Combined acupuncture and HuangDiSan treatment affects behavior and synaptophysin levels in the hippocampus of senescence-accelerated mouse prone 8 after neural stem cell transplantation
Chun-lei Zhou, Lan Zhao , Hui-yan Shi Jian-wei Liu, Jiang-wei Shi Bo-hong Kan Zhen Li Jian-chun Yu Jing-xian Han
1 Tianjin First Center Hospital, Tianjin, China
2 First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
3 Tianjin Key Laboratory of Acupuncture and Moxibustion, Tianjin, China
4 Tianjin University of Traditional Chinese Medicine, Tianjin, China
Introduction
Neural stem cells (NSCs) show great promise for the treatment of damaged nerves in inherited and injury-related diseases (Elliott Donaghue et al., 2014; Novak et al., 2016; Ma et al., 2017). Endogenous NSCs are usually in a resting state and can be activated under certain physiological or pathological stimuli (Harris et al., 2016; Yu et al., 2016). After implantation into the brain, exogenous NSCs can repair and replace damaged nerve cells, and secrete various factors that promote the proliferation and survival of NSCs (Xiong et al.,2017). However, NSC self-renewal, activation, proliferation and differentiation are mainly dependent on the surrounding microenvironment (Llorens-Bobadilla and Martin-Villalba, 2017; Wang et al., 2017). When the environment changes, the self-renewal, proliferation, and differentiation capacity of stem cells is greatly altered (Park et al., 2015).Thus, improving the outcome of exogenous NSC transplantation is an urgent problem to be solved.
Encephalopathy can potentially be treated by NSC transplantation, aided by acupuncture to promote NSC proliferation and differentiation through the Jing-Qi-Shen conversion, marrow filling and spirit clearing (Zhao et al., 2015).Based on the theory of “Sanjiao dysfunction inducing aging”, Professor Han (First Teaching Hospital of Tianjin University of Traditional Chinese Medicine) developed Sanjiao acupuncture and HuangDiSan prescription for the treatment of Alzheimer’s disease and vascular dementia, with a good curative effect (Yu et al., 2006; Zhao et al., 2017).
Our previous study shows that Sanjiao acupuncture can promote the proliferation, migration and differentiation of endogenous NSCs in the main distribution area, the subventricular and subgranular zones, in senescence-accelerated mouse prone 8 (SAMP8) mice (Cheng et al., 2008). Sanjiao acupuncture improved learning and memory impairment,as well as behavior in dementia-model mice, and has a positive effect on NSC migration and expression of differentiation-related proteins and genes (Cheng et al., 2008). These findings indicate that acupuncture might affect the NSC microenvironment to have a positive impact on the transplantation of exogenous NSCs.
To further assess the signi ficance of the Sanjiao theory in aging and dementia, and to improve treatment methods,we evaluated the effects of Sanjiao acupuncture and Huang-DiSan on SAMP8 mice behavior and synaptophysin expression after hippocampal NSCs transplantation.
Materials and Methods
NSC isolation, culture, and transplantation
Embryos were removed from 12—16-day pregnant senescence-accelerated mouse resistance 1 (SAMR1) mice [provided by the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Animal production license No. SCXK (Jin) 2015-0003] under sterile conditions. Hippocampal samples were placed in NSC medium, containing 2% B27, 20 ng/mL epidermal growth factor, 20 ng/mL basic fibroblast growth factor, 100 U/mL 100 g/mL penicillin and streptomycin, and DMEM/F12, in a 5% CO2incubator at 37°C. After subculture and expansion, Nestin-positive cells were evaluated, and the proliferation of NSCs was observed.Proliferating NSCs were adjusted to 1 × 106/mL, and seeded in 24-well plates with coverslips. The medium was replaced by DMEM/F12 with 20% fetal bovine serum, 100 U/mL penicillin, and 100 g/mL streptomycin to induce NSC differentiation. Immunocytochemical staining was used to confirm NSCs differentiation. A neuronal nuclei (NeuN) antibody suggested differentiation into neurons, while a glial fibrillary acidic protein (GFAP) antibody suggested differentiation into astrocytes. NSC density was adjusted to 5 × 105/μL and bilateral NSC transplantation into the hippocampal dentate gyrus cell sparse areas was conducted according to the following stereotaxic coordinates relative to the anterior fontanelle: anteroposterior: ?2.06, mediolateral: ±1.75, dorsoventral: ?1.75 (Blurton-Jones et al., 2009). The left and right hippocampal regions were injected with 1 μL NSCs over 1 minute. After the operation, mice were allowed to recover for 24 hours.
Group assignment of experimental animals
The experimental procedures were carried out according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1985). The study protocol was approved by the Ethics Committee of Tianjin Medical University of China (approval number: TMUaMEC 2016007). Senescence-accelerated mice (SAM) with different phenotypes were selected from a common background strain, AKR/J, in Kyoto University,Japan. Among the SAM mice, SAMP8 mice show deficits in learning and memory abilities (Takeda et al., 1981). Fifty 8-month-old clean healthy male SAMP8 mice [provided by the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Animal production license No.SCXK (Jin) 2015-0003] were randomized into five groups,with ten mice per group. Ten 8-month-old healthy male SAMR1 mice [provided by First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Animal production license No. SCXK (Jin) 2015-0003] were used as controls. Five mice were housed per cage, with free access to food and water under a 12-hour light/dark cycle at 24 ± 2°C and 45% humidity. The groups were:
a) SAMR1 control group (RC): catching-grasping stimulation.
b) SAMP8 control group (PC): catching-grasping stimulation.
c) SAMP8 NSC transplantation group (PT): 15 days of catching-grasping stimulation before NSC transplantation,and an additional 15 days of catching-grasping stimulation post-surgery.
d) SAMP8 NSC transplantation with acupuncture group(PTA): 15 days of acupuncture intervention before NSC transplantation, and an additional 15 days of acupuncture intervention post-surgery.
e) SAMP8 NSC transplantation with HuangDiSan group(PTD): 15 days of HuangDiSan administration before NSC transplantation, and an additional 15 days of HuangDiSan administration post-surgery.
f) SAMP8 NSC transplantation with acupuncture and HuangDiSan combination group (PTC): 15 days of combined acupuncture and HuangDiSan treatment before NSCs transplantation, and an additional 15 days of combined acupuncture and HuangDiSan treatment post-surgery.
Acupuncture method
HuangDiSan delivery
Eight grams of HuangDiSan (composed with Rehmannia Root and Chinese Angelica; provided by the First Hospital Affiliated to Tianjin Medical University, license number:Z20100942) were dissolved in 8 mL 0.9% saline. Each mouse was gavaged with 0.2 mL using a 1 mL blunt syringe, once a day for 15 days, with a day off on day 7.
Morris water maze
After the 15-day treatments, behavioral changes were assessed using the Morris water maze (Tomás Pereira and Burwell, 2015). This consists of three parts: a circular pool,a platform, and a recording system. The pool was 90 cm in diameter, with a height of 50 cm. At the beginning of each experimental day, water made opaque with milk was added to the pool to a height of 30 cm, and adjusted to a temperature of 24 ± 1°C. The pool was divided into four quadrants(northeast, southeast, southwest and northwest). A cylindrical platform 9 cm in diameter and 28 cm high was placed in the center of any quadrant, such that the platform was 2 cm under the water. A camera was placed 2 m above the center of the pool. Swimming animals were automatically recorded and data were processed with an automatic image collection and analysis system. All equipment for the Morris-water maze test was provided by the Chinese Academy of Medical Sciences (Beijing, China). The mice were trained for the hidden platform trial for 5 days, for the probe trial for 1 day, for the reversal trial for 3 days, and for the visible platform trial for 1 day.
Hidden platform trial
The day before the trial, mice were allowed to swim freely for 90 seconds in the pool without the platform once in the morning and once in the afternoon to become familiar with the maze environment. During the trial, the platform had a fixed position; the midpoint of the platform from the pool wall was 22.5 cm. On the offside of the platform, two points at the same distance from the point of entry were selected as the entry points. Animals were placed into the water, facing the pool wall. The time elapsed from entering the water to finding the platform was recorded as escape latency. If a mouse could not find the platform within 90 seconds, escape latency was recorded as 90 seconds. Each mouse was tested twice daily using two different water entry points. Statistical analysis was performed by the arithmetic mean of two escape latency durations. The trial was performed for 5 consecutive days.
Probe trial
Twenty-four hours after the hidden platform trial, the platform was removed. Then, the mice were placed into the water from any entry point, and retention time in the former quadrant as well as the frequency of crossing the former platform was recorded for 60 seconds.
Reversal trial
The platform was moved to the quadrant opposite to that in the hidden platform trial, and the mice were assessed as in the hidden platform trial.
Visible platform trial
To eliminate sensory, visual or motor dysfunction effects on spatial learning and memory abilities, a 1-day visual platform trial was used. The platform was marked with yellow tape and placed 2 cm above the water. The tests were performed as described for the hidden platform trial.
Western blot assay
When the Morris water maze trial was over, mice were sacrificed by cervical dislocation after anesthesia, and the hippocampal tissue was dissected out and homogenized to a 10% homogenate in radioimmune precipitation assay lysis buffer. After centrifugation for 10 minutes at 12,000× g, the total protein concentration was determined with a bicinchoninic acid protein assay kit. After electrophoresis and transfer to a membrane, proteins were incubated with a rabbit monoclonal anti-synaptophysin antibody (1:5,000;Abcam, Cambridge, UK) at 4°C overnight and then with a goat anti-rabbit IgG (1:8,000; Abcam) secondary antibody at room temperature for 1 hour. Blots were developed using the chemiluminescent method. Protein bands were quantified using Quantity One software (Bio-Rad, Hercules, CA,USA) and the synaptophysin/GAPDH ratio calculated to show relative protein expression.
Real-time PCR
Total hippocampal RNA was extracted from 10% hippocampal homogenate using TRIzol reagent (Invitrogen, Carlsbad,CA, USA) and 2 μg RNA was used for reverse transcription with a first-strand cDNA synthesis kit (Bio-Rad, USA).Real-time PCR was performed using the absolute quantitative 2-ΔΔCtmethod with a SYBR Green PCR Master Mix kit(Applied Biosystems, Foster City, CA, USA) on a Bio-Rad iCycler system to detect synaptophysin gene expression.
Statistical analysis
All data were analyzed with SPSS 11.5 software (SPSS Inc,Chicago, IL, USA) and are expressed as the mean ± standard deviation (SD). Data of multiple groups were compared using one-way analysis of variance. Comparison between twogroups was conducted by the Student-Newman-Keuls test.A value of P < 0.05 was considered statistically signi ficant.
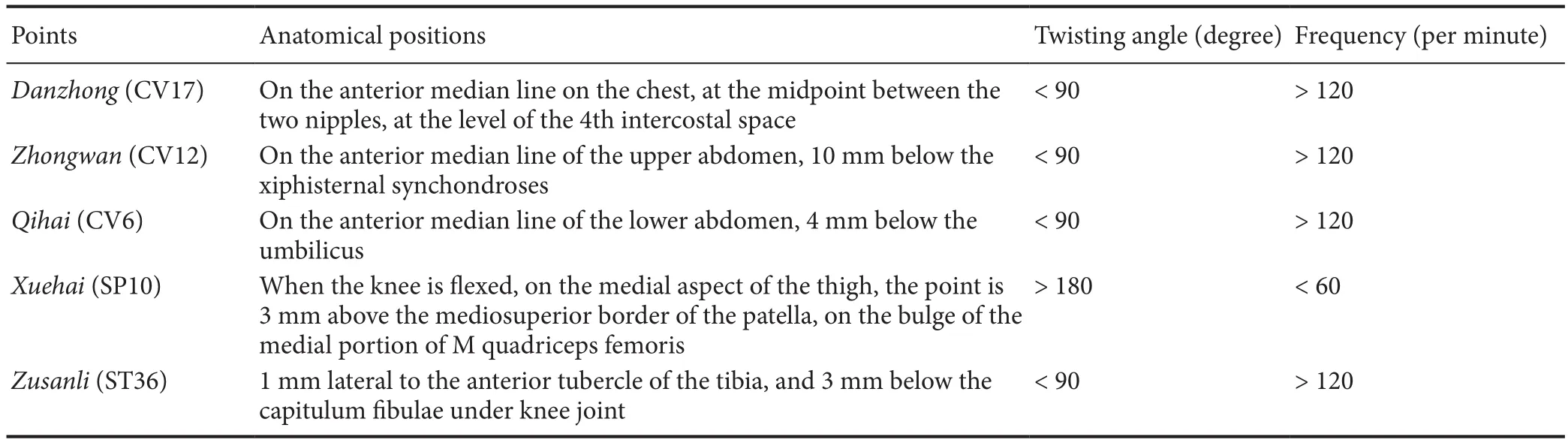
Table 1 Acupuncture points and manipulation (Zhao et al., 2013)
Results
Spatial memory and cognitive changes in SAMP8 mice
The Morris water maze is used to evaluate the learning ability of animals to find the position of a hidden platform (Vorhees and Williams, 2014; Huang et al., 2016). In the hidden platform trial, compared with the RC group, the average escape latency in the PC group increased signi ficantly from day 2 (P < 0.05). Compared with the PC group, escape latency in the PTC group was signi ficantly shorter from day 3(P< 0.05); from day 4, escape latencies in the PT, PTA, PTD,and PTC groups were significantly shorter compared with the PC group (P < 0.05). On day 5, compared with the PT group, escape latencies in the PTA, PTD and PTC groups were shorter (P < 0.05). Compared with PTA animals, escape latency of the PTC group was signi ficantly shorter (P <0.05). The PTC group showed signi ficantly shorter escape latency compared with the PTD group (P < 0.05;Figure 1A).The probe trial mainly reflects memory retention (Gómez et al., 2017). The results showed that retention time in the former quadrant of the hidden platform trial among all groups was significantly different. Compared with the RC group, retention time in the former platform quadrant in the PC, PT, PTA and PTD groups was significantly shorter (P< 0.05). Compared with the PC group, retention time in the former platform quadrant in the PT, PTA, PTD, and PTC groups was significantly prolonged (P < 0.05). Compared with the PT group, retention time of the PTA, PTD and PTC groups in the former platform quadrant was significantly prolonged (P < 0.05). The PTC group had a significantly prolonged retention time in the former platform quadrant compared with the PTA group (P < 0.05). Compared with the PTD group, retention time of the PTC group in the former platform quadrant was signi ficantly prolonged (P < 0.05).The frequencies of crossing the former platform in the RC,PT, PTA, PTD and PTC groups were significantly higher compared with that in the PC group (P < 0.05;Figure 1B).
In the reversal trial for 3 consecutive days, escape latency signi ficantly varied among different groups. Compared with the RC group, average escape latency in the PC, PT, PTA and PTD groups was signi ficantly increased (P < 0.05). Compared with the PC group, average escape latency in the PT, PTA,PTD, and PTC groups was significantly shorter (P < 0.05).Compared with the PT group, average escape latency in the PTD and PTC groups was significantly shorter compared with the PT group (P < 0.05); from day 2, average escape latency of PTA, PTD and PTC groups was signi ficantly shorter(P < 0.05). The PTC group showed signi ficantly shorter average escape latency compared with the PTA group (P < 0.05).Compared with the PTD group, average escape latency of the PTC group was decreased signi ficantly from day 2 (P < 0.05;Figure 1C).
Visible platform trials showed no signi ficant difference in escape latency among groups (P > 0.05;Figure 1D).
Protein levels of synaptophysin in the hippocampus of SAMP8 mice
Compared with the RC group, the levels of synaptophysin in the PC, PT, PTA, PTD and PTC groups were signi ficantly reduced (P < 0.05). Compared with the PC group, synaptophysin levels in the PTC group were signi ficantly increased(P < 0.05). Compared with the PT group, synaptophysin levels in the PTA, PTD and PTC groups were not changed significantly (P > 0.05). Compared with the PTA group,synaptophysin levels in the PTD and PTC groups were not signi ficantly different (P > 0.05;Figure 2).
Gene expression of synaptophysin in the hippocampus of SAMP8 mice
Compared with the RC group, synaptophysin mRNA levels in the PC, PT, PTA, PTD and PTC groups were signi ficantly decreased (P < 0.05). Meanwhile, synaptophysin mRNA levels in the PTC group were signi ficantly increased compared with the PC group (P < 0.05). Compared with the PT, PTA,and PTD groups, synaptophysin mRNA levels in the PTC group were signi ficantly increased (P < 0.05;Figure 3).
Discussion
The multi-differentiation properties of NSCs may enable them to be used for replacement therapy in neurodegenerative tissues, which provides hope for overcoming degenerative diseases of the central nervous system (Lemmens and Steinberg, 2013; Zhong et al., 2016; Pen and Jensen, 2017). A large number of studies have shown that injured nerve cells are usually repaired and replaced; several types of cytokines are secreted to promote the proliferation and survival of NSCs, and cellular circuits and functions are partially regenerated after the implantation of exogenous NSCs into the brain (Ahmed et al., 2016; Jablonska et al., 2016; Ye et al.,2016; Hou et al., 2017). Transplantation of exogenous NSCs has the advantage of targeting a limited and de fined site to replace injured or lost cells, promote self-repair, and restore brain function (Dong and Yi, 2010; Chen et al., 2014; Cai et al., 2015). We selected the hippocampus, which is strongly associated with dementia, for exogenous NSC transplantation, and the status of mice with dementia was considerably improved. Morris water maze visible platform trials indicated that differences in sensory, visual or motor functions did not signi ficantly affect spatial learning or memory. SAMP8 mice had cognitive impairment in learning and memory acquisition, lower memory retention and re-learning ability.Hippocampal transplantation of NSCs significantly improved learning and memory dysfunction, memory retention, and re-learning ability of SAMP8 mice.
Sanjiao is the pathway for ascending, descending, exiting and entering and the source for the production of Qi,blood, essence and body fluid. As the commander for Qi activity, Sanjiao controls functional activities of internal organs to maintain normal human life activities. Therefore,the abnormal function of “Qi activity in Sanjiao” is the basic mechanism for aging (Han, 2007). Based on the dysfunction of Qi activity of Sanjiao, which is the fundamental pathological mechanism of senile dementia, Professor Han Jingxian proposed that “dysfunction of Qi activity of Sanjiao leads to aging”, and created “Sanjiao acupuncture” and “Huang-DiSan” prescription. Treatment was based on Qi, with the acupuncture acupoints CV17, CV12, CV6, SP10 and ST36.Regulating the Qi of Sanjiao modulates spleen and stomach functions, enriching Qi and blood, tonifying the kidney, promoting blood circulation for blood stasis removal, eliminating phlegm, improving dementia symptoms, and retarding brain aging. Acupuncture was used as the fundamental prescription, and could be changed according to different conditions. The main ingredients of HuangDiSan are Chinese Angelica and Rehmannia, with the effects of dredging Sanjiao, tonifying Qi and regulating blood, as well as strengthening the root and tonifying the kidney. The combination of acupuncture and HuangDiSan for the treatment of Alzheimer’s disease as well as de ficiency illness of Qi and blood had signi ficant effects (Shi et al., 2015). In this study, three methods of acupuncture, Chinese medicine, and a combination of acupuncture/Chinese medicine, further improved cognitive impairment, memory retention and re-learning in SAMP8 mice. Furthermore, the effects of acupuncture combined with HuangDiSan were stronger than those of either modality administered alone. Thus, the above combination therapy might be the best way to improve cognitive function in SAMP8 mice after NSCs transplantation. Chinese medical interventions act upon nerve cells themselves and on their microenvironments, affecting multiple targets and processes, including proliferation, differentiation, migration, and synapse formation (Lu et al., 2014; Li et al., 2016; Nie et al.,2016; Zhang et al., 2016, 2017).
The synapse is the fundamental connection between neurons for information transmission (Barbash and Sakmar, 2017;Fink et al., 2017; Pietronigro et al., 2017). In AD patients,there is neuronal loss and reduced amounts of synaptophysin in the hippocampus (Tannenberg et al., 2004; Kirvell et al.,2006; Wang et al., 2007). As an important marker of synaptic reconstruction, synaptophysin is widely used for labeling axon terminals, reflecting the occurrence and density of synapses(Dan et al., 2008; Brewer et al., 2009; Hong et al., 2016; Pan et al., 2016; Stoyanova et al., 2016). Synaptophysin reduction in the hippocampus of SAMP8 mice indicates a decreased transport capacity of synaptic vesicles, resulting in deficient synaptic transmission and altered transmission, processing,and storage of information in the nervous system. Synapse formation and plasticity in hippocampal neurons are strongly associated with learning and memory abilities (Howland and Wang, 2008; Leal-Galicia et al., 2008; Rae and O’Malley, 2016).A decreased number of synapses in the hippocampus would affect the contact between neural pathways (Duyckaerts et al.,2009; Logue et al., 2016; Wu et al., 2016), which might lead to various dysfunctions, especially cognitive and memory de ficits(Kleeman et al., 2016; Muhia et al., 2016). Functional synaptic connections can be formed between neurons differentiated from NSCs transplanted into the hippocampus of rats (Gao et al., 2007; Hou et al., 2008), as well as among neurons differentiated from NSCs in vitro (Liebau et al., 2007). As shown above, learning and memory, memory retention, and relearning abilities in SAMP8 mice were improved, and synaptophysin levels in the hippocampus were signi ficantly increased after transplantation of NSCs. These findings indicate that the significant improvement of cognitive impairment in dementia model mice might be correlated with the formation of new synapses in the hippocampus. All three intervention approaches, including acupuncture, HuangDiSan, and combined acupuncture/HuangDiSan, significantly alleviated cognitive impairment and upregulated synaptophysin expression in dementia model mice. However, the combined acupuncture/HuangDiSan positively affected behavior and synaptophysin levels in dementia model mice. This indicates that the combination of acupuncture and HuangDiSan (based on the Sanjiao theory) is more effective than the use of acupuncture or Chinese medicine alone, and might be the optimal method for treating cognitive impairment after NSCs transplantation in the hippocampus of SAMP8 mice.

Figure 1 Morris water maze assay of SAM mice.
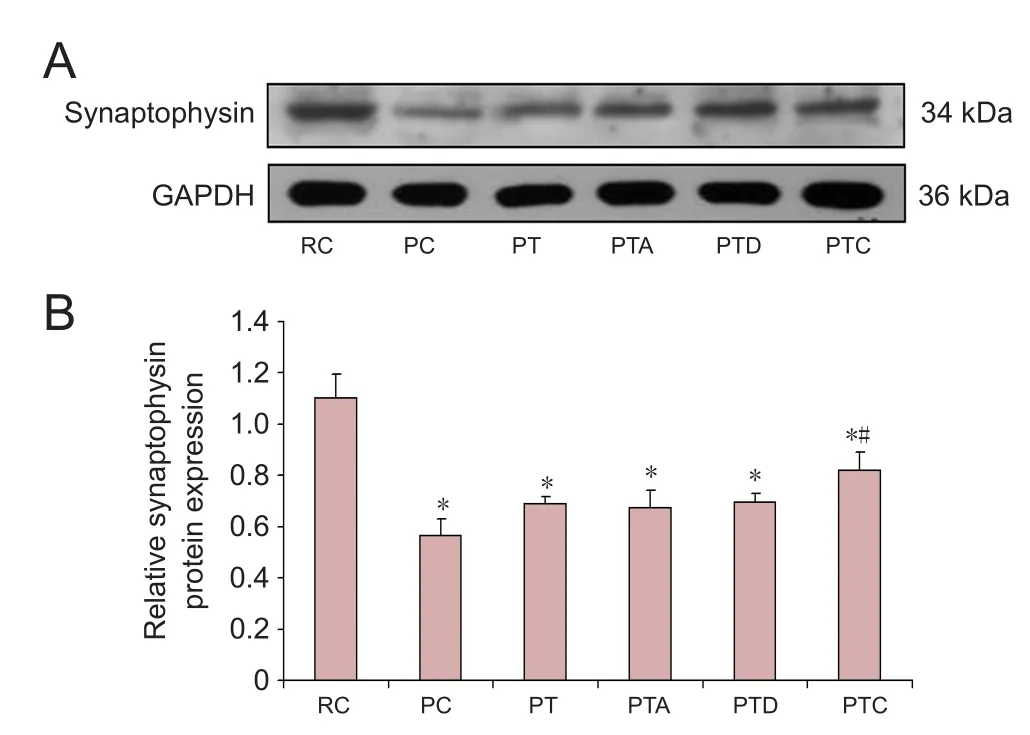
Figure 2 Western blot assay for synaptophysin protein levels.
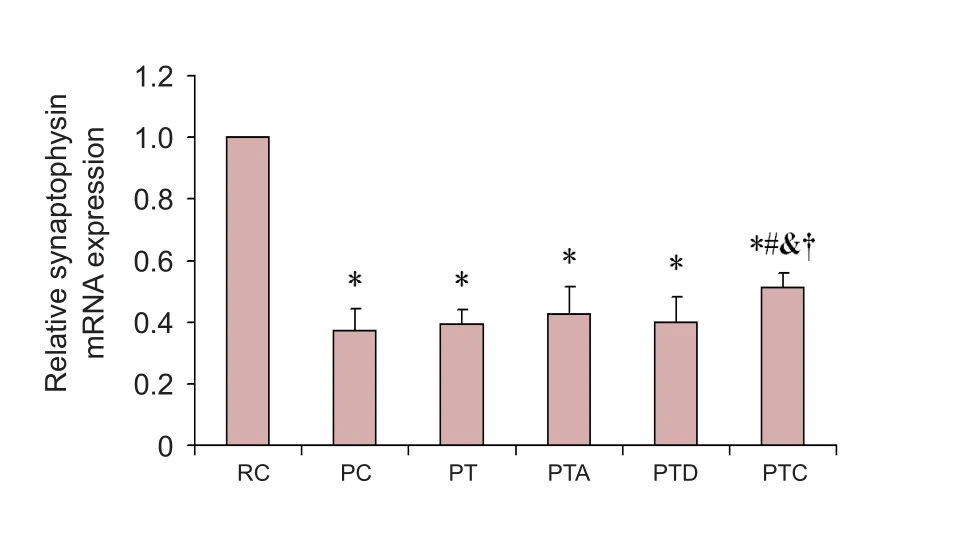
Figure 3 Real-time PCR for synaptophysin mRNA.
In conclusion, combined acupuncture and HuangDiSan treatment might act on components of the microenvironment that regulate transplanted exogenous NSCs, for example, promoting the secretion of nutrients necessary for injury repair,facilitating myelination of unmyelinated or new-born axons,providing substrates for axon growth, promoting synapse formation and neurotransmitter release, enhancing synaptic transmission, and improving learning ability and memory in dementia model mice.
Author contributions:LZ was responsible for study design. CLZ and LZ performed western blot assay and real-time PCR, participated in statistical analysis and wrote the paper. LZ, CLZ and BHK were responsible for research funding. HYS, JWL and BHK were involved in data collection, and performed NSCs transplantation and Morris Water Maze. JCY and JXH were responsible for mechanism analysis of Sanjiao acupuncture and HuangDiSan. All authors approved the final version of the paper.
Con flicts of interest:None declared.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81202740 and 81603686; the Natural Science Foundation of Tianjin of China No. 17JCYBJC26200 and 12JCQNJC07400; the Public Health Bureau Science and Technology Foundation of Tianjin of China No. 2014KY15; and the Specialized Research Foundation for the Doctoral Program of Higher Education, No.20121210120002. Funders had no involvement in the study design; data collection, management, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Research ethics:The study protocol was approved by the Ethical Committee of Tianjin Medical University of China (approval number:TMUaMEC 2016007).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Ahmed AI, Gajavelli S, Spurlock MS, Chieng LO, Bullock MR (2016)Stem cells for therapy in TBI. J R Army Med Corps 162:98-102.
Barbash S, Sakmar TP (2017) Length-dependent gene misexpression is associated with Alzheimer’s disease progression. Sci Rep 7:190.
Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA,Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM (2009) Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A 106:13594-13599.
Brewer GJ, Boehler MD, Pearson RA, DeMaris AA, Ide AN, Wheeler BC (2009) Neuron network activity scales exponentially with synapse density. J Neural Eng 6:014001.
Cai Q, Chen Z, Song P, Wu L, Wang L, Deng G, Liu B, Chen Q (2015)Co-transplantation of hippocampal neural stem cells and astrocytes and microvascular endothelial cells improve the memory in ischemic stroke rat. Int J Clin Exp Med 8:13109-13117.
Chen L, Qiu R, Li L, He D, Lv H, Wu X, Gu N (2014) The role of exogenous neural stem cells transplantation in cerebral ischemic stroke.J Biomed Nanotechnol 10:3219-3230.
Cheng H, Yu J, Jiang Z, Zhang X, Liu C, Peng Y, Chen F, Qu Y, Jia Y, Tian Q, Xiao C, Chu Q, Nie K, Kan B, Hu X, Han J (2008) Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice. Neurosci Lett 432:111-116.
Dan C, Jian-Bin T, Hui W, Le-Ping Z, Jin Z, Ju-Fang H, Xue-Gang L(2008) Synaptophysin expression in rat retina following acute high intraocular pressure. Acta Histochem Cytochem 41:173-178.
Dong MM, Yi TH (2010) Stem cell and peripheral nerve injury and repair. Facial Plast Surg 26:421-427.
Duyckaerts C, Delatour B, Potier MC (2009) Classi fication and basic pathology of Alzheimer disease. Acta Neuropathol 118:5-36.
Elliott Donaghue I, Tam R, Sefton MV, Shoichet MS (2014) Cell and biomolecule delivery for tissue repair and regeneration in the central nervous system. J Control Release 190:219-227.
Fink KL, López-Giráldez F, Kim IJ, Strittmatter SM, Cafferty WBJ(2017) Identi fication of intrinsic axon growth modulators for intact CNS neurons after injury. Cell Rep 18:2687-2701.
Gómez C, Carrasco C, Redolat R (2017) Adolescent and adult mice display differential sensitivity to the effects of bupropion on the acquisition of a water maze task. Pharmacol Rep 69:162-167.
Gao J, Coggeshall RE, Chung JM, Wang J, Wu P (2007) Functional motoneurons develop from human neural stem cell transplants in adult rats. Neuroreport 18:565-569.
Han JX (2007) Acupuncture principle of tonifying qi and regulating blood, supporting the root and fostering the source on aging and senile diseases. Chin J Integr Med 13:166-167.
Harris L, Zalucki O, Piper M, Heng JI (2016) Insights into the biology and therapeutic applications of neural stem cells. Stem Cells Int 2016:9745315.
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA,Selkoe DJ, Stevens B (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352:712-716.
Hou B, Ma J, Guo X, Ju F, Gao J, Wang D, Liu J, Li X, Zhang S, Ren H(2017) Exogenous neural stem cells transplantation as a potential therapy for photothrombotic ischemia stroke in Kunming mice model. Mol Neurobiol 54:1254-1262.
Hou SW, Wang YQ, Xu M, Shen DH, Wang JJ, Huang F, Yu Z, Sun FY (2008) Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke 39:2837-2844.
Howland JG, Wang YT (2008) Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res 169:145-158.
Huang KY, Liang S, Yu ML, Fu SP, Chen X, Lu SF (2016) A systematic review and meta-analysis of acupuncture for improving learning and memory ability in animals. BMC Complement Altern Med 16:297.
Jablonska A, Drela K, Wojcik-Stanaszek L, Janowski M, Zalewska T,Lukomska B (2016) Short-lived human umbilical cord-blood-derived neural stem cells in fluence the endogenous secretome and increase the number of endogenous neural progenitors in a rat model of lacunar stroke. Mol Neurobiol 53:6413-6425.
Kirvell SL, Esiri M, Francis PT (2006) Down-regulation of vesicular glutamate transporters precedes cell loss and pathology in Alzheimer’s disease. J Neurochem 98:939-950.
Kleeman E, Nakauchi S, Su H, Dang R, Wood MA, Sumikawa K (2016)Impaired function of alpha2-containing nicotinic acetylcholine receptors on oriens-lacunosum moleculare cells causes hippocampus-dependent memory impairments. Neurobiol Learn Mem 136:13-20.
Leal-Galicia P, Casta?eda-Bueno M, Quiroz-Baez R, Arias C (2008)Long-term exposure to environmental enrichment since youth prevents recognition memory decline and increases synaptic plasticity markers in aging. Neurobiol Learn Mem 90:511-518.
Lemmens R, Steinberg GK (2013) Stem cell therapy for acute cerebral injury: what do we know and what will the future bring? Curr Opin Neurol 26:617-625.
Li Y, Xu J, Xu P, Song S, Liu P, Chi T, Ji X, Jin G, Qiu S, Hou Y, Zheng C, Wang L, Meng D, Zou L (2016) Xanthoceras sorbifolia extracts ameliorate dendritic spine deficiency and cognitive decline via upregulation of BDNF expression in a rat model of Alzheimer’s disease. Neurosci Lett 629:208-214.
Liebau S, Vaida B, Storch A, Boeckers TM (2007) Maturation of synaptic contacts in differentiating neural stem cells. Stem Cells 25:1720-1729.
Llorens-Bobadilla E, Martin-Villalba A (2017) Adult NSC diversity and plasticity: the role of the niche. Curr Opin Neurobiol 42:68-74.
Logue OC, Cramer NP, Xu X, Perl DP, Galdzicki Z (2016) Alterations of functional properties of hippocampal networks following repetitive closed-head injury. Exp Neurol 277:227-243.
Lu Y, Sun XD, Hou FQ, Bi LL, Yin DM, Liu F, Chen YJ, Bean JC, Jiao HF, Liu X, Li BM, Xiong WC, Gao TM, Mei L (2014) Maintenance of GABAergic activity by neuregulin 1-ErbB4 in amygdala for fear memory. Neuron 84:835-846.
Ma F, Zhu T, Xu F, Wang Z, Zheng Y, Tang Q, Chen L, Shen Y, Zhu J (2017) Neural stem/progenitor cells on collagen with anchored basic fibroblast growth factor as potential natural nerve conduits for facial nerve regeneration. Acta Biomater 50:188-197.
Muhia M, Thies E, Labonté D, Ghiretti AE, Gromova KV, Xompero F,Lappe-Sieかe C, Hermans-Borgmeyer I, Kuhl D, Schweizer M, Ohana O, Schwarz JR, Holzbaur EL, Kneussel M (2016) The Kinesin KIF21B regulates microtubule dynamics and is essential for neuronal morphology, synapse function, and learning and memory. Cell Rep 15:968-977.
Nie J, Tian Y, Zhang Y, Lu YL, Li LS, Shi JS (2016) Dendrobium alkaloids prevent Abeta25-35-induced neuronal and synaptic loss via promoting neurotrophic factors expression in mice. PeerJ 4:e2739.
Novak I, Walker K, Hunt RW, Wallace EM, Fahey M, Badawi N (2016)Concise review: stem cell interventions for people with cerebral palsy: systematic review with meta-analysis. Stem Cells Transl Med 5:1014-1025.
Pan W, Han S, Kang L, Li S, Du J, Cui H (2016) Effects of dihydrotestosterone on synaptic plasticity of the hippocampus in mild cognitive impairment male SAMP8 mice. Exp Ther Med 12:1455-1463.
Park D, Lim J, Park JY, Lee SH (2015) Concise review: stem cell microenvironment on a chip: current technologies for tissue engineering and stem cell biology. Stem Cells Transl Med 4:1352-1368.
Pen AE, Jensen UB (2017) Current status of treating neurodegenerative disease with induced pluripotent stem cells. Acta Neurol Scand 135:57-72.
Pietronigro EC, Della Bianca V, Zenaro E, Constantin G (2017) NETosis in Alzheimer’s disease. Front Immunol 8:211.
Rae MG, O’Malley D (2016) Cognitive dysfunction in Duchenne muscular dystrophy: a possible role for neuromodulatory immune molecules. J Neurophysiol 116:1304-1315.
Shi JW, Meng Y, Liu XX, Jia YJ, Yu T, Han JX (2015) Clinical observation of combination of Sanjiao acupuncture with Huangdisan on vascular dementia: a randomized controlled trial. Tianjin J Tradit Chin Med 32: 401-404.
Stoyanova, II, Hofmeijer J, van Putten M, le Feber J (2016) Acyl ghrelin improves synapse recovery in an in vitro model of postanoxic encephalopathy. Mol Neurobiol 53:6136-6143.
Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T (1981) A new murine model of accelerated senescence.Mech Ageing Dev 17:183-194.
Tannenberg RK, Scott HL, Westphalen RI, Dodd PR (2004) The identi fication and characterization of excitotoxic nerve-endings in Alzheimer disease. Curr Alzheimer Res 1:11-25.
Tomás Pereira I, Burwell RD (2015) Using the spatial learning index to evaluate performance on the water maze. Behav Neurosci 129:533-539.
Vorhees CV, Williams MT (2014) Value of water mazes for assessing spatial and egocentric learning and memory in rodent basic research and regulatory studies. Neurotoxicol Teratol 45:75-90.
Wang R, Tang Y, Feng B, Ye C, Fang L, Zhang L, Li L (2007) Changes in hippocampal synapses and learning-memory abilities in age-increasing rats and effects of tetrahydroxystilbene glucoside in aged rats. Neuroscience 149:739-746.
Wang RG, Pi LH, Chen HY, Zhang HZ (2017) Effects of Bushen Huoxue Recipe combined with neural stem cell transplantation in rats with tinnitus. Zhongguo Zuzhi Gongcheng Yanjiu 21:730-735.
Wu T, He K, Ang S, Ying J, Zhang S, Zhang T, Xue Y, Tang M (2016)Impairments of spatial learning and memory following intrahippocampal injection in rats of 3-mercaptopropionic acid-modified CdTe quantum dots and molecular mechanisms. International journal of nanomedicine 11:2737-2755.
Xiong LL, Hu Y, Zhang P, Zhang Z, Li LH, Gao GD, Zhou XF, Wang TH (2017) Neural stem cell transplantation promotes functional recovery from traumatic brain injury via brain derived neurotrophic factor-mediated neuroplasticity. Mol Neurobiol doi: 10.1007/s12035-017-0551-1.
Ye LJ, Bian H, Fan YD, Wang ZB, Yu HL, Ma YY, Chen F (2016)Rhesus monkey neural stem cell transplantation promotes neural regeneration in rats with hippocampal lesions. Neural Regen Res 11:1464-1470.
Yu J, Zhang X, Liu C, Meng Y, Han J (2006) Effect of acupuncture treatment on vascular dementia. Neurol Res 28:97-103.
Yu JH, Seo JH, Lee JY, Lee MY, Cho SR (2016) Induction of neurorestoration from endogenous stem cells. cell Transplant 25:863-882.
Zhang H, Zhao C, Lv C, Liu X, Du S, Li Z, Wang Y, Zhang W (2017)Geniposide alleviates amyloid-induced synaptic injury by protecting axonal mitochondrial trafficking. Front Cell Neurosci 10:309.
Zhang W, Zhu Y, Li J, Guo Q, Peng J, Liu S, Yang J, Wang Y (2016)Cell-derived extracellular matrix: basic characteristics and current applications in orthopedic tissue engineering. Tissue Eng Part B Rev 22:193-207.
Zhao L, Jia Y, Yan D, Zhou C, Han J, Yu J (2013) Aging-related changes of triose phosphate isomerase in hippocampus of senescence accelerated mouse and the intervention of acupuncture. Neurosci Lett 542:59-64.
Zhao L, Zhou CL, Liu YH, Pan P, Han JX, Yu JC (2017) Clinical study on the effect of Sanjiao acupuncture on immune function in Alzheimer’s disease. Tianjin Zhongyiyao 34:32-36.
Zhao YQ, Yuan LJ, Qiao LJ, Kang NN (2015) Discussion on acupuncture and neural stem cells’ transplantation in the treatment of encephalopathy from the perspective of Chinese Medicine of “Spirit”theory. Liaoning Zhongyi Zazhi 42:1331-1334.
Zhong Q, Ren BX, Tang FR (2016) Neurogenesis in the hippocampus of patients with temporal lobe epilepsy. Curr Neurol Neurosci Rep 16:20.
- 中國神經(jīng)再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Optic radiation injury in patients with aneurismal subarachnoid hemorrhage: a preliminary diffusion tensor imaging report
- Regulatory role of calpain in neuronal death
- The ROCK pathway inhibitor Y-27632 mitigates hypoxia and oxidative stress-induced injury to retinal Müller cells
- Endoplasmic reticulum stress transducer old astrocyte speci fically induced substance contributes to astrogliosis after spinal cord injury
- DTI and pathological changes in a rabbit model of radiation injury to the spinal cord after 125I radioactive seed implantation

