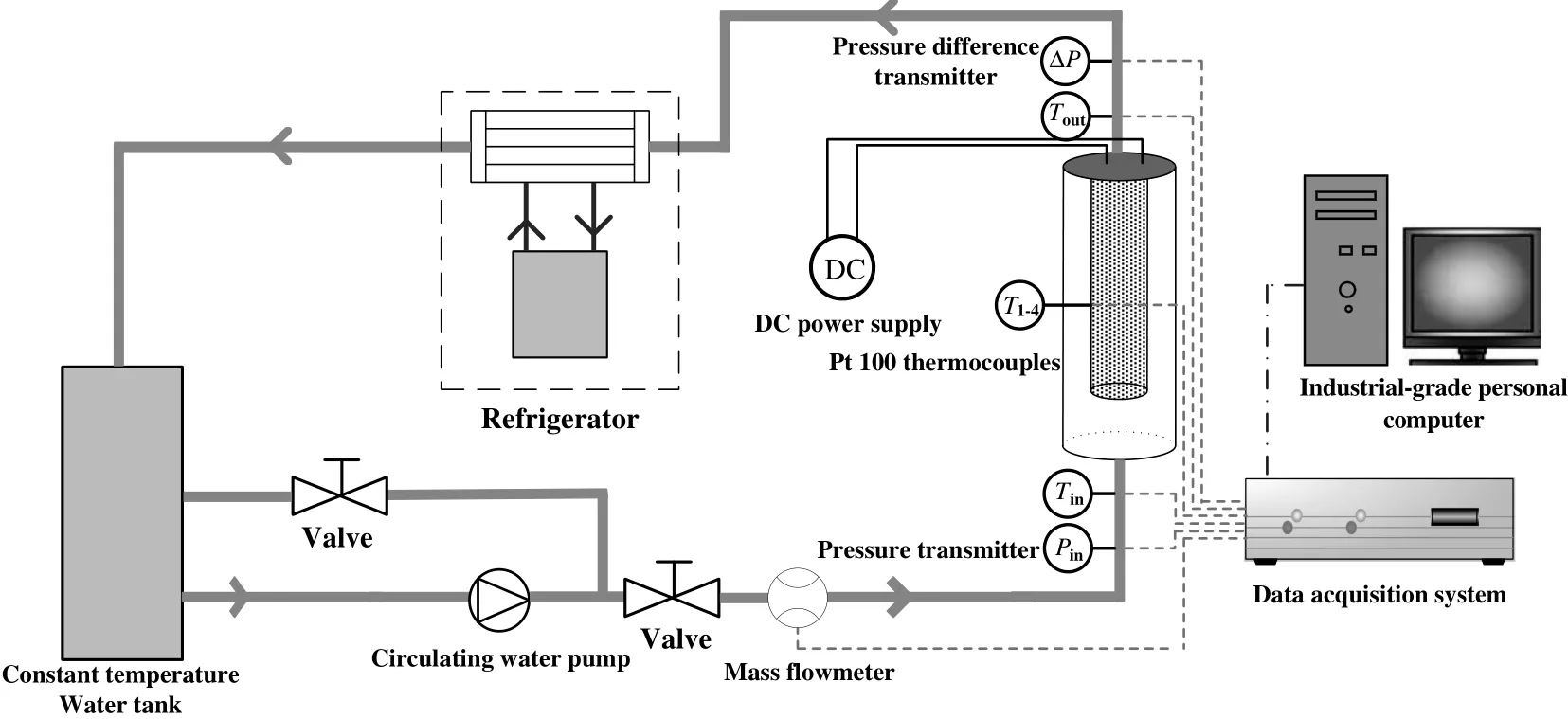
Fig.1.A schematic of the experimental setup.
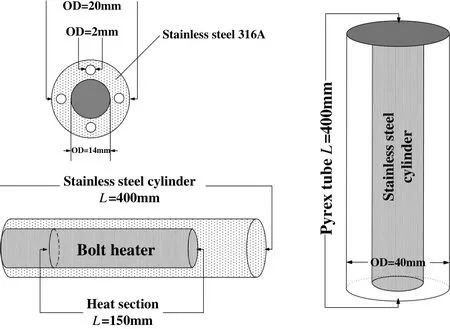
Fig.2.Mechanical drawings of the test section.
Mechanical drawings of experimental apparatus are depicted in Fig.2.The heat transfer cylinder is made of stainless steel type 316 L.The cylinder is heated by a DC bolt heater inserted into the middle of the cylinder,and the bolt heater(manufactured by HZLD Automation Equipment Co.)has a diameter of 14 mm,a length of 250 mm,and a heated length of 150 mm.The stainless steel cylinder is only uniformly and radially heated over the first 150 mm.The gap between the heat transfer cylinder and the quartz glass tube is the channel for the experimental working fluid,and the annular gap diameter is 20 mm.In order to avoid the influence of gravity on the fouling deposition process,the working fluid flows upward through the vertical experimental section.Both the boiling and the fouling processes can be observed through the transparent quartz glass tube.
2.2.Method of sedimentation experiment
In order to compare the variation of the experiment working fluid for different temperatures over time,a visible spectrophotometer(721-100,JINGHUA Instruments Co.)was used to measure the optical density value(OD)of the γ-Al2O3/water suspension in the sedimentation experiments.ODisthe abbreviation of opticaldensity,which represents the density of light absorbed by the detected object,and the OD value can indirectly reflect the settlement velocity of the particles in suspension.The OD value is based on the photoelectric turbidimetry principle,and the stability of γ-Al2O3/water suspension for different temperatures over time corresponds to differing OD values.The γ-Al2O3nanoparticles with diameters of 20 nm were added into the base fluid,deionized water here is considered as base fluid.
The experimental steps were as follows:the suspensions were prepared with deionized water under three different temperatures,and the concentrations were kept the same as those during the flowing experiments.An ultrasonic cleaner was used to disperse the suspension after a specific amount of time.Afterwards,the suspension was placed in the constant temperature water tank to control temperature.The interval for each measurement was 20 min,and the beaker should keep standing until the next measurement.
2.3.Data reduction
The heat flux of the heated surface can be calculated by the power of the heating rod and the heated surface

The temperature of the heated surface can be calculated by

The overall heat transfer coefficient can be calculated by

where Q is the heat transfer rate in W;q is the heat flux in kW·m-2;Twis the surface temperature in°C;Tthis the arithmetic mean temperature of the four thermometers readings around the rod circumference in°C;Tbis the mean temperature of the fluid in the experimental loop;s is the distance between the thermometers location and the heat transfer surface in mm;λwis the thermal conductivity of stainless steel in W·m?1·K?1.
The fouling resistance Rfis defined as:

where k0and k are the overall heat transfer coefficient of the clean surfaces and the fouled surfaces in W·m-2·K?1,respectively.
2.4.Analysis of uncertainty
The main sources of uncertainty for the heat transfer coefficient spur from the uncertainty of the direct measured values and the uncertainty of the wire resistance during data transmission.The root mean square value is selected in the error analysis[23],and it can be calculated by

where σiis the uncertainty of the measured value,andis the transfer coefficient for the uncertainty.
The uncertainty for the measurement devices used in this study are as follows:the maximum uncertainty for the wire wound resistors is 0.05%;the accuracy of Pt100 resistance thermometers is class A with an uncertainty of 0.15±0.005|t|;the maximum uncertainty for the mass flow meter is 0.1%and the measurement error of the outputcurrent and output voltage from the DC power supply is 0.1%.The uncertainties associated with the utilized sensors can be calculated from Eq.(5)with the results shown in Table 1.
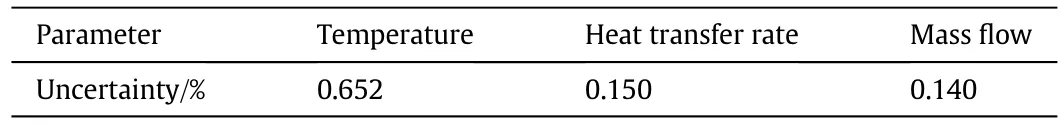
Table 1 Accuracy for sensors and parameters
The uncertainty of fouling resistance can be calculated by
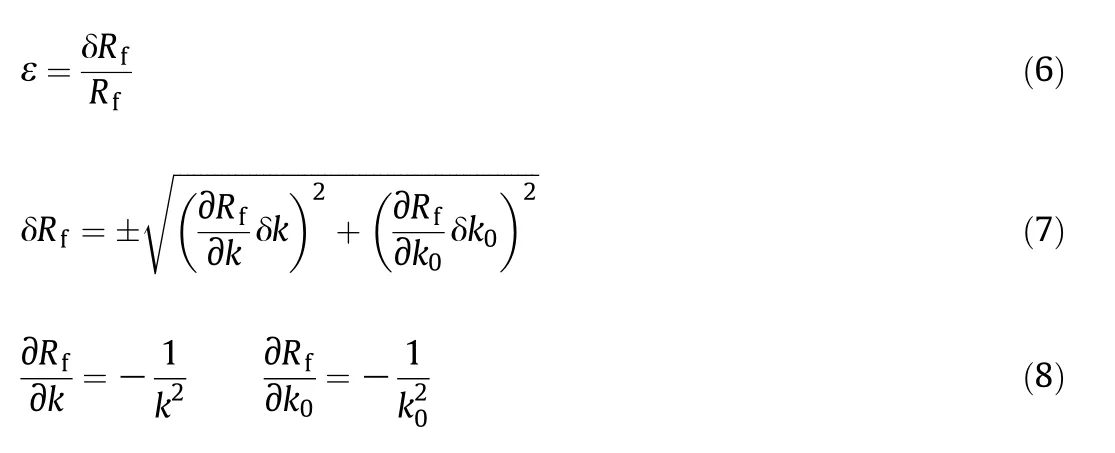
The uncertainty of fouling resistance calculated through Eq.(6)is 5.07%,and the uncertainty in the measured parameters is less than 1%.
3.Results and Discussions
3.1.The effect of heat flux on γ-Al2O3 particulate fouling characteristics
3.1.1.The effect of heat flux for single-phase flow
The experiments to investigate the effect of heat flux on γ-Al2O3particulate fouling characteristics were carried out with an inlet temperature of 60°C.The measured heat flux of four experiments(A,B,C and D)were 10.61 kW·m?2,21.22 kW·m?2,31.83 kW·m?2,and 63.66 kW·m?2,respectively.The effect of heat flux on γ-Al2O3particulate fouling characteristics for single-phase flow is shown in Fig.3.As can be seen,there is no obvious induction period during the process of fouling formation,and the asymptotic value of fouling resistance decreases with increasing heat flux through the heated surface.Experiment D lies between the single-phase flow and the subcooled- flow boiling conditions,and therefore the bubble formation is not clear.
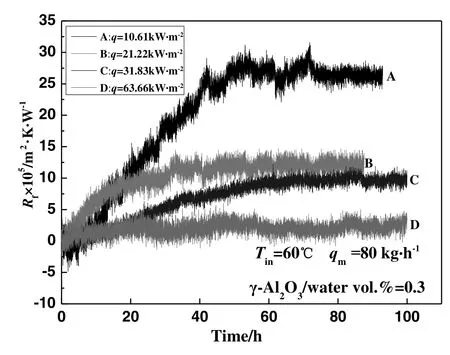
Fig.3.The effect of heat flux on γ-Al2O3 particulate fouling characteristics under singlephase flow.
The effects of heat flux on fouling characteristics include two aspects.On the one hand,the difference of heat flux may result in a temperature difference between the heated surface and the working fluid.As a result,the thermophoresis force is changes.When the nanoparticles exist in an environment with a temperature gradient,different temperature gradients will lead to altered thermal motion of the molecules.Driven by the temperature gradient,the nanoparticles will move from the high temperature area to that of the low temperature.The smaller the particle size and the greater the temperature gradient,the more obvious the thermophoresis force becomes[24].Comparing the four experiments with different heat fluxes,the temperature differences between the heat transfer surface and the working fluid for A,B,C,and D are 15 °C,22 °C,36 °C and 56 °C respectively.Therefore,the temperature difference between the heated surface and the working fluid increases with increasing heat flux;thus,the nanoparticles become affected by a stronger thermophoresis force caused by the high wall temperature.With this increase in the thermophoresis force,the nanoparticles are prevented from adhering to the heat transfer surface for the fouling formation.
On the other hand,the increased heat flux may affect the temperature of the working fluid in the experimental loop.When the inlet temperature of the working fluid is constant,it can be considered that the stability of the working fluid is equivalent,that is,the number of nanoparticles in the suspension is considerable,and there is no significant difference in the number of nanoparticles which transfer to the heat transfer surface.However,with an increase in the heat flux,the mean fluid temperature in the experimental section rises.Thus,the Brownian motion and the kinetic energy of the nanoparticles increase.Finally,there will be more particles that exceed the potential barrier and form agglomerated particles,and these agglomerated particles cannot adhere well to the surface for fouling formation.Accordingly,the heat flux effects can be understood from two previously described aspects:the increase in temperature of the experimental loop and the increased thermophoresis force,both caused by the increase in heat flux,explain the decrease in fouling resistance.
3.1.2.The effect of heat flux under subcooled- flow boiling
The experiments for investigating the effect of heat flux on γ-Al2O3particulate fouling characteristics were carried out at a constant inlet temperature,while increasing the power of the heating rod to change the flow state from the single-phase flow condition to the subcooled flow condition.The heat flux of the three experiments A,B,and C were 74.59 kW·m?2,95.49 kW·m?2,and 116.71 kW·m?2,respectively.The effect of heat flux on γ-Al2O3particulate fouling characteristics is shown in Fig.4.As can be seen,there is no obvious induction period in the process of fouling formation,and the asymptotic value of fouling resistance increases with increasing the heat flux of the heated surface.
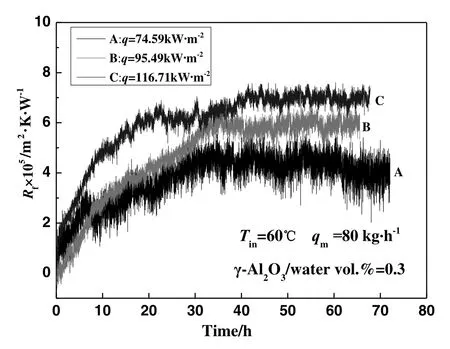
Fig.4.The effect of heat flux on γ-Al2O3 particulate fouling characteristics under subcooled- flow boiling.
In the state of subcooled- flow boiling,a large number of bubbles are generated from the heat transfer surface that violently disturbs the fluid near the surface.Bubble formation is one of the most important factors affecting fouling formation in subcooled- flow boiling condition.As can be seen in Fig.5,bubble formation is a strong function of heat flux.The effect of the bubbles on the particles is more significant than the effect of the thermophoresis force,and the boiling intensity therefore has the greatest control of the fouling process,instead of the temperature.Thus,the effect of heat flux on particulate fouling characteristics for subcooled- flow boiling mainly reflects the difference in boiling intensity.Firstly,in the case of the same inlet temperature,the boiling intensity of the fluid near the heat transfer surface increases with increasing heat flux,and the greater the boiling intensity is,the more bubbles that are generated.When the bubbles detach from the surface,the surrounding fluid flows to the heat transfer surface,with the amount of corresponding fluid movement increasing with increasing boiling intensity.As a result,more working fluid is supplied to the heated surface,thereby transferring more particles for the fouling formation.In addition,the particle concentration of the working fluid increases at the moment of bubble formation.From this point,the particulate concentration increases accordingly and results in the increase of the fouling at the heat transfer surface.Photos of the heat transfer surface at the end of the experiment are shown in Fig.6.Seen in the photos,that the amount of fouling increases with increasing heat flux,and there are many circular areas on the heat transfer surface where fouling is thicker.Once fouling deposits form on the heat transfer surface,these areas become the new evaporation cores,and the bubbles continue generating in these areas.Consequentially,the amount of fouling in the evaporation core becomes larger than for the other.Secondly,the increase in heat flux may cause a fluid temperature increase near the heated surface.The intensity of Brownian motion increases with increasing working fluid temperature,and it increases collision probability for the nanoparticles on the heat transfer surface.Meanwhile,the nanoparticles would have greater kinetic energy to collide with the surface.

Fig.5.Bubble formation as a function of heat flux.(a)q=74.59 kW·m?2.(b)q=95.49 kW·m?2.(c)q=116.71 kW·m?2.
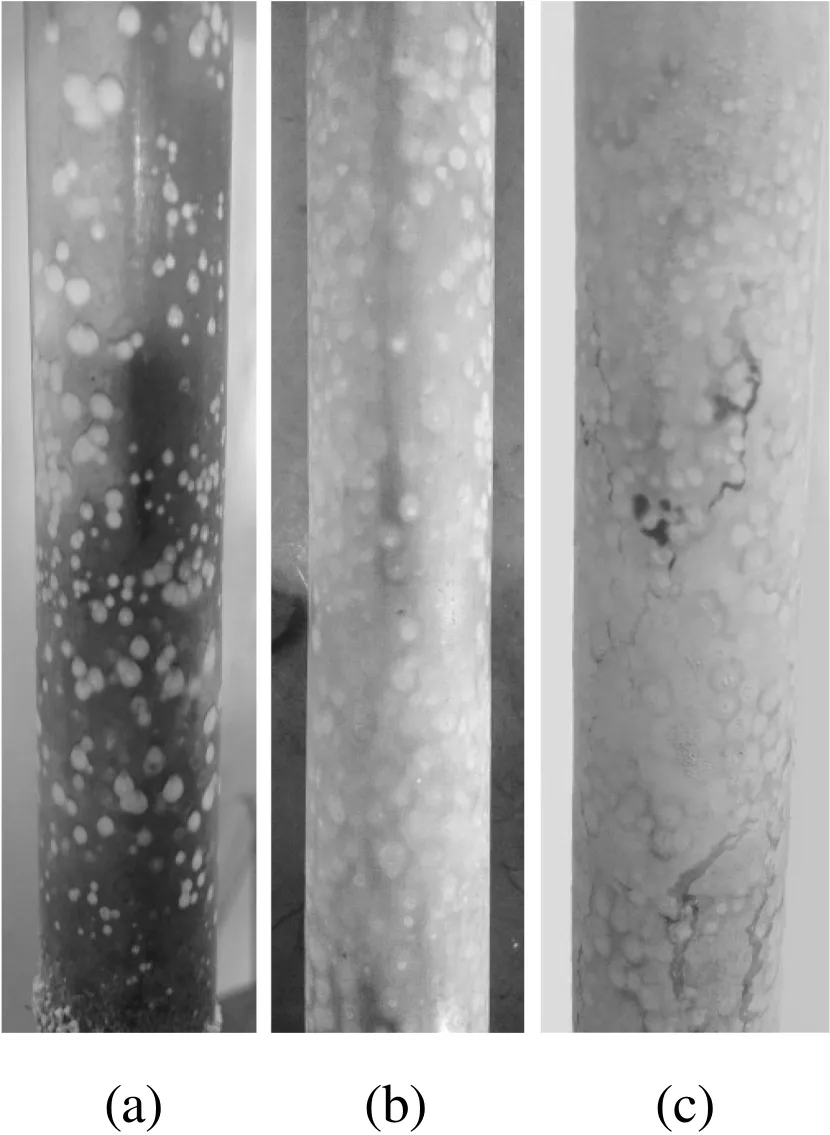
Fig.6.The photos of the heat transfer surface.(a)q=74.59 kW·m?2.(b)q=95.49 kW·m?2.(c)q=116.71 kW·m?2.
3.1.3.The trend in γ-Al2O3 particulate fouling resistance under different heat fluxes
Comparing the experimental results in Figs.3 and 4,reveals the trend in γ-Al2O3particulate fouling resistance under different heat fluxes,as shown in Fig.7.It can be seen from this figure that the asymptotic value of fouling resistance for the single-phase flow condition is much higher than for the subcooled- flow boiling condition.The effect of heat flux on the fouling resistance under the two flow states has an inverse trend,and there exists a minimum value of fouling resistance between the single-phase flow and the subcooled- flow boiling conditions.The fouling resistance reaches a minimum at the heat flux of 63.66 kW·m-2.In this operating condition,the effect of fouling resistance on the heat transfer performance is at its lowest.
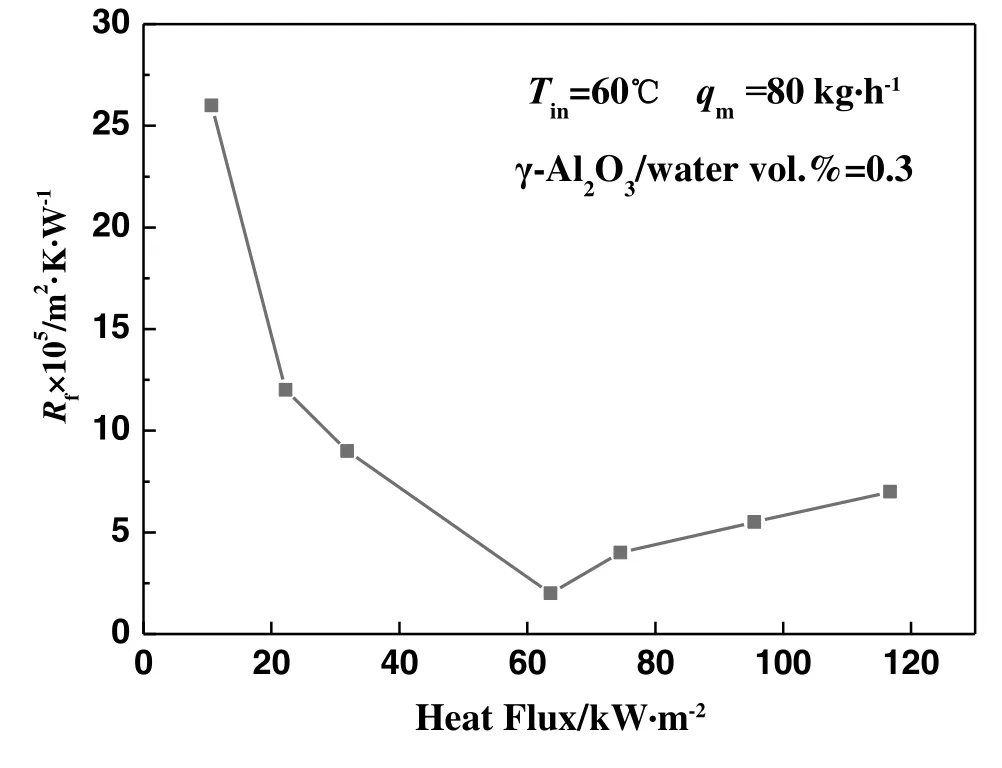
Fig.7.The trend in γ-Al2O3 particulate fouling resistance under different heat fluxes.
3.2.The effect of inlet temperature on γ-Al2O3 particulate fouling characteristics
3.2.1.The effect of inlet temperature under single-phase flow condition
The experiments to investigate the effect of inlet temperature on γ-Al2O3particulate fouling characteristics were carried out at a constant heat flux of 10.61 kW·m?2,with the flow state being singlephase.The inlet temperatures of the three experiments A,B,and C,were 40 °C,60 °C and 80 °C,respectively.The effect of inlet temperature on γ-Al2O3particulate fouling characteristics under single-phase flow is shown in Fig.8.In the figure,there is no obvious induction period in the fouling formation process,and the inlet temperature significantly affects the particulate fouling characteristics.The asymptotic value of fouling resistance clearly decreases with an increase in the inlet temperature.
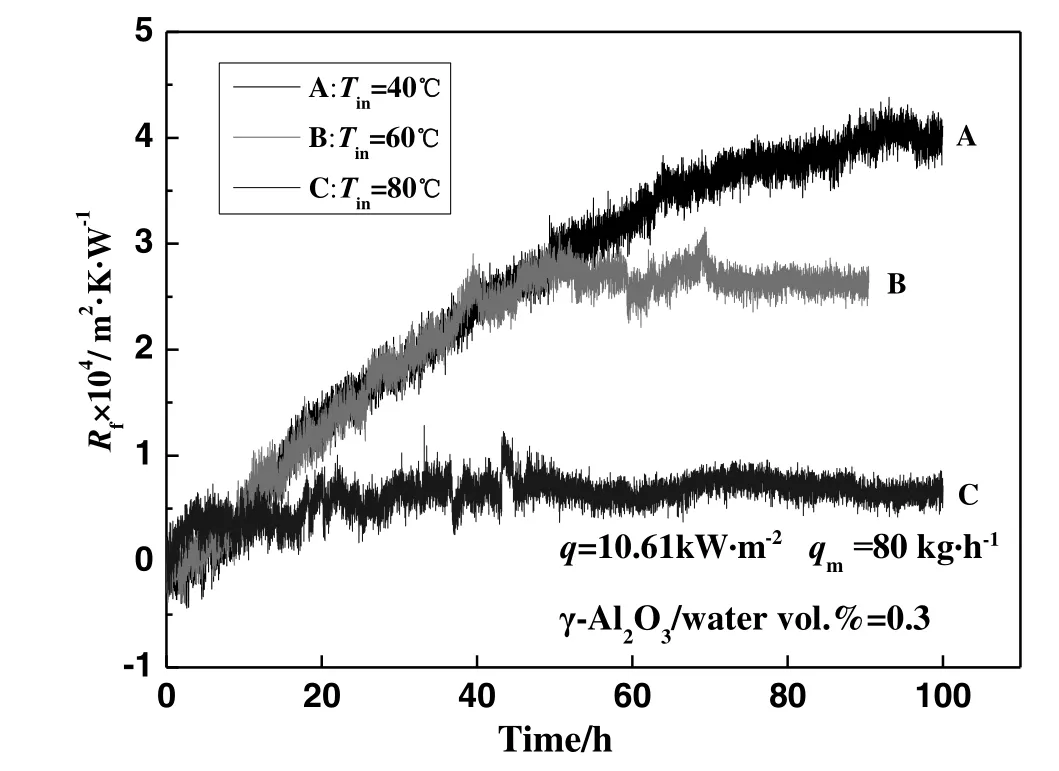
Fig.8.The effect of inlet temperature on γ-Al2O3 particulate fouling characteristics under the single-phase flow.
In our opinion,two possible reasons for this observation are:(1)as previously mentioned above,the thermophoresis force may affect the adsorption of the particles,and the inlet temperature has an influence on the thermophoresis force.Thus,different inlet temperatures result in different fouling resistance.Comparing the three experiments A,B,and C,the temperature differences between heated surface and the working fluid were 16 °C,15 °C and 13 °C,respectively.These differences were not significant,so it can be concluded that the effects of the thermophoresis force on nano particulates motion were similar.
(2)The difference in inlet temperature corresponds to a temperature difference in the working fluid.The nano γ-Al2O3particles in suspension have a large specific surface,and the system of suspension has a high surface energy.Consequently,the nanoparticles have a tendency to aggregate for the purpose of reducing the surface energy of the system.In addition,some physical and chemical factors may also lead to the aggregation phenomenon of the nanoparticles,such as light,electricity,heat,the addition of electrolytes,and other factors.
Next,the sedimentation experiments were used to compare the effects of three temperatures on the stability of the γ-Al2O3suspension.The OD value of the γ-Al2O3suspension at different temperatures is shown in Fig.9,and it can be seen that temperature significantly affects the coagulation of γ-Al2O3particles.The OD value decreases more rapidly with the increase of suspension temperature.
Gravity and buoyancy are no longer important for nanoparticles[25]at this size scale.The nanoparticles are in random motion under the action of Brownian forces,which is a main difference between nanoparticles and regular sized particles.The velocity of the particles is proportional to the temperature of the system,and it is irrelevant to the chemical composition of the particles.The effect of Brownian motion on the nanoparticles has two aspects.On the one hand,the nanoparticles are in random motion under the action of Brownian forces,and the higher temperature,the more obvious their movement.From this point of view,the high temperature may promote a discrete distribution of nanoparticles in the suspension.On the other hand,in the process of disordered motion of nanoparticles,the collision of particles is inevitable.With the increase of temperature,it will provide the opportunity for particles to collide.This collision may result in the agglomeration of particles over time,forming agglomerated particles.There are two reasons for the spontaneous formation of agglomerated particles.One is that there exists mutual attraction among particles,and the other is that the surface energy of the system will reduce with an increase in particle size,and the system tends to stabilize.Because of the effect of gravity,the agglomerated particles will exhibit an obvious sedimentation phenomenon.So with increasing temperature,the suspension undergoes coagulation phenomenon,which may lead to a reduction in the number of independent nanoparticles.
Nanoparticles also collide with each other under the action of the Brownian motion force,but there exists a certain condition for nanoparticles to aggregate into agglomerated particles.At present,DLVO theory is relatively acceptable for explaining the colloidal stability and the effect of electrolytes.According to the DLVO theory,the suspension's stability or coagulation under certain conditions depends on the sum of the repulsive force and the attractive force between particles,where both of forces are functions of distance.If the repulsive force is greater than the attractive force,the system will be in a stable condition.On the contrary,coagulation may occur and endanger the stability of the system.The potential energy of the system is equal to the sum of the attractive potential and repulsive potential,and the highest point on the potential energy curve is called the repulsion barrier.The potential energy is determined by the Hamaker constant,surface potential,and the concentration of the electrolyte.Coagulation only occurs when the kinetic energy of the particles exceeds the potential barrier.Therefore,the height of the potential barrier determines the stability of the suspension.The kinetic energy of the particles must exceed the potential barrier so that they could agglomerate.If the kinetic energy of the particles cannot exceed the potential barrier,the agglomeration of the particles could not occur.In these sedimentation experiments,the three factors mentioned above are invariant and will affect the potential energy,with exception of the suspension temperature.Thus,the potential energy of the suspensions with three different temperatures is constant.The kinetic energy of the particles' thermal motion significantly increases with increasing temperature,and there will be more particles with a kinetic energy exceeding the potential barrier able to form into agglomerated particles.For the suspension system,an obvious agglomeration behavior may occur.This is the reason that the OD value rapidly decreases with increasing the system temperature.
The previous analysis provides one possible explanation for why the higher the inlet temperature,the more obvious the coagulation phenomenon in the suspension system.The coagulation results in a decrease in the fouling resistance,and it can be explained by the processes of fouling formation,which involves transportation,adhesion,and erosion.For the transportation process,the higher the temperature is,the more obvious the agglomeration phenomenon.This reduces the number of independent nanoparticles in the suspension.For the agglomerated particles,Brownian motion is not obvious,that is to say,the number of nanoparticles transported to the heated surface for fouling formation decreases.For the adhesion process,it is difficult for the agglomerated particles to adhere to the heat transfer surface due to of their large gravity force,whereas,it is extremely easy for nanoparticles to adhere to the heat transfer surface to reduce the interface energy of the suspension system.For the erosion process,the particles are removed from the heat transfer surface under the action of the shear stress in the fluid.The particles can adhere to the heat transfer surface only when the adhesive force is greater than the shearstress.The smaller the particle size,the smaller the fluid shear stress,and the gravity force can be ignored.Therefore,the nanoparticles can easily adhere to the heat transfer surface for fouling formation.When higher temperatures result in more agglomerated particles,because of their larger diameters,the effects of the gravity force and the shear stress are more significant.Even if they adhere to the heat transfer surface,they are also easily removed from the heated surface by the large erosion force.Therefore,the number of the suspended independent nanoparticles in the working fluid determines the value of the fouling resistance.
3.2.2.The effect of inlet temperature under subcooled- flow boiling
The experiments to investigate the effect of inlet temperature of the working fluid on the γ-Al2O3particulate fouling characteristics were carried out with a heat flux of 95.49 kW·m?2.The inlet temperatures of three experiments A,B,and C were 60 °C,70 °C and 80 °C,respectively.The effect of inlet temperature on γ-Al2O3particulate fouling characteristics for subcooled- flow boiling is shown in Fig.10.There is no obvious induction period in the process of fouling formation,and the effect of inlet temperature on nano-particulate fouling characteristics under subcooled flow boiling is not obvious.The asymptotic value of fouling resistance increases slightly with increasing inlet temperature.In order to verify the accuracy of the experiment,another group of experiments with a heat flux of 74.59 kW·m?2were conducted.As seen in Fig.10b,the results for different conditions demonstrate the same phenomenon as the results documented in Fig.10a.
This result could be analyzed from two aspects.The previous analysis results show that the variation of inlet temperature significantly affects the coagulation of the suspension.This coagulation becomes more obvious when increasing the inlet temperature.This will lead to a reduction in the number of nanoparticles and a decrease in the fouling resistance.Furthermore,when the heat flux is constant,the boiling intensity increases with inlet temperature,thereby increasing the fouling resistance value.The previous analysis offers more detailed explanations.
To summarize,with the increase of inlet temperature,the effect of the variation in the suspension coagulation decreases the fouling resistance,whereas variation in boiling intensity results in an increase in the fouling resistance.Each effect has an opposing influence on the fouling characteristics,so these two factors reflect little change in the fouling resistance.As for the slight increase in fouling resistance,it can be considered that the effect of boiling intensity is slightly greater than the effect of temperature on suspension coagulation.
4.Conclusions
In this investigation,the effects of heat flux and inlet temperature on particulate fouling characteristics were studied in detail.Several important results were obtained and can be summarized as follows:
(1)For single-phase flow,the asymptotic value of fouling resistance decreases with increasing heat flux.Its effect on fouling characteristics is mainly caused by the difference in thermophoretic force on the particles and the variation in the agglomeration phenomenon within the experiment apparatus.
(2)For subcooled- flow boiling,the asymptotic value of fouling resistance increases with increasing heat flux.The effect of heat flux on the fouling characteristics mainly reflects the difference in boiling intensity.
(3)The asymptotic value of fouling resistance for single-phase flow is much higher than for subcooled- flow boiling.The effect of heat flux on the fouling resistance under the two flow states has an inverse trend,and there exists a minimum value for the fouling resistance between the two flow states.
(4)In the condition of single-phase flow,the asymptotic value of fouling resistance decreases with increasing inlet temperature,which is mainly causes by the working fluid stability.
(5)In the condition of subcooled- flow boiling,inlet temperature has a negligible effect on the fouling resistance.The result is synthetically affected by the variation of suspension coagulation and the boiling intensity.
[1]B.D.Bowen,N.Epstein,Fine particle deposition in smooth paralleleplate channels,J.Colloid Interface Sci.72(1979)81–97.
[2]R.Williamson,I.Newson,T.R.Bott,The deposition of hematite particles from flowing water,Can.J.Chem.Eng.66(1)(1988)51–54.
[3]A.J.Karabelas,S.G.Yiantsios,B.Thonont,et al.,Liquid-side fouling of heat exchangers.An integrated R&D approach for conventional and novel designs,Appl.Therm.Eng.17(8–10)(1997)727–737.
[4]C.W.Turner,S.J.Klimas,Deposition of magnetite particles from flowing suspensions under flow-boiling and single-phase forced-convective heat transfer,Can.J.Chem.Eng.78(6)(2000)1065–1075.
[5]M.Basset,J.Mcinerney,N.Arbeau,et al.,The fouling of alloy-800 heat exchange surfaces by magnetite particles,Can.J.Chem.Eng.78(1)(2000)40–52.
[6]C.Henry,J.P.Minier,G.Lefèvre,Towards a description of particulate fouling:from single particle deposition to clogging,Adv.Colloid Interf.Sci.185(2012)34–76.
[7]S.Grandgeorge,C.Jallut,B.Thonon,Particulate fouling of corrugated plate heat exchangers.Global kinetic and equilibrium studies,Chem.Eng.Sci.53(17)(1998)3050–3071.
[8]G.M.Zhang,G.Q.Li,W.Li,et al.,Particulate fouling and composite fouling assessment in corrugated plate heat exchangers,Int.J.Heat Mass Transf.60(2013)263–273.
[9]S.G.Yiantsios,A.J.Karabelas,Deposition of micron-sized particles on flat surfaces:Effects of hydrodynamic and physicochemical conditions on particle attachment efficiency,Chem.Eng.Sci.58(14)(2003)3105–3113.
[10]P.Lipp,U.Müller,B.Hetzer,Characterization of nanoparticulate fouling and breakthrough during low-pressure membrane filtration,Desalin.Water Treat.9(1–3)(2009)234–240.
[11]B.O.Hasan,G.J.Nathan,P.J.Ashman,et al.,The effects of temperature and hydrodynamics on the crystallization fouling under cross flow conditions,Appl.Therm.Eng.36(2012)210–218.
[12]T.M.P??kk?nen,M.Riihim?ki,C.J.Simonson,et al.,Crystallization fouling of CaCO3—Analysis of experimental thermal resistance and its uncertainty,Int.J.Heat Mass Transf.55(23–24)(2012)6927–6937.
[13]D.A.Al-Otaibi,M.S.J.Hashmi,B.S.Yibas,Fouling resistance of brackish water:Comparison of fouling characteristics of coated carbon steel and titanium tubes,Exp.Thermal Fluid Sci.55(2014)158–165.
[14]M.S.Abd-Elhady,S.H.Clevers,T.N.G.Adriaans,et al.,Influence of sintering on the growth rate of particulate fouling layers,Int.J.Heat Mass Transf.50(1–2)(2007)196–207.
[15]M.G.Mwaba,C.C.M.Rindt,A.A.Van Steenhoven,et al.,Experimental investigation of CaSO4crystallization on a flat plate,Heat Transfer Eng.27(3)(2006)42–54.
[16]S.M.Peyghambarzadeh,A.Vatani,M.Jamialahmadi,Application of asymptotic model for the prediction of fouling rate of calcium sulfate under subcooled flow boiling,Appl.Therm.Eng.39(2012)105–113.
[17]S.M.Peyghambarzadeh,A.Vatani,M.Jamialahmadi,Influences of bubble formation on different types of heat exchanger fouling,Appl.Therm.Eng.50(2013)848–856.
[18]L.Zhao,Y.Zou,Y.D.Liu,et al.,Effect of temperature on formation of crystalline fouling on heat exchanger surface,J.Chem.Ind.Eng.60(8)(2009)1938–1943.
[19]Z.M.Xu,J.T.Wang,Y.T.Jia,et al.,Experimental study on microbial fouling characteristics of the plate heat exchanger,Appl.Therm.Eng.108(2016)150–157.
[20]Z.M.Xu,Y.T.Jia,B.L.Wang,etal.,Experimental analysis on bio-fouling ofiron bacteria on plate heat exchanger,CIESC J.65(8)(2014)3178–3183(in Chinese).
[21]Z.M.Xu,Y.L.Zhang,X.Xu,Simulation study on in fluent of temperature and concentration on crystallization fouling deposition,Proc.CSEE 34(35)(2014)6263–6270.
[22]Z.M.Xu,J.T.Wang,L.Wang,etal.,Experimental analysis on particulate fouling characteristics of alternating ellipticalaxis tube,Chem.Ind.Eng.Prog.33(4)(2014)831–836.
[23]H.Q.Zhang,Testing and Calculate Method Study on Thermal Performance and Flow Pressure Drop Characteristics of a Plate Heat Exchanger,PhD.Thesis Harbin Institute of Technology,Harbin,2006(in Chinese).
[24]V.Nikkhah,M.M.Sarafraz,F.Hormozi,et al.,Particulate fouling of CuO-water nano fluid at isothermal diffusive condition inside the conventional heat exchanger experimental and modeling,Exp.Thermal Fluid Sci.60(2015)83–95.
[25]D.M.Nie,Research on the Particles Sedimentation and Brownian Motion,PhD.Thesis Zhejiang University,Hangzhou,2011(in Chinese).
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis of butter fly-like BiVO4/RGO nanocomposites and their photocatalytic activities☆
- Fabrication of chitosan microspheres for efficient adsorption of methyl orange☆
- The combined effects of lysozyme and ascorbic acid on microstructure and properties of zein-based films☆
- Effects of nitrogen doping on surface-enhanced Raman scattering(SERS)performance of bicrystalline TiO2 nano fibres☆
- Parametric study and effect of calcination and carbonation conditions on the CO2 capture performance of lithium orthosilicate sorbent
- Variation of toxic pollutants emission during a feeding cycle from an updraft fixed bed gasifier for disposing rural solid waste☆
