Ultrasensitive electrochemical determination of metronidazole based on polydopamine/carboxylic multi-walled carbon nanotubes nanocomposites modified GCE
Satar Tursynbolat,Yrysgul Bakytkarim,Jianzhi Huang,Lishi Wang
School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510641,China
1.Introduction
Metronidazole,one of nitroimidazole derivative drugs(Fig.1)well-known for its antimicrobial properties, is effective against trichomonas[1–3],Vincent's organisms[4]and anaerobic bacteria [5–7].However,overuse and long-term use of metronidazole will cause toxicity[8],peripheral neuropathies[9]and opticneuropathy[10,11].Therefore,itisnecessary to monitor metronidazole concentration in patients under antibiotic therapy.Several analytical methods have been reported for the determination of metronidazole,including spectrophotometry[12,13]and chromatography[14–17].However,these methods could not realize high selectivity of metronidazole determination,and such determination processes were costly and time consuming.Hence,it is important to develop an alternative method for metronidazole determination with high sensitivity and selectivity.
Nowadays,electrochemical methods have been widely used in environmental analysis and biological samples analysis[18–22].Particularly,electrochemical sensors and biosensors have been developed for pharmaceutical, food, agricultural and environmental analyses due to the advantages of fast response and good sensitivity[23–26].
Electrochemical determination based on electrochemical sensor possesses the advantages of high sensitivity low cost and easy operation,which was widely used in analytical chemistry,and separation step is usually used to increase the selectivity prior to the determination[27–29].Electrochemical sensors fabricated by different modified electrode materials have been developed for electrochemical determination [27,29].Poly-dopamine is a conductive and biocompatible polymer,which has versatile applications due to its many attractive properties[30–33].Polydopamine can be coated on different materials and can be a good support for loading metal nanoparticle to form nanocomposites[34,35],which finally was applied in various electrochemical biosensors[36–39].Moreover,the polymerization method of dopamine was facile,and its surface morphology and layer thickness can be better controlled[40–42].Furthermore,polydopamine can be easily coated on the materials surface through a very strong chemical bond[43,44].Carboxylic muti-walled carbon nanotubes(MWCNTs–COOH)have been widely applied for the development of chemical sensors due to their excellent electrical conductivity,high surface area,remarkable mechanical strength and good chemical stability[45,46].
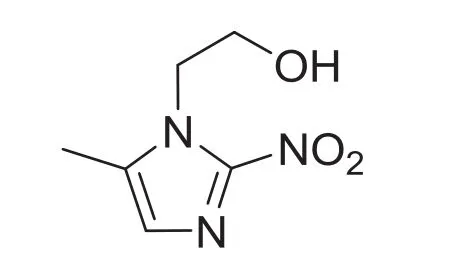
Fig.1.Chemical structure of metronidazole.
In this work,we developed a novel electrochemical sensor based on polydopamine/MWCNTs–COOH nanocomposites,where polydopamine can be easily electropolymerized to the surface of MWCNTs–COOH to form nanocomposites,and f i nally successfully realized the ultrasensitive determination for metronidazole with a wide linear detection range from 5 to 5000 μmol/dm3and a low detection limit of 0.25 μmol/dm3(S/N=3).Most importantly,the proposed sensor has been successfully applied for the quantitative determination of metronidazole in real drug samples.This work would provide an effective analytical strategy for metronidazole determination in application of real pharmaceutical and biological samples analysis.
2.Experimental
2.1.Reagents
Metronidazole(99%,analytical grade)was purchased from Macklin Biochemical Co.,Ltd.(Shanghai,China).Carboxylic multiwalled carbon nanotubes were purchased from Aladdin Industrial Company(Shanghai,China).Dopamine hydrochloride(98%,analytical grade)was purchased from J&K Chemical(Beijing,China).Drug samples were obtained from Huayueyang Biotechnology Co.,Ltd.(Beijing,China).All other reagents were of analytical grade and used without further purification.0.1 M phosphate buffer solution(PBS)was prepared by mixing NaH2PO4and Na2HPO4,and then adjusted to the required pH values with H3PO4or NaOH solution.All aqueous solutions were prepared with doubly distilled water.
2.2.Fabrication of polydopamine/MWCNTs–COOH nanocomposites/GCE sensor
First,the bare GCE was polished with 0.3 and 0.05 μm of alumina powders,then rinsed ultrasonically with absolute alcohol and distilled water,and finally dried in the nitrogen stream.5μL of 0.5 mg/mL MWCNTs–COOH homogeneous suspension was dropped onto the electrode surface and then was dried under the infrared lamp,thus obtaining MWCNTs–COOH/GCE.Finally the polydopamine was electropolymerized onto thesurfaceof MWCNTs–COOH by cyclic voltammetry in 5 mmol/dm3dopamine in 0.1 M PBS(pH=5)between-0.4 V and+0.7 V at a scan rate of 50 mV/s for 10 cycles,thus obtained polydopamine/MWCNTs–COOH nanocomposites/GCE sensor.
2.3.Apparatus and method
Cyclic voltammetry(CV),electrochemical impedance spectroscopy(EIS)and differential pulse voltammetry(DPV)experiments were performed on a CHI 660B electrochemical workstation,purchased from Chenhua Co,Ltd.(Shanghai,China).A conventional three-electrode system was used with a glassy carbon electrode(3 mm diameter)as the working electrode,a saturated calomel reference electrode(SCE)and a Pt wire as the counter electrode.The differential pulse voltammetry scans ranged from-0.4 V to-1.0 V with amplitude of 0.05 V,pulse width of 0.05 s,pulse period of 0.5 s,sampling width of 0.0167,and increment of 0.004 V.For CV,scan rate was 50 mV/s,sample interval was 0.001 V.Electrochemical impedense spectroscopy was obtained in 5 mmol/dm3K3[Fe(CN)6]/K4[Fe(CN)6]solution containing 0.1 M KCl under open circuit potential with frequency range from 0.1 Hz to 100 kHz and 5 mV amplitude.The surface morphology was characterized using a field emission scanning electron microscope(FE-SEM;Zeiss Ultra55,Germany).
For the determination of metronidazole,the detection limit(Cm)was obtained using the following equation:

Where m is the slope of the calibration plot in the linear range,and Sbis the standard deviation of the blank response which was obtained from 20 replicate measurements of the blank PBS buffer solution.
3.Results and discussion
3.1.Characterization of polydopamine/MWCNTs–COOH nanocomposites modified GCE
The SEM images of MWCNTs–COOH/GCE and polydopamine/MWCNTs–COOH nanocomposites/GCE are shown in Fig.2.The MWCNTs–COOH can be obviously observed in Fig.2A,when the polydopamine was electropolymerized onto the electrode surface,a rough polymer film could be obviously observed on the surface of MWCNTs–COOH,indicating the successful preparation of polydopamine/MWCNTs–COOH nanocomposites/GCE sensor(Fig.2B).
Fig.3A shows cyclic voltammograms of bare GCE,MWCNTs–COOH/GCE and polydopamine/MWCNTs–COOH nanocomposites/GCE in the presence of 5 mmol/dm3K3Fe(CN)6]/K4[Fe(CN)6]solution containing 0.1 M KCl.A pair of reversible oxidation and reduction peaks were observed at 0.26 and 0.17 V,respectively,for the bare GCE(curve a).After being modified with the MWCNTs–COOH(curve b),it showed obvious increased redox peak currents because MWCNTs–COOH can dramatically increase the electrode surface area and possesses good electrical conductivity[47].Moreover,the polydopamine/MWCNTs–COOH nanocomposites/GCE(curve c)showed further enhanced redox peak currents compared with MWCNTs–COOH/GCE because polydopamine can accelerate the electron transfer eff i ciency between the electrode surface and solution.
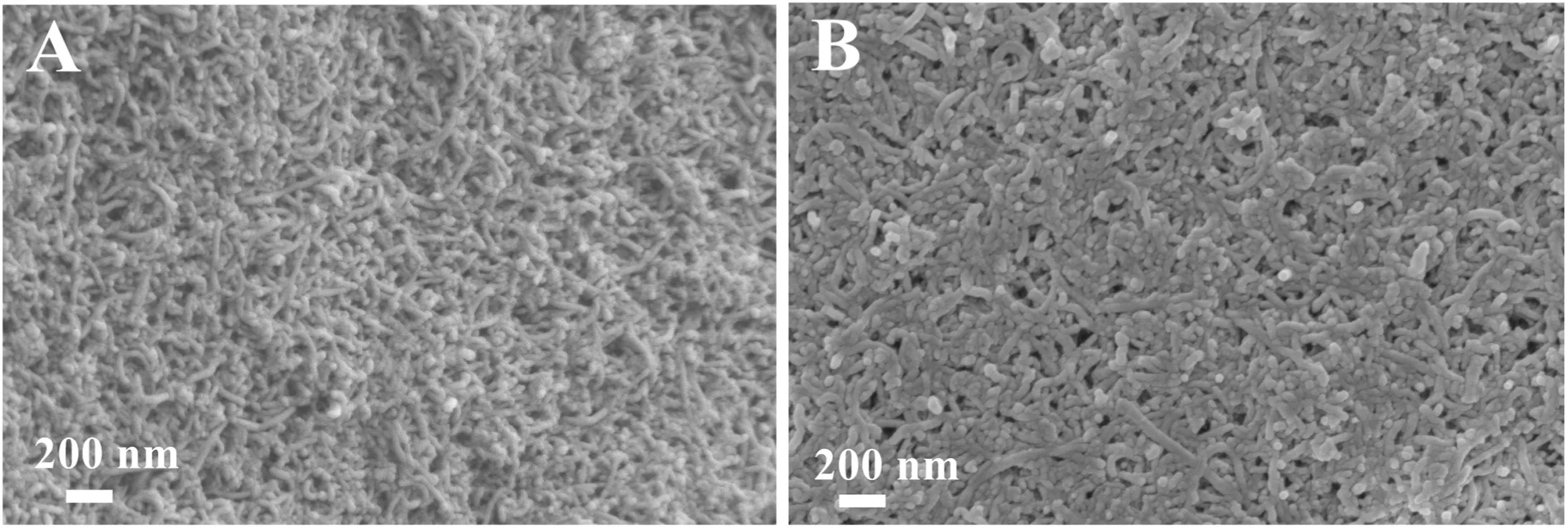
Fig.2.SEM images of(A)MWCNTs–COOH/GCE and(B)polydopamine/MWCNTs–COOH nanocomposites/GCE.
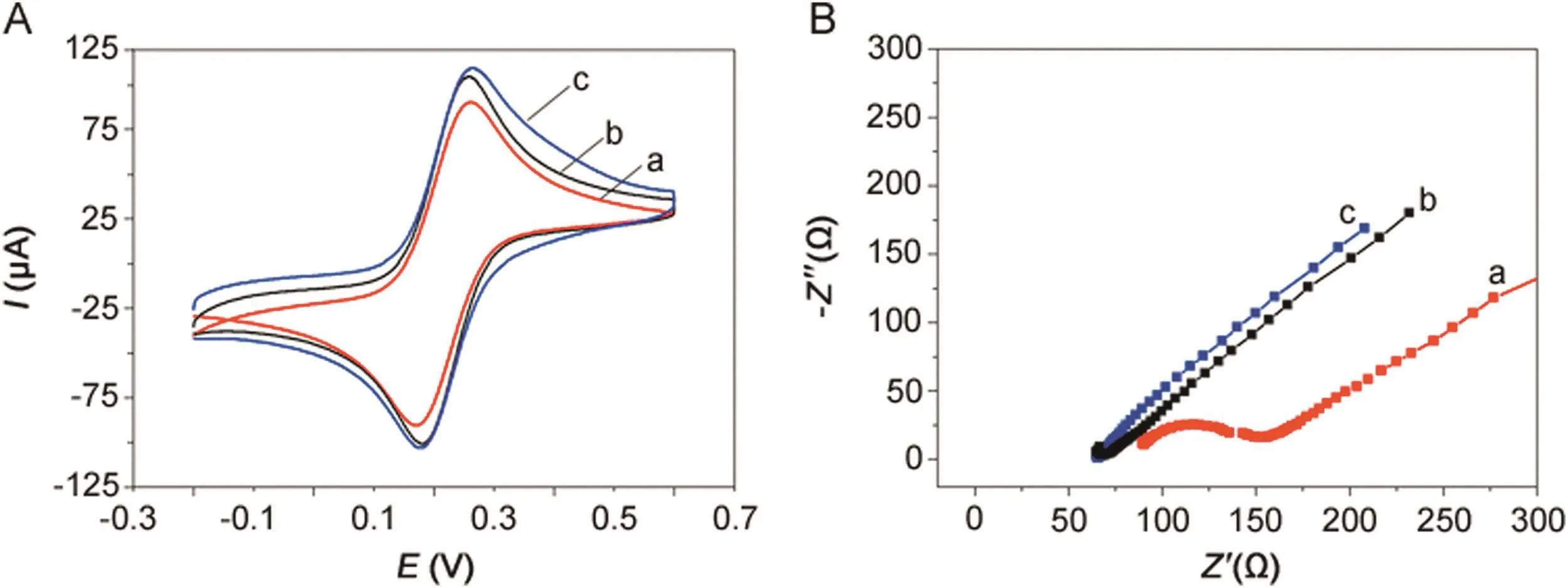
Fig.3.(A)Cyclic voltammograms and(B)Electrochemical impedance spectroscopy obtained at(a)bare GCE,(b)MWCNTs–COOH/GCE and(c)polydopamine/MWCNTs–COOH nanocomposites/GCE in 5 mmol/dm3K3[Fe(CN)6]/K4[Fe(CN)6]solution containing 0.1 M KCl.
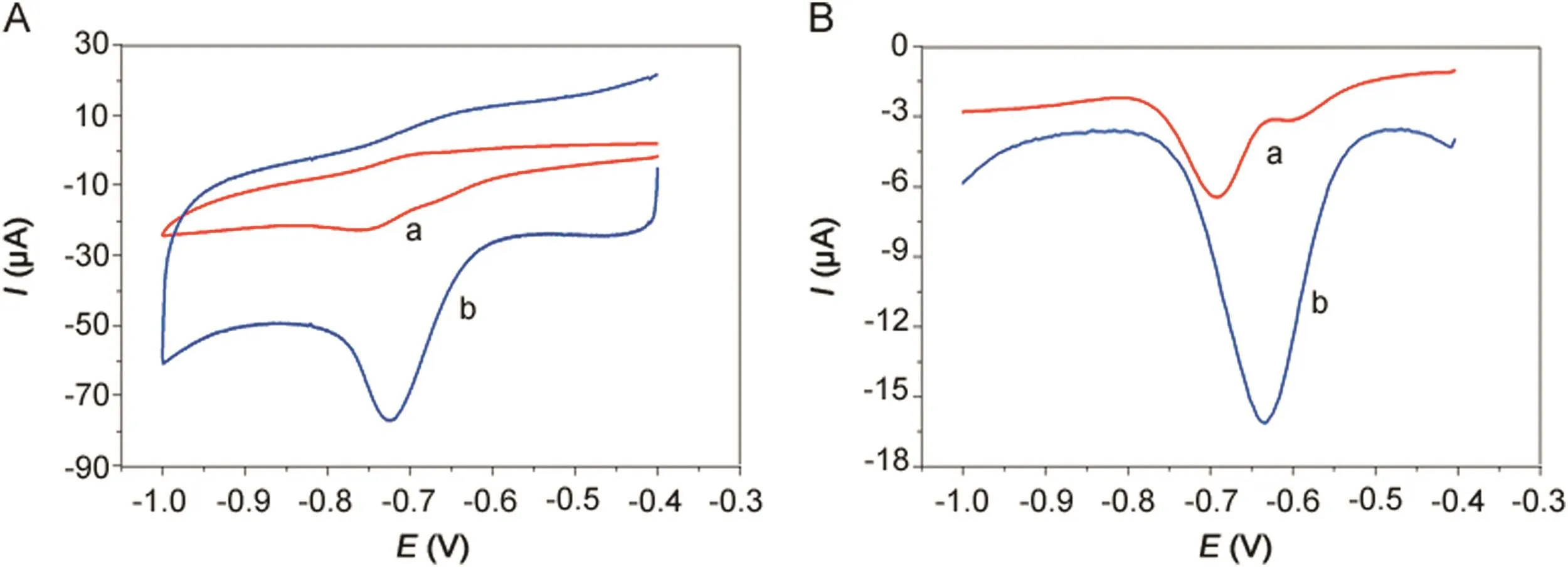
Fig.4.(A)CVs and(B)DPVs of 500 μmol/dm3metronidazole in 0.1 M PBS(pH=10)buffer solution at(a)bare GCE and(b)polydopamine/MWCNTs–COOH nanocomposites/GCE.
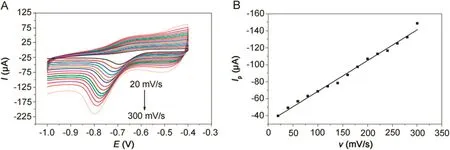
Fig.5.(A)CVs of 500 μmol/dm3metronidazole at the polydopamine/MWCNTs–COOH nanocomposites/GCE in 0.1 M PBS(pH=10)buffer solution at different scan rates.(B)The relationship between the reduction peak currents and scan rates.
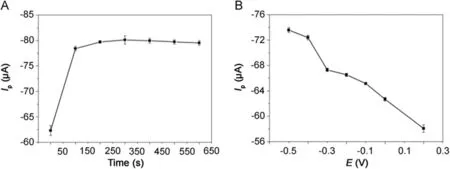
Fig.6.The effect of(A)accumulation time and(B)accumulation potential on the reduction peak current of 500 μmol/dm3metronidazole in 0.1 M PBS(pH=10)buffer solution at the polydopamine/MWCNTs–COOH nanocomposites/GCE.
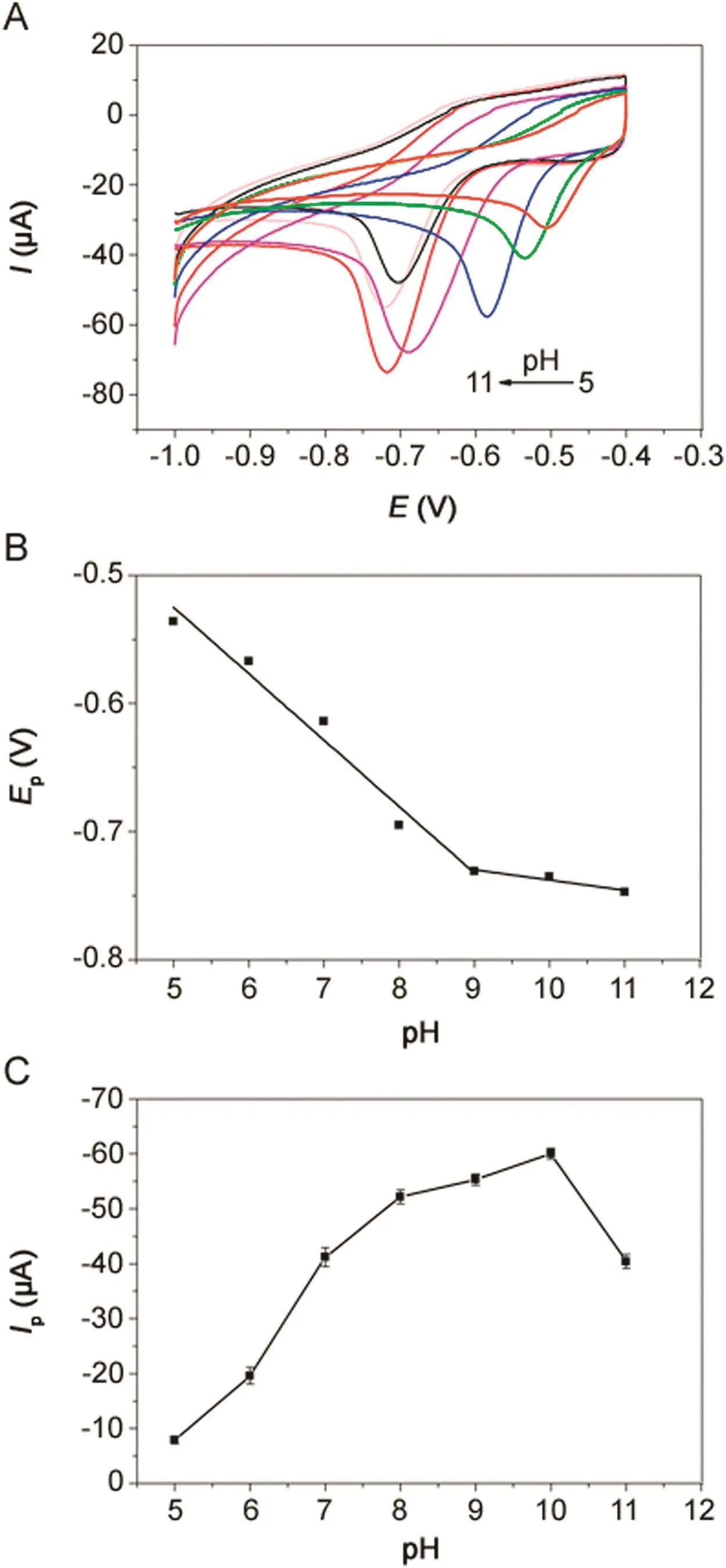
Fig.7.(A)CVs of 500 μmol/dm3metronidazole in 0.1 M PBS(pH=10)buffer solution at different pH values at the polydopamine/MWCNTs–COOH nanocomposites/GCE.The relationship of(B)reduction peak potentials vs.pH values and(C)reduction peak currents vs.pH values.
Electrochemical impedance spectroscopy(EIS)is a powerful tool for studying the surface-modified electrode.Fig.3B shows the EISplotsofbareGCE,MWCNTs–COOH/GCE,polydopamine/MWCNTs–COOH nanocomposites/GCE at 5 mmol/dm3K3[Fe(CN)6]/K4[Fe(CN)6]in 0.1 M KCl.The bare GCE(curve a)possesses a small resistance.When MWCNTs–COOH was modified onto the bare GCE surface(curve b),it displayed a straight line in the Nyquist plot because the resistance was significantly decreased.Moreover,thepolydopamine/MWCNTs–COOHnanocomposites/GCE(curve c)also displayed a straight line in the Nyquist plot,which almost showed the resistance same as MWCNTs–COOH/GCE,because polydopamine/MWCNTs–COOH nanocomposites also possess excellent electron transfer efficiency.Therefore,both the CV and EIS plots proved the successful preparation of polydopamine/MWCNTs–COOH nanocomposites/GCE sensor.
3.2.Electrochemical behavior of metronidazole at the polydopamine/MWCNTs–COOH nanocomposites/GCE sensor
The electrochemical behavior of bare GCE and polydopamine/MWCNTs–COOH nanocomposites/GCE for determination of 500 μmol/dm3metronidazole in 0.1 M PBS(pH 10.0)buffer solution is shown in Fig.4A.The reduction peak current and peak potential of metronidazole at the bare GCE(curve a)were Ip=-8.44 μA and Ep=-0.749 V.However,compared to the bare GCE,the polydopamine/MWCNTs–COOH nanocomposites/GCE(curve b)exhibited significantly increased reduction peak current(Ip=-41.12 μA)and significantly increased reduction peak potential(Ep=-0.721 V)of metronidazole.The significantly increased reduction peak potential and significantly increased reduction peak current both confirmed the polydopamine/MWCNTs–COOH nanocomposites possess strong catalytic activity towards the reduction of metronidazole.Moreover,the DPVs results in Fig.4B correspond with the CVs in Fig.4A.Therefore,the polydopamine/MWCNTs–COOH nanocomposites/GCE sensor can be successfully utilized for the determination of metronidazole.
3.3.The effect of scan rate
The CVs of polydopamine/MWCNTs–COOH nanocomposites/GCE in 500 μmol/dm3metronidazole at different scan rates are shown in Fig.5A,where the reduction peak currents showed linearity with the scan rates.And the linear regression equation can be expressed as Ip(μA)=-0.363ν(mV/s)-32.399(R=-0.9914)in Fig.5B,indicating that the reduction of the metronidazole is a typical adsorption controlled process.Therefore,it is necessary to study the effect of accumulation time and accumulation potential in order to obtain more sensitive determination for metronidazole.
3.4.The effect of accumulation time and accumulation potential
The effect of accumulation time and accumulation potential for the determination of metronidazole was studied by DPVs in Fig.6.As shown in Fig.6A,at the accumulation potential of-0.5 V,the reduction peak current increased gradually with the accumulation time and reached the maximum value when the accumulation time was 200 s.However,the reduction peak current almost remained the same after 200 s due to the saturation of surface active catalytic sites of polydopamine/MWCNTs–COOH nanocomposites/GCE.Thus,the optimal accumulation time of 200 s was employed in our experiments.With the optimal accumulation time determined above,we further studied the effect of accumulation potential on reduction peak current of metronidazole.As shown in Fig.6B,the reduction peak current decreased gradually with the increase of accumulation potential;therefore,the accumulation potentialwaschosen at-0.5 V fordetermination ofmetronidazole in our later experiments.
3.5.The pH effect
The effect of pH value on the electrochemical response of 500 μmol/dm3metronidazole in 0.1 M PBS with pH value ranging from 5.0 to 11.0 at the polydopamine/MWCNTs–COOH nanocomposites/GCE was investigated by CV(Fig.7A).The reduction peak potentials showed linearity with pH values ranging from 5.0–9.0 and 9.0–11.0,with the linear regression equations of Ep=-0.0518pH–0.266(R=-0.9687)and Ep=-0.008pH–0.658(R=-0.9462),respectively(Fig.7B),indicating two different reaction mechanisms of metronidazole.According to previous reports[39,48],the reaction mechanisms of metronidazole are listed below:
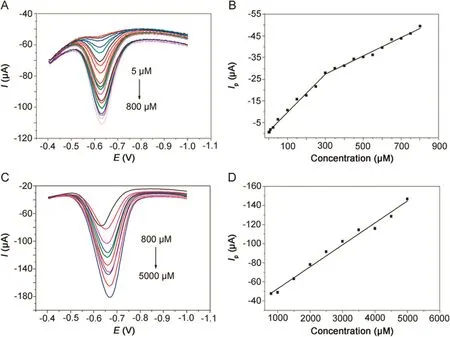
Fig.8.DPVs of metronidazole at(A)5–800 μmol/dm3and(C)800–5000 μmol/dm3in 0.1 M PBS(pH=10)buffer solution at the polydopamine/MWCNTs–COOH nanocomposites/GCE.Linear relationships between reduction peak currents and concentrations at(B)5–800 μmol/dm3and(D)800–5000 μmol/dm3.
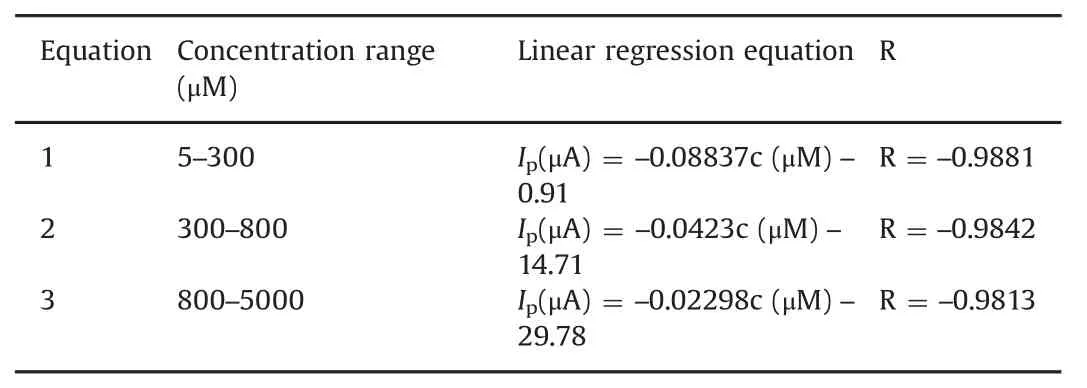
Table 1 Linear regression equations of metronidazole under different concentration rangs.
In pH values of 5·0–9·0:
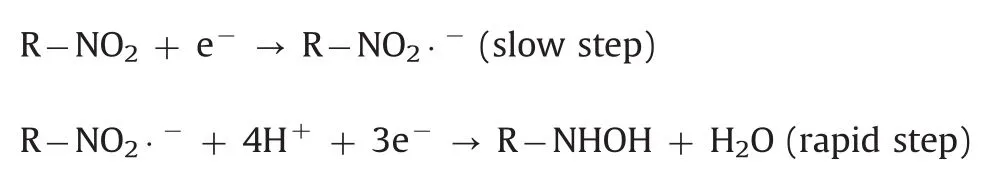
In pH values of 9·0–11·0:
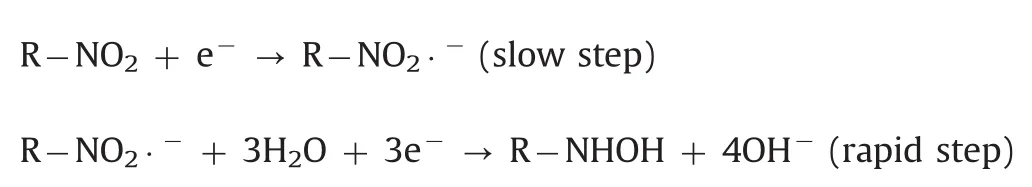
Moreover,as shown in Fig.7C,because the reduction peak current achieved the maximum value in pH=10.0,the pH value of 10.0 was chosen as the best pH value for the determination of metronidazole.
3.6.The quantitative determination of metronidazole
The quantitative determination of metronidazole at the polydopamine/MWCNTs–COOH nanocomposites/GCE was achieved by DPV under optimal conditions addressed above.As shown in Fig.8,the reduction peak currents of metronidazole at the polydopamine/MWCNTs–COOH nanocomposites/GCE increased linearly with concentration ranges of 5–300 μmol/dm3,300–800 μmol/dm3and 800–5000 μmol/dm3,and their corresponding linear regression equations are listed in Table 1.
The detection limit of metronidazole was determined to be 0.25 μmol/dm3(S/N=3).Moreover,compared with recently most reported electrochemical sensors[49–55]for determination of metronidazole,our proposed nanocomposites sensor could fi nish the ultrasensitive determination of metronidazole with a much widerlinearrangesand a much lowerdetection limits(Table 2).
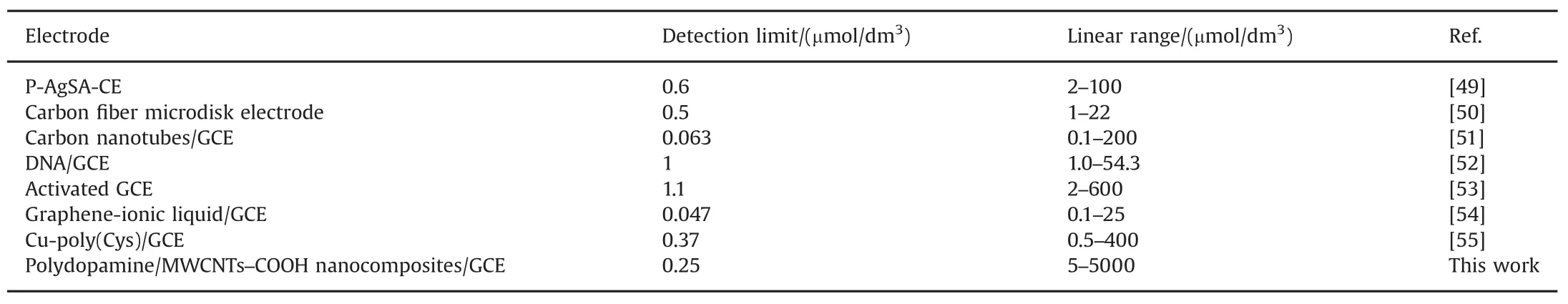
Table 2 Comparison of performances of the polydopamine/MWCNTs–COOH nanocomposites/GCE with other modif i ed electrodes.
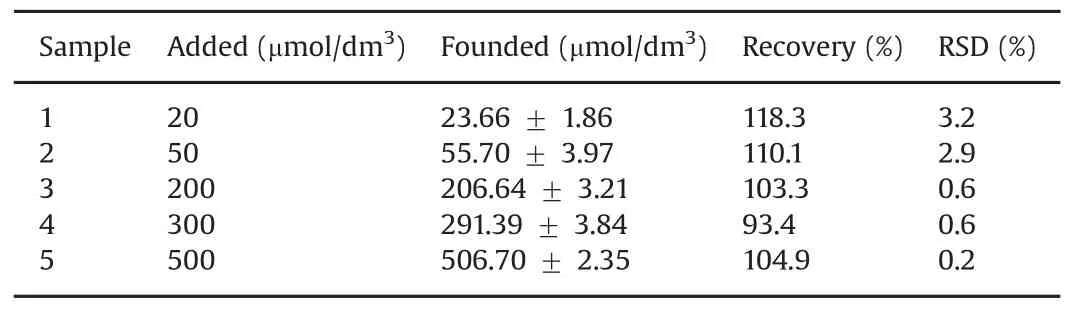
Table 3 Practical determination of metronidazole in real drug samples(n=3).(Sample responses are expressed as a confidence interval of 95%probability).
3.7.Selectivity,stability and reproducibility of the polydopamine/MWCNTs–COOH nanocomposites/GCE sensor
Selectivity,stability and reproducibility of the proposed sensors are key factors for their practical application.The proposed sensor was not affected by additions of 100-fold concentrations of various inorganic ions(K+,Mg2+,Zn2+,Na+,Ca2+,PO43-,SO42-,F-,CO32-,NO3-and Cl-,signal change below 3%)and 10-fold concentrations of some organic compounds(oxalic acid,ascorbic acid,glucose,citric acid,cystine,alanine and tartaric acid,signal change below 6%).This results suggested that the proposed sensor possesses excellent selectivity for the determination of metronidazole.After the prepared electrode was stored at 4°C in a refrigerator for 1 month,the reduction peak current of metronidazole remained 95.2%of its initial value,indicating that the proposed sensor possesses good stability.Moreover,four modified electrodes were fabricated to estimate the sensor's reproducibility,and the relative standard deviation(RSD)of detection measurements was calculated to be 2.5%for metronidazole,suggesting that the proposed sensor possesses high reproducibility.Therefore,the polydopamine/MWCNTs–COOH nanocomposites/GCE sensor is promising for determination of metronidazole with excellent selectivity,stability and reproducibility.
3.8.Real samples determination
The practical analytical application of the polydopamine/MWCNTs–COOH nanocomposites/GCE sensor was evaluated by determination of metronidazole in real drug samples by standardaddition technique.Three parallel experiments were performed on all measurements.As shown in Table 3,the recovery of the real samples ranged between 93.4%and 118.3%,and the RSD values were less than 4%,indicating that the our proposed sensor can be successfully applied forthepracticaldetermination ofmetronidazole in real samples.
4.Conclusions
In summary,we successfully develop an ultrasensitive electrochemical sensor for metronidazole determination,which was based on polydopamine/MWCNTs–COOH nanocomposites.Moreover,the fabrication of polydopamine/MWCNTs–COOH nanocomposites/GCE sensor was simple,where polydopamine can coat on the surface ofMWCNTs–COOH via asimple electropolymerization process.Under optimized conditions,the proposed sensorshowed widerlineardetection range from 5 to 5000 μmol/dm3and a low detection limit of 0.25 μmol/dm3(S/N=3)for metronidazole,and was successfully applied for the practical determination of metronidazole in real drug samples.The proposed sensor shows broad potential in application of real pharmaceutical and biological samples analysis.
Conflicts of interest
The authors declare that there are no conflicts of interest.
This work was financially supported by the National Natural Science Foundation of China(Grant Nos.21475046,21427809).
[1]N.C.Desai,A.S.Maheta,K.M.Rajpara,et al.,Green synthesis of novel quinolone based imidazole derivatives and evaluation of their antimicrobial activity,J.Saudi Chem.Soc.18(2014)963–971.
[2]A.M.Jarrad,T.Karoli,A.Debnath,et al.,Metronidazole–triazole conjugates:activity against Clostridium difficile and parasites,Eur.J.Med.Chem.101(2015)96–102.
[3]L.A.Dunn,K.T.Andrews,J.S.McCarthy,et al.,The activity of protease inhibitors against Giardia duodenalis and metronidazole-resistant Trichomonas vaginalis,Int.J.Antimicrob.Agents 29(2007)98–102.
[4]A.H.Davies,J.A.Mafadzean,S.Squires,Treatment of Vincent's stomatitis with metronidazole,Br.Med.J.5391(1964)1149–1150.
[5]N.Dione,S.Khelai fi a,J.C.Lagier,et al.,The aerobic activity of metronidazole against anaerobic bacteria,Int.J.Antimicrob.Agents 45(2015)537–540.
[6]A.Katsandri,A.Avlamis,A.Pantazatou,et al.,In vitro activities of Tigecycline against recently isolated Gram-negative anaerobic bacteria in Greece,including metronidazole-resistant strains,Diagn.Microbiol.Infect.Dis.55(2006)231–236.
[7]A.V.Scorza,M.R.Lappin,Metronidazole for the treatment of feline giardiasis,J.Feline Med.Surg.6(2004)157–160.
[8]M.W.Carroll,D.Jeon,J.M.Mountz,et al.,Ef fi cacy and safety of metronidazole for pulmonary multidrug-resistant tuberculosis,J.Antimicrob.Agents Chemother.57(2013)3903–3909.
[9]A.Etxeberria,S.Lonneville,M.P.Rutgers,et al.,Metronidazole-cerebellopathy associated with peripheral neuropathy,downbeat nystagmus and bilateral ocular abduction de fi cit,Rev.Neurol.168(2012)193–195.
[10]N.M.McGrath,B.Kent-Smith,D.M.Sharp,Reversible optic neuropathy due to metronidazole,Clin.Exp.Ophthalmol.35(2007)585–586.
[11]M.P.Prabhakaran,M.Zamani,B.Felice,et al.,Electrospraying technique for the fabrication of metronidazole contained PLGA particles and their release profile,Mater.Sci.Eng.C 56(2015)66–73.
[12]A.K.Mishra,A.Kumar,A.Mishra,et al.,Development of ultraviolet spectroscopic method for the estimation of metronidazole benzoate from pharmaceutical formulation,J.Nat.Sci.Biol.Med.5(2014)261–264.
[13]G.O.El-Sayed,Polarographic determination of metronidazole in pharmaceutical formulations and urine,Microchem.J.55(1997)110–114.
[14]W.Tian,L.Gao,Y.Zhao,et al.,Simultaneous determination of metronidazole,chloramphenicol and 10 sulfonamide residues in honey by LC–MS/MS,Anal.Methods 5(2013)1283–1288.
[15]C.Ho,D.W.M.Sin,K.M.Wong,et al.,Determination of dimetridazole and metronidazole in poultry and porcine tissues by gas chromatography–electron capture negative ionization mass spectrometry,Anal.Chim.Acta 530(2005)23–31.
[16]H.M.Maher,R.M.Youssef,R.H.Khalil,et al.,Simultaneous multi residue determination of metronidazole and spiramycin in fi sh muscle using high performance liquid chromatography with UV detection,J.Chromatogr.B 876(2008)175–181.
[17]J.Li,Y.B.Wang,L.Wu,et al.,Fabrication of multi-walled carbon nanotubes/oxide reinforced hollow fi bers by sol–gel technique for rapid determination of metronidazole in milk,Anal.Methods 6(2014)1401–1411.
[18]M.M.Ardakani,H.Beitollahi,Z.Taleat,et al.,Selective voltammetric determination of D-penicillamine in the presence of tryptophan at a modified carbon paste electrode incorporating TiO2nanoparticles and quinizarine,J.Electroanal.Chem.644(2010)1–6.
[19]M.M.Ardakani,Z.Taleat,H.Beitollahi,et al.,Electrocatalytic oxidation and nanomolar determination of guanine at the surface of a molybdenum(VI)complex-TiO2nanoparticle modified carbon paste electrode,J.Electroanal.Chem.624(2008)73–78.
[20]S.Tajika,M.A.Taher,H.Beitollahi,Simultaneous determination of droxidopa and carbidopa using a carbon nanotubes paste electrode,Sens.Actuators B Chem.188(2013)923–930.
[21]V.Vyskocil,J.Barek,Polarographic and voltammetric study of genetoxic 2,7-dinitro fluoren-9-one and its determination using mercury electrodes,Collect.Czech Chem.C 74(2009)1675–1696.
[22]O.Yosypchuk,J.Barek,V.Vyskocil,Voltammetric determination of carcinogenic derivatives of pyrene using a boron-doped diamond fi lm electrode,Anal.Lett.45(2012)449–459.
[23]H.Beitollahi,H.K.Maleh,H.Khabazzadeh,Nanomolar and selective determination of epinephrine in the presence of norepinephrine using carbon paste electrode modified with carbon nanotubes and novel 2-(4-Oxo-3-phenyl-3,4-dihydro-quinazolinyl)-N-phenyl-hydrazinecarbothioamide,Anal.Chem.80(2008)9848–9851.
[24]H.Beitollahi,M.M.Ardakani,H.Naeimi,et al.,Electrochemical characterization of 2,2′-[1,2-ethanediylbis(nitriloethylidyne)]-bis-hydroquinone-carbon nanotube paste electrode and its application to simultaneous voltammetric determination of ascorbic acid and uric acid,J.Solid State Electrochem.13(2009)353–363.
[25]M.M.Ardakani,H.Beitollahi,M.K.Amini,et al.,Simultaneous determination of epinephrine and uric acid at a gold electrode modified by a 2-(2,3-dihydroxy phenyl)-1,3-dithiane self-assembled monolayer,J.Electroanal.Chem.651(2011)243–249.
[26]M.Baghayeri,M.Namadchian,H.K.Maleh,et al.,Determination of nifedipine using nanostructured electrochemical sensor based on simple synthesis of Ag nanoparticles at the surface of glassy carbon electrode:application to the analysis of some real samples,J.Electroanal.Chem.697(2013)53–59.
[27]V.Vyskocil,J.Barek,Mercury electrodes-possibilities and limitations in environmental electroanalysis,Crit.Rev.Anal.Chem.39(2009)173–188.
[28]V.Vyskocil,J.Barek,Electroanalysis of nitro and amino derivatives of polycyclic aromatic hydrocarbons,Curr.Org.Chem.15(2011)3059–3076.
[29]J.Gajdar,E.Horakova,J.Barek,et al.,Recent applications of mercury electrodes for monitoring of pesticides:a critical review,Electroanalysis 28(2016)2659–2671.
[30]M.L.Lynge,R.van der Westen,A.Posta,et al.,Polydopamine a nature-inspired polymer coating for bilchemical scince,Nanoscale 3(2011)4916–4928.
[31]Y.Li,Y.Su,X.Zhao,et al.,Antifouling,high-flux nanofiltration membranes enabled by dual functional polydopamine,ACS Appl.Mater.Interfaces 6(2014)5548–5557.
[32]C.Wang,J.Zhou,P.Wang,et al.,Robust nanoparticle-DNA conjugates based on mussel-inspired polydopamine coating for cell imaging and tailored self-assembly,Bioconjug.Chem.27(2016)815–823.
[33]Q.Liu,N.Wang,J.Caro,et al.,Bio-inspired polydopamine:a versatile and powerful platform for covalent synthesis of molecular sieve membranes,J.Am.Chem.Soc.135(2013)17679–17682.
[34]J.Ryu,S.H.Ku,H.Lee,et al.,Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization,Adv.Funct.Mater.20(2010)2132–2139.
[35]W.Zhang,Y.Tang,J.Liu,et al.,An electrochemical sensor for detecting triglyceride based on biomimetic polydopamine and gold nanocomposite,J.Mater.Chem.B 2(2014)8490–8495.
[36]M.Amiri,E.Amali,A.Nematollahzadeh,et al.,Poly-dopamine films:voltammetric sensor for pH monitoring,Sens.Actuators B-Chem.228(2016)53–58.
[37]M.Amiri,E.Amali,A.Nematollahzadeh,Poly-dopamine thin film for voltammetric sensing of atenolol,Sens.Actuators B-Chem.216(2015)551–557.
[38]L.Zheng,L.Xiong,Y.Li,et al.,Facile preparation of polydopamine-reduced graphene oxide nanocomposite and its electrochemical application in simultaneous determination of hydroquinone and catechol,Sens.Actuators B-Chem.177(2013)344–349.
[39]H.B.Ammar,M.B.Brahim,R.Abdelhedi,et al.,Boron doped diamond sensor for sensitive determination of metronidazole:mechanistic and analytical study by cyclic voltammetry and square wave voltammetry,Mater.Sci.Eng.C 59(2016)604–610.
[40]J.Z.Huang,X.L.Shen,R.L.Wang,et al.,A highly sensitive metronidazole sensor based on a Pt nanospheres/polyfurfural film modified electrode,RSC Adv.7(2017)535–542.
[41]E.L.Ciolkowski,B.R.Cooper,J.A.Jankowski,et al.,Direct observation of epinephrine and norepinephrine cosecretion from individual adrenal medullary chromaf fin cells,J.Am.Chem.Soc.114(1992)2815–2821.
[42]E.L.Ciolkowski,K.M.Maness,P.S.Cahill,et al.,Disproportionation during electrooxidation of catecholamines at carbon- fiber microelectrodes,Anal.Chem.66(1994)3611–3617.
[43]Y.S.Choi,H.Kang,D.G.Kim,et al.,Mussel-inspired dopamine-and plant-based cardanol-containing polymer coatings for multifunctional filtration membranes,ACS Appl.Mater.Interfaces 6(2014)21297–21307.
[44]H.Lee,S.M.Dellatore,W.M.Miller,et al.,Mussel-inspired surface chemistry for multifunctional coatings,Science 318(2007)426–430.
[45]D.Eder,Carbon nanotube-inorganic hybrids,Chem.Rev.110(2010)1348–1385.
[46]H.Beitollahi,S.Mohammadi,Selective voltammetric determination of norepinephrine in the presence of acetaminophen and tryptophan on the surface of a modi fi ed carbon nanotube paste electrode,Mater.Sci.Eng.C 33(2013)3214–3219.
[47]J.Z.Huang,S.L.Bai,G.Q.Yue,et al.,Coordination matrix/signal amplifier strategy for simultaneous electrochemical determination of cadmium(II),lead(II),copper(II),and mercury(II)ions based on polyfurfural film/multi-walled carbon nanotube modified electrode,RSC Adv.7(2017)28556–28563.
[48]A.Hajkova,J.Hranicek,J.Barek,et al.,Voltammetric determination of trace amounts of 2-amino fl uoren-9-one at a mercury meniscus modi fi ed silver solid amalgam electrode,Electroanalysis 25(2013)295–302.
[49]V.Vyskocil,T.Navratil,A.Danhel,et al.,Voltammetric determination of selected nitro compounds at a polished silver solid amalgam composite electrode,Electroanalysis 23(2011)129–139.
[50]P.Bartlett,E.Ghoneim,G.El-Hefnawy,et al.,Voltammetry and determination of metronidazole at a carbon fiber microdisk electrode,Talanta 66(2005)869–874.
[51]A.Salimi,M.Izadi,R.Hallaj,et al.,Simultaneous determination of ranitidine and metronidazole at glassy carbon electrode modified with single wall carbon nanotubes,Electroanalysis 19(2007)1668–1676.
[52]A.M.Brett,S.H.Serrano,I.G.Gutz,et al.,Comparison of the voltammetric behavior of metronidazole at a DNA-modified glassy carbon electrode,a mercury thin film electrode and a glassy carbon electrode,Electroanalysis 9(1997)110–114.
[53]S.A.?zkan,Y.?zkan,Z.?entürk,Electrochemical reduction of metronidazole at activated glassy carbon electrode and its determination in pharmaceutical dosage forms,J.Pharm.Biomed.Anal.17(1998)299–305.
[54]J.Peng,C.Hou,X.Hu,Determination of metronidazole in pharmaceutical dosage forms based on reduction at graphene and ionic liquid composite film modified electrode,Sens.Actuators B-Chem.169(2012)81–87.
[55]Y.Gu,X.Y.Yan,W.L.Liu,et al.,Biomimetic sensor based on copper-poly(cysteine) film for the determination of metronidazole,Electrochim.Acta 152(2015)108–116.
 Journal of Pharmaceutical Analysis2018年2期
Journal of Pharmaceutical Analysis2018年2期
- Journal of Pharmaceutical Analysis的其它文章
- Surrogate potency assays:Comparison of binding profiles complements dose response curves for unambiguous assessment of relative potencies
- Eco-friendly reduced graphene oxide for the determination of mycophenolate mofetil in pharmaceutical formulations
- Quantitative determination of erlotinib in human serum using competitive enzyme-linked immunosorbent assay
- Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L.fruit:In silico and in vivo approaches
- Structural confirmation of sulconazole sulfoxide as the primary degradation product of sulconazole nitrate
- Physicochemical characterization,the Hirshfeld surface,and biological evaluation of two meloxicam compounding pharmacy samples
