17α-Ethinylestradiol removal from water by magnetic ion exchange resin☆
Liang Wang ,Lu Liu ,Zhaohui Zhang ,*,Bin Zhao ,Junjing Li,Bingjie Dong ,Nian Liu
1 State Key Laboratory of Separation Membranes and Membrane Processes,Tianjin 300387,China
2 Department of Environmental Engineering,Tianjin Polytechnic University,Tianjin 300387,China
1.Introduction
In recentyears,magnetic ion exchange(MIEX)has been increasingly employed by water treatmentplants for the removalofdissolved organic carbon(DOC)as an emerging technology[1–3].Morran and Allpike et al.[4,5]evaluated a full-scale treatment with MIEX at the Mount Pleasant Water Treatment Plant in South Australia and the Wanneroo Water Treatment Plant in Western Australia,respectively,and reported that pre-treatment with MIEX followed by coagulation was very effective at removing a wide range of molecular weight fractions of DOC and reducing the subsequent coagulant demand.MIEX has two main features differentfromtraditionalones.The firstfeature is theirmagnetic agglomeration effect.MIEXbeads contain magnetized cores thatcould enhance agglomeration into larger,fast-settling particles during the separation process[6].The second feature relates to their higher surface area,MIEX beads(150–180 μm in diameter)are 2–5 times smaller than conventional ones,which provide a higher surface area,thus allowing rapid adsorption kinetics[7].MIEX is a strong base anion exchange resin with a macro-porous polyacrylic matrix in the chloride form that can be used to adsorb negatively charged aquatic contaminants through exchange ofanions.Some work has shown thatmagnetic resin pretreatment can remove from 50%to 70%of the DOC from water[8,9].Use of the resin as a pre-treatment before coagulation has shown significant reduction in key DBPs of between 50%and 70%for trihalomethanes(THMs)and>60%for haloacetic acids(HAAs)when compared with single coagulation alternatives[10].
The removal efficiencies of MIEX for micro-pollutants from water were also studied.Micro-pollutants,which include pesticides,natural and synthetic hormones and pharmaceutically active products,were considered ubiquitous in surface and wastewater[11].Low concentrations of many micro-pollutants(ng·L-1to μg·L-1)can have a signi ficant effect on vertebrate and ecosystem.Humbert et al.[12]evaluated the removal of two pesticides(i.e.,atrazine and isoproturon)from surface water using a series of strong anion exchange resins.The results showed that the MIEX resin dosage of 8 ml·L-1only brought about the removal of 7%and 5%for atrazine and isoproturon,respectively.They attributed the removal efficiencies to the micro-pore adsorption of MIEX instead of ion exchange.Mastrup and Sch?fer[13]and Sch?fer et al.[14]studied estrone removal by MIEX and found its removal was in fluenced by pH and ionic strength.Below pH 11 MIEX could only remove 30%of estrone from solution,since in the environment,estrone has a neutral charge and is non-ionic.17α-Ethinylestradiol(EE2),as a typical micro-pollutant,is often discovered from surface water and the effluent of sewage treatment plant.EE2 has many negative effects at trace concentrations(i.e.,< 10 ng·L-1)such as developmental anomalies in wildlife(such as feminized male fish),which makes it significant to remove EE2 from water body[15].As a typical non-ionic organic compound,EE2 is almost insoluble in water.However,in our previous experiment it was found that the removal of EE2 by MIEX was still over 40%even as the resin has been regenerated twenty times.The results seem to be contradictory with the removal mechanism of MIEX.
The objective of this study was to elucidate the removal mechanism of EE2 by MIEX.The charge density of EE2 as a function of pH was determined with the potentiometric titration method.Multi-cycle adsorption-regeneration experiments were conducted,and the ion exchange stoichiometry of EE2 and nitrate for chloride was analyzed.The internal micro-environment of the resin bead was discussed according to the Donnan Theory.Based on the above results,the removal mechanism of EE2 by MIEX was illuminated.
2.Materials and Methods
2.1.Water samples
Since EE2 hardly dissolved in water,the aqueous solution of EE2 must be prepared as follows:EE2 was dissolved with ethanol to prepare the 1 g·L-1stock solution,then hermetically kept in a brown reagent bottle and used within a month.Experimental solutions whose pH values adjusted by 0.1 mol·L-1hydrochloric acid(HCl)and 0.1 mol·L-1sodium hydroxide(NaOH)were obtained by successive dilutions with ultrapure water when the concrete concentrations were desired.Table 1 shows the composition of the model waters used in this work.Control(i.e.,EE2-free)model water was prepared by adding sodium nitrate to ultrapure water.The EE2 removal experiments atdifferent MIEXdosages or initialEE2 concentrations were conducted using EE2 I model water.The experiment about the relationship between pH,charge density and EE2 removal was finished using EE2 II model water,and multi-cycle adsorption-regeneration experiment also used EE2 II model water as in fluent.The experiment about ternary ion exchange of EE2 and nitrate for chloride was conducted with EE2 III and Control model water.
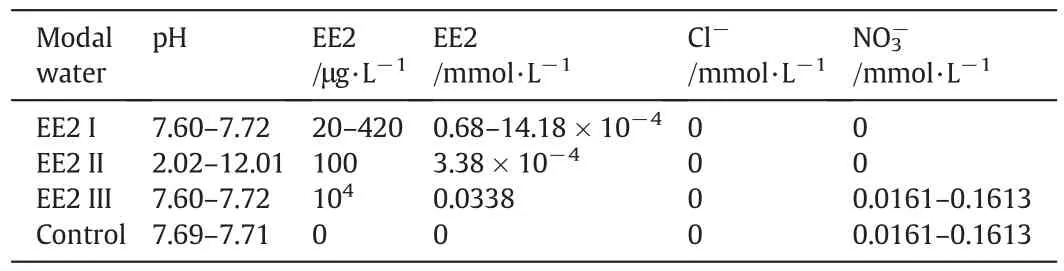
Table 1 Composition of model waters
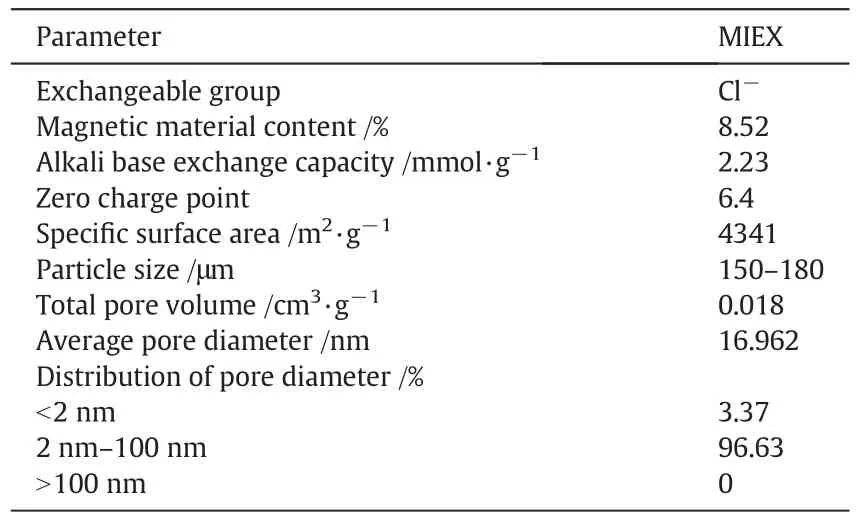
Table 2 Parameters of MIEX resin used in the experiments[16]
2.2.MIEX adsorption experiments
MIEX resin was provided by Orica Watercare of Australia.Basic physicochemical parameters of MIEX are shown in Table 2.Normally,MIEX resin was stored in Amber bottles with 5%NaCl solution.Prior to use,the resin was repeatedly rinsed with ultrapure water until the supernatant conductivity could reach 1 μS·cm-1.MIEX resin dosages in the range of 5–15 ml·L-1were tested after being settled for 30 min.Batch experiments were performed in a mixer(ZR4-6,China).In the adsorption stage,the mixing speed was set at 150 r·min-1,and the temperature was kept at(25 ± 0.5)°C.The adsorption stage was continued for 1 h and then settling for 15 min.The supernatant was decanted and filtered through 0.45μm membrane,which could preventthe destroyed MIEX fines.The filtrate was used to determine the EE2 concentration after MIEX.Each condition of MIEX experiments was tested in triplicate and the average value of the triplicate results was reported.
2.3.Analytical methods
EE2 was determined by High Performance Liquid Chromatography(HPLC)directly.Water samples were first filtered through a 0.45 μm cellulose acetate membrane filter.The filtrates were analyzed on HPLC(America Waters 2695-2489)with a UV-detector to determine EE2 concentration.Isocratic separation was performed on a Symmetry C18 column(4.6 mm × 150 mm,5 μm-Waters)at 30 °C.Mixtures of acetonitrile and ultrapure water(50/50,V/V)were used as the mobile phase at 1 ml?min-1.The detection wavelength was 280 nm.The sample volume injected was 20 μl.
Charge density of EE2[meq?(μg EE2)-1]was determined by the potentiometric titration according to the method described by Boyer et al.[17].Brie fly,a solution containing 10 mg·L-1EE2 and 0.1 mol·L-1KCl was purged with nitrogen gas for 30 min to eliminate carbon dioxide and then titrated with 0.04 mol·L-1NaOHsolution under a nitrogen atmosphere.Allsamples were titrated from approximately pH 2 to 12.The charge density of EE2 was calculated based on the pH measurements and a charge balance of the solution as follows:
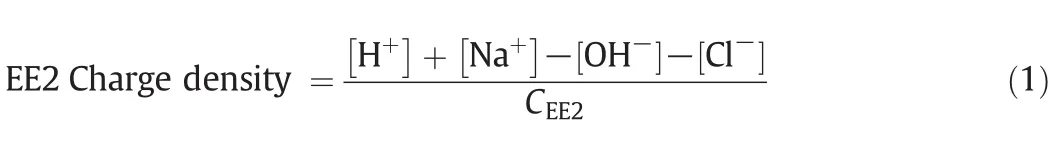
where the concentrations of H+,Na+,OH-and Cl-are in meq·L-1and CEE2is the EE2 concentration of the solution(μg·L-1).[Cl-]is from HCl rather than KCl.Potassium and chloride for KCl were not included in the charge balance since they were opposite in charge and equal in concentration.
The concentrations of Cl-and NO3-were determined by ion chromatograph(America Dionex,ICS-1100).The pH values were measured using automatic potentiometric titrator(Switzerland Mettler,S40).
3.Results and Discussions
3.1.The experiment of EE2 removal by MIEX
EE2 removalexperiments by MIEXwere conducted with three initial EE2 concentrations,and the results are shown in Fig.1.The removal efficiency of EE2 increased with MIEX dosages,but it was no longer obviously promoted as the MIEX dosage increased to 10 ml·L-1.At such a dosage,even at the highest initial concentration of EE2(100 μg·L-1),which showed the lowest removal rate,the removal efficiency of EE2 was found to be still more than 50%.Additionally,the relationship between EE2 removal efficiency and initial EE2 concentrations was further investigated,and the results also are shown in Fig.1.Obviously,a removal of at least 50%was maintained for initial EE2 concentrations in the range of 20–420 μg·L-1.In the meantime,EE2 removal increased as initial EE2 concentration decreased because of limited surface area(exchange sites)on MIEX.The maximum removal of 75.3%for EE2 was obtained at the initial EE2 concentration of 20 μg·L-1.Such a high removal of EE2 was markedly different from other micropollutants removal reported in the previous research[12–14].
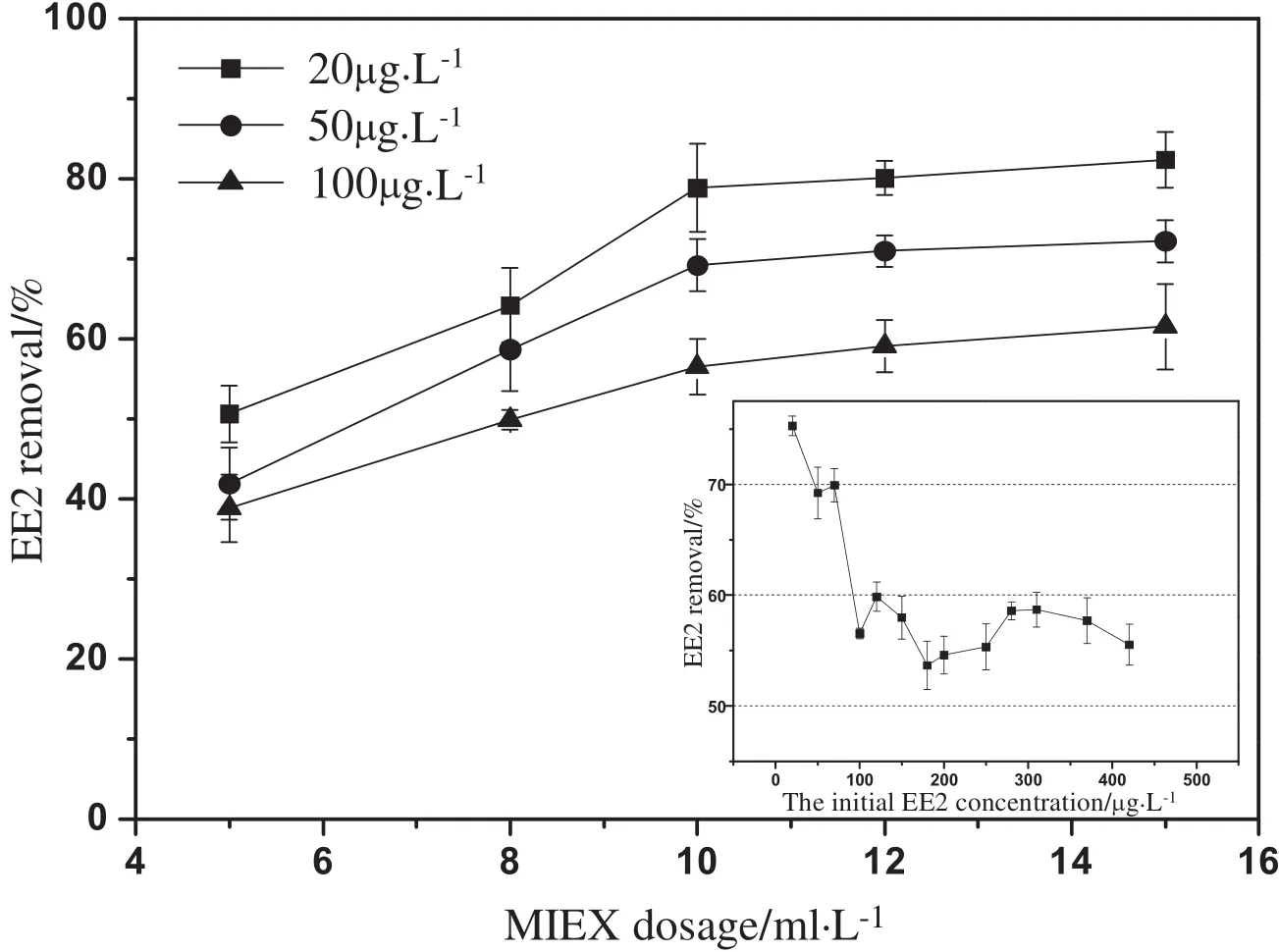
Fig.1.The effects of MIEX dosages and initial EE2 concentrations on EE2 removal efficiency.
3.2.The removal mechanism of EE2 by MIEX
Fig.2 showed EE2 removal as a function of regeneration times of MIEX.Twenty cycles of adsorption-regeneration were conducted.During the adsorption stage,10 ml MIEX resin was dosed into 1 L EE2 solution with an initial concentration of 100 μg·L-1.The mixing speed was set at 150 r·min-1.The residual concentration of EE2 in water was determined every hour,until the concentration was no longer decreased.This means the MIEX resin has been saturated by EE2 and the adsorption stage was finished.The final removal of EE2 was recorded.Then the MIEX resin were separated from the solution through a 0.45 μm filter and were regenerated with 1 mol·L-1sodium chloride solution.Up flow regeneration method was adopted,and the volume ratio of the regenerating solution(1 mol·L-1sodium chloride solution)and MIEX was 250:1.After regeneration,the MIEX resin was used to adsorb EE2 again.Repeat the above experiments(adsorption–regeneration)twenty times.
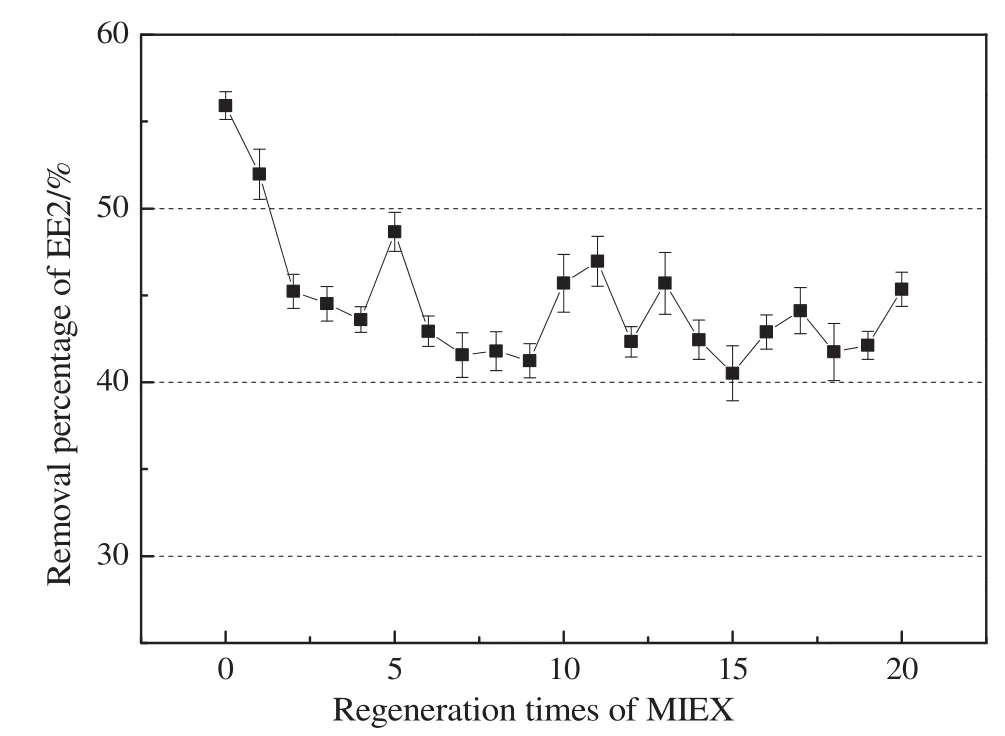
Fig.2.The effect of regeneration times of MIEX on EE2 removal efficiencies.
The results in Fig.2 suggested that the removal of EE2 by fresh MIEX resin was(55.9±0.8)%.In the next twenty times regeneration,the EE2 removal was maintained at an average of(43.7±1.2)%.This implied the contribution ratio of irreversible adsorption only accounted for a small fraction of the total removal,and reversible adsorption still was the main removal mechanism of EE2 by MIEX in neutral water.
Since the reversible adsorption of MIEX consist of ion exchange and reversible micro-pore adsorption,to quantify the contributions of ion exchange and reversible micro-pore adsorption to EE2 removal,the stoichiometry of removal of EE2 by ion exchange was analyzed according to the method described by Boyer etal.[17].The ion exchange reaction of interestis the equilibration ofan anion exchange resin in the chloride form with a solution containing EE2,nitrate,and chloride.The charge balance equation for this reaction is written as follows:
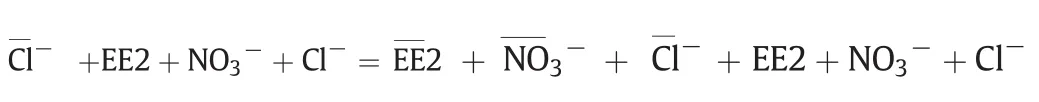
where over bars represent resin-phase species in meq·g-1of resin and all aqueous-phase concentrations are in meq·L-1.At equilibrium,for a purely ion exchange reaction,the decrease in the aqueous-phase concentrations of EE2 and nitrate must equal the increase in the aqueousphase concentration of chloride.Fig.3 shows the results of multiple batch equilibrium experiments using MIEX resin and the model waters(Controland EE2 III)shown in Table 1.The x-axis shows the netincrease in the aqueous concentration of chloride(i.e.,equilibrium chloride concentration minus initial chloride concentration),while the y-axis shows the net decrease in the aqueous concentrations of EE2 andtogether.Fig.3 indicates the chemical species taking parting in the ion exchange reaction,e.g.,/Cl-indicates nitrate–chloride exchange.The y=x line in Fig.3 represents the stoichiometric exchange of chloride on the resin for nitrate and EE2 in the solution.The nitrate–chloride exchange experiments in the absence of EE2 were conducted as a control since it was expected that nitrate would be removed exclusively via ion exchange.Fig.3 shows that all of the data points for/Clare clustered about the y=x line and veri fies that nitrate was indeed removed via ion exchange(where Control in Table 1 was used as the model water).Of particular interest are the data points for the EE2//Cl-experiments(where EE2 III in Table 1 was used as the model water).For the systems,the data points are also tightly clustered about the y=x line.The average relative difference between chloride release and “EE2+”uptake was only 6.5%.The results of the EE2/exchange experiments mirrored the results of the control experiments and clearly show that,at approximately pH 7–8,EE2 was predominantly removed by ion exchange.
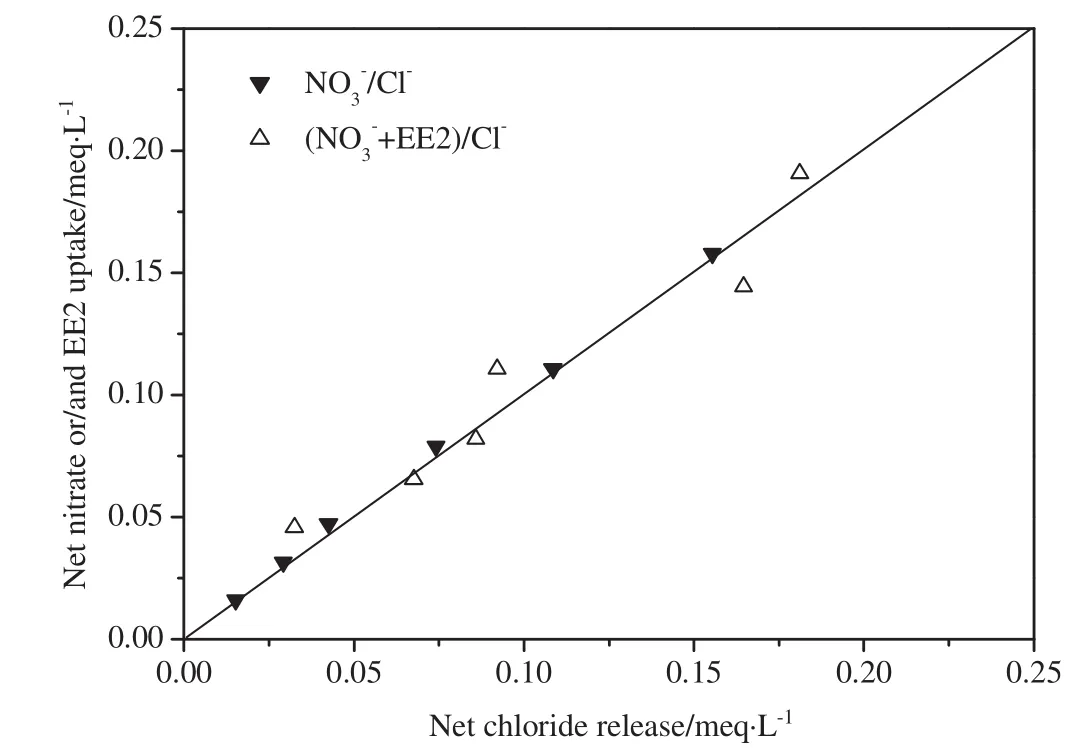
Fig.3.Illustrative ion exchange stoichiometry for MIEXresin.Corresponding modelwaters are as follows:Control(/Cl-)and EE2 III(EE2+/Cl-).
Ion exchange has been recognized as the main removal mechanism of DOC and ionic-pollutants by MIEX since these pollutants would ionize into the negatively charged groups or anions in water[18].However,differentfrom DOC and ionic-pollutants,EE2 hardly dissolved in water.To elucidate the occurrence form of EE2 in water,the charge density of EE2 as a function of pH was determined by direct potentiometric titration in a carbon-dioxide-free solution.The titration of EE2 was done in duplicate;the agreement between the two titration curves was very good,as shown in Fig.4.The duplicate titration curves were averaged using an interpolation routine in MATLAB.The results in Fig.4 suggested that in the pH range of 7–8 the charge density of EE2 is almost zero[(0.00000219 ± 0.00000015)meq·μg-1],which means in neutralwaterenvironmentEE2 molecules hardly generate ionization.The result seemed to be contradictory with their removal mechanism(ion exchange).
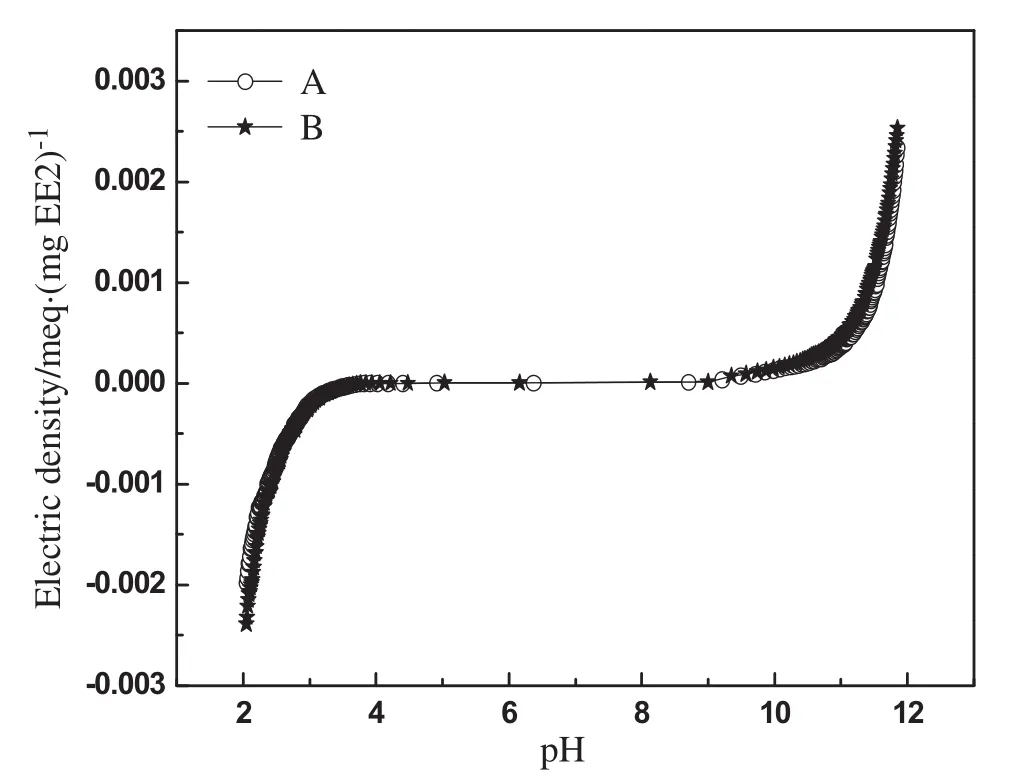
Fig.4.Charge density of EE2 resulting from direct potentiometric titration.
In addition,the results in Fig.4 also showed that EE2 electric density was strongly correlated with pH value.In strong acidic environment(pH<4)or strong alkaline environment(pH>11),the charge density of EE2 sharply increased.This can be explained by the reactions shown in Fig.5.EE2 molecule possesses one phenolic hydroxyl on benzene ring and one alcoholic hydroxyl linking to quaternary carbon atom,easily affected by the pH value.In the strong acidic environment,uncharged EE2 molecules transformed into positively charged molecular ions because of the protonation of the alcoholic hydroxyl groups of EE2.In contrast,in the strong alkaline conditions,the intense ionization of the phenolic hydroxyl group on EE2 molecule was caused by the neutralization between the free hydroxyl and the hydrion from the phenolic hydroxyl,which make EE2 ionized into negatively charged groups.
The EE2 removal by MIEX as functions of pH was further investigated and the results are shown in Fig.6.Obviously,EE2 removal by MIEX closely related with the charge density of EE2 in the pH range of 2–12.In neutral water environment(pH 6–8)the charge density of EE2 was close to zero,which corresponded to the lowest removal(about 50%).In strong alkaline conditions,the high charge density of EE2 solution accelerated ion exchange between the negatively charged groups of EE2 and chloride ions of MIEX,lead to a much higher removal of EE2 from water.The highest removal of EE2 was 100%in pH 12,which means the alkaline water environment was very favorable to EE2 removal by MIEX.Similarly,in the strong acidic conditions a higher removal of EE2 was also obtained.This might be the result of electrostatic interaction between the positively charged molecular ions of EE2 and the active sites of MIEX.
According to the Donnan theory[19],MIEXresin was considered as a kind of polyelectrolyte.The interface between MIEX particle and bulk solution can be considered as a semipermeable membrane,one side of which was the resin phase and the other side was the aqueous phase.The fixed ions of MIEX resin can't penetrate the semipermeable membrane,while the exchangeable ions of MIEX resin(i.e.,chloride ions)and the anions in the bulk solution can penetrate the semipermeable membrane.Based on the theory,it can be concluded that a pH change in solution would be observed after MIEX resin was put into ultrapure water.To verify the conclusion,the following experiments were conducted.Ten milliliters of MIEX resin was put into 1 L of ultrapure water after the resin was fully regenerated with NaCl solution,and then the pH values of the ultrapure water were determined.The above experiment was done in duplicate.Fig.7 showed the pH of the ultrapure water as a function of time.The results suggested that pH decrease in the ultrapure water occurred.This can be elucidated as follows:as the MIEX resin in the chloride form was dosed into ultrapure water,some chloride ions in the resin-phase desorbed and diffused into the bulk solution.In the meantime,to keep the balance of charges on both sides of the semipermeable membrane,water molecules in the bulk solution ionized into hydrogen ions and hydroxide ions,and then hydroxide ions with the same amount of charges passed through the semipermeable membrane and into the resin-phase.The hydrogen ions were left to keep the electric neutrality in the aqueous-phase.As a result,pH of the bulk solution decreased and that of the resin interior increased.Since the pore volume of the resin interior was much smaller than thatofthe bulk solution,a more significantincrease ofpHoccurred in the resin beads,i.e.,a strong alkaline environment was obtained in the internal space of resin beads.
According to the results in Fig.4,the solution only containing EE2 can be regarded as ultrapure water from the standpoint of ion composition,because almost no ionization of EE2 molecules occurred.Therefore,As MIEX resin in chloride form was dosed into EE2 solution,a similar pH change with ultrapure water would occur.Actually,in the multi-cycle adsorption–regeneration experiment,pHvalues have been measured during the adsorption process ofthe first cycle and last cycle(Fig.8).A very similar pH evolution with ultrapure water only containing MIEX was observed.The result indicated that some hydroxide ions have entered into the inner space of the resin beads.According to the results in Fig.4,as EE2 molecules entered into the inner space of the resin beads due to concentration diffusion,the strong alkaline environmentmade EE2 molecules ionize into negatively charged groups,then the ion exchange reaction occurred between chloride in resin-phase and the negatively charged groupsofEE2.As a result,EE2 was removed from the liquid phase by means of ion exchange of MIEX even in neutral water environment.
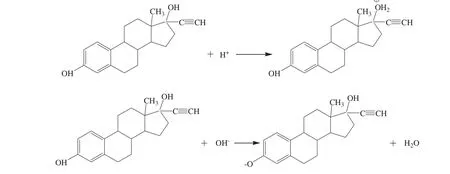
Fig.5.Schematic of EE2 structure altered by acid or alkali.
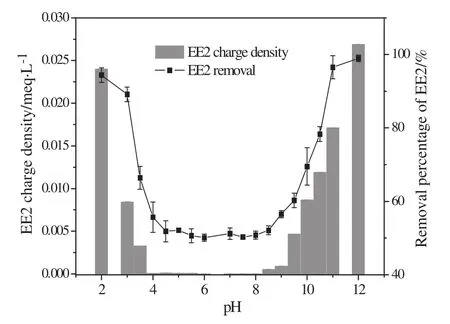
Fig.6.Effect of pH on charge density and removal of EE2.
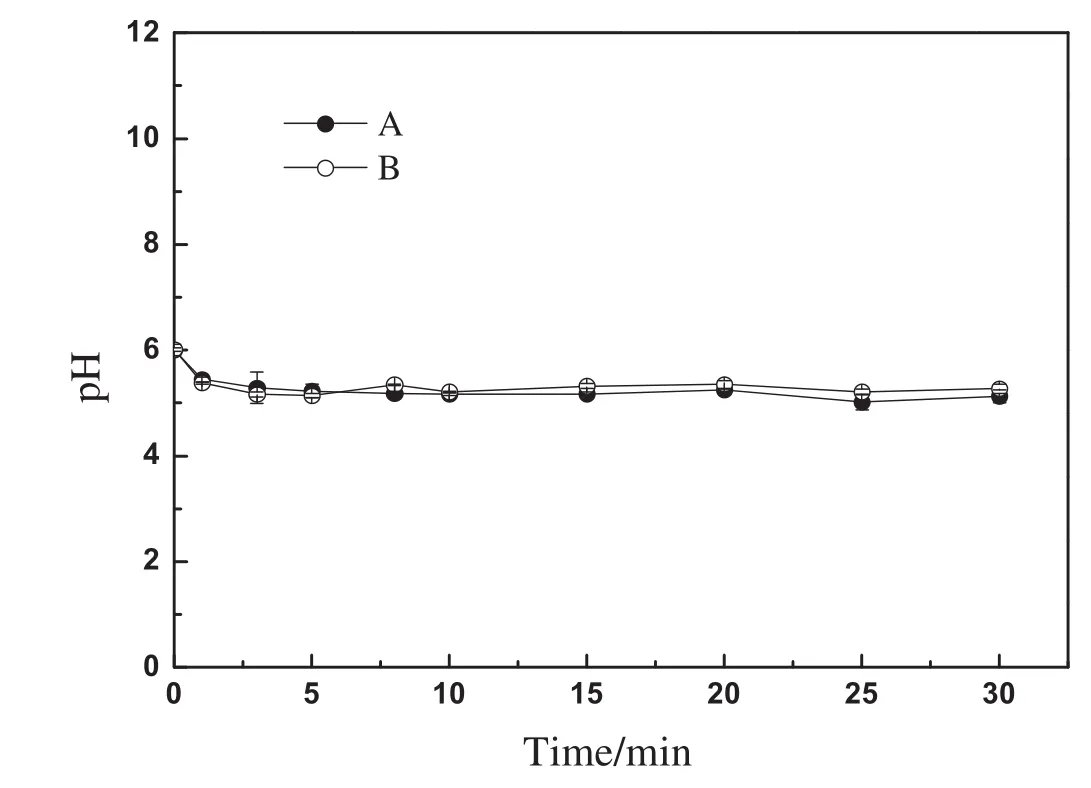
Fig.7.Kinetic curves of MIEX desorption as a function of the pH value in ultrapure water.
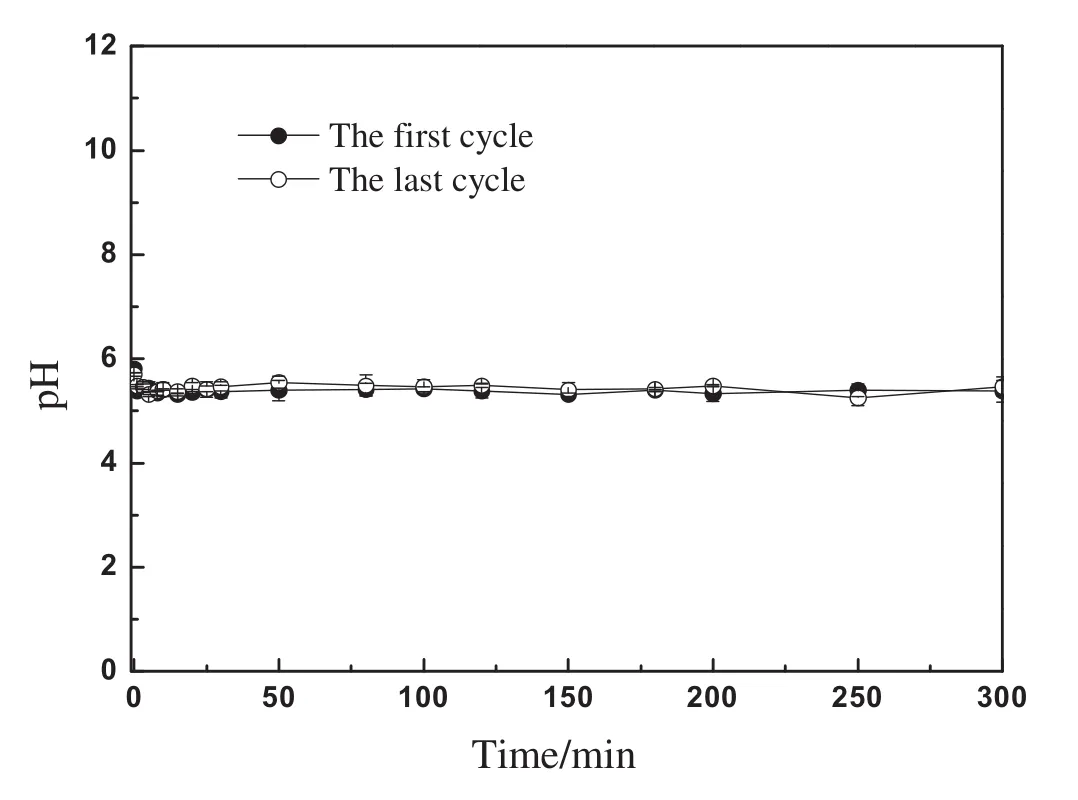
Fig.8.pH evolution of EE2 solution in the adsorption process of multi-cycle adsorptionregeneration experiment.
4.Conclusions
As a typicalnon-ionized micro-pollutant,EE2 can be removed with a much higher removal by MIEX than other micro-pollutants reported.The irreversible micro-pore adsorption of EE2 was excluded according to a multi-cycle adsorption-regeneration experiment.Ion exchange stoichiometry analysis further testified that the dominant mechanism of EE2 removal by MIEX was ion exchange instead of reversible micropore adsorption.However,The charge density of EE2 was almost zero[(0.00000219 ± 0.00000015)meq·(μg EE2)-1]in water according to the potentiometric titration method.This means EE2 normally exists in the form of neutral molecule in water,and the existence form of EE2 in water seemed to be contradictory with its removal mechanism(ion exchange)by MIEX.However,the existence form of EE2 in water was significantly affected by pH values.In alkaline environment EE2 can be intensively ionized into negatively charged groups.The experimentalresults based on the Donnan theory proved the aqueous solution in the internal pore of the resin was alkali.Therefore,EE2 molecules would be ionized into negatively charged groups in the alkali environment as they diffused into the internal pore of the resin and then removed from water through ion exchange.
References
[1]M.Drikas,M.Dixon,J.Morran,Long term case study of MIEX pre-treatment in drinking water;understanding NOM removal,Water Res.45(2011)1539–1548.
[2]M.Arias-Paic,K.M.Cawley,S.Byg,F.L.Rosario-Ortiz,Enhanced DOC removal using anion and cation ion exchange resins,Water Res.88(2016)981–989.
[3]H.Huang,H.H.Cho,K.J.Schwab,J.G.Jacangelo,Effects of magnetic ion exchange pretreatment on low pressure membrane filtration of natural surface water,Water Res.46(2012)5483–5490.
[4]J.Y.Morran,M.Drikas,D.Cook,D.B.Bursill,Comparison of MIEX treatment and coagulation on NOM character,Water Sci.Technol.Water Supply 4(2004)129–137.
[5]B.P.Allpike,A.Heitz,C.A.Joll,R.I.Kagi,G.Abbt-Braun,F.H.Frimmel,T.Brinkmann,N.Her,G.Amy,Size exclusion chromatography to characterize DOC removal in drinking water treatment,Environ.Sci.Technol.39(2005)2334–2342.
[6]L.Ding,C.Wu,H.P.Deng,X.X.Zhang,Adsorptive characteristics of phosphate from aqueous solutions by MIEX resin,J.Colloid Interface Sci.376(2012)224–232.
[7]T.H.Boyer,P.C.Singer,Bench-scale testing of a magnetic ion exchange resin for removal of disinfection by-product precursors,Water Res.39(2005)1265–1276.
[8]S.E.H.Comstock,T.H.Boyer,Combined magnetic ion exchange and cation exchange for removal of DOC and hardness,Chem.Eng.J.241(2013)366–375.
[9]K.C.Graf,D.A.Cornwell,T.H.Boyer,Removal of dissolved organic carbon from surface water by anion exchange and adsorption:bench-scale testing to simulate a two-stage countercurrent process,Sep.Purif.Technol.122(2014)523–532.
[10]M.R.Mergen,B.Jefferson,S.A.Parsons,P.Jarvis,Magnetic ion-exchange resin treatment:impact of water type and resin use,Water Res.42(2008)1977–1988.
[11]R.P.Schwarzenbach,B.I.Escher,K.Fenner,T.B.Hofstetter,C.A.Johnson,U.von Gunten,B.Wehrli,The challenge of micropollutants in aquatic systems,Science 313(2006)1072–1077.
[12]H.Humbert,H.Gallard,H.Suty,J.P.Croue,Natural organic matter(NOM)and pesticides removal using a combination of ion exchange resin and powdered activated carbon(PAC),Water Res.42(2008)1635–1643.
[13]M.Mastrup,A.I.Sch?fer,Removal of Estrone in Solution by Magnetic Ionic Exchange Resin(MIEX),Environmental Engineering Research Event,2001.
[14]A.I.Sch?fer,M.Mastrup,S.Venkatesh,Trace contaminant removal using magnetic ion exchange and microfiltration,Recent Adv.Water Recycl.Technol.,Brisbane 26(2001)41–54(小).
[15]T.Yi,S.Mackintosh,D.S.Aga,W.F.Harper Jr.,Exploring 17a-ethinylestradiol removal,mineralization,and bioincorporation in engineered bioreactors,Water Res.45(2011)1369–1377.
[16]C.Liu,B.Wang,Y.Deng,J.Wang,W.Chen,Y.Liu,M-PGMA as a new water treatment agent to remove oxytetracycline from water,Water Sci.Technol.Water Supply 16(2016)295–304.
[17]T.H.Boyer,P.C.Singer,Stoichiometry of removal of natural organic matter by ion exchange,Environ.Sci.Technol.42(2007)608–613.
[18]O.Gibert,N.Pages,X.Bernat,J.L.Cortina,Removal of dissolved organic carbon and bromide by a hybrid MIEX-ultra filtration system:insight into the behaviour of organic fractions,Chem.Eng.J.312(2017)59–67.
[19]F.Helfferich,Ion Exchange,McGraw-Hill Book Co.,Inc.,New York,1962.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- Oil field produced water treatment in internal-loop airlift reactor using electrocoagulation/ flotation technique
- From pollutant to solution of wastewater pollution:Synthesis of activated carbon from textile sludge for dye adsorption
- Transesteri fication of sun flower oil in microchannels with circular obstructions
- The extraction of potassium from K-feldspar ore by low temperature molten salt method☆
- Decomposition behavior of CaSO4 during potassium extraction from a potash feldspar-CaSO4 binary system by calcination☆
