Optogenetics and its application in neural degeneration and regeneration
Josue D. Ordaz, Wei Wu, Xiao-Ming Xu,
1 Spinal Cord and Brain Injury Research Group, Stark Neurosciences Research Institute, Indiana University School of Medicine, Indianapolis, IN, USA
2 Department of Neurological Surgery, Indiana University School of Medicine, Indianapolis, IN, USA
3 Goodman Campbell Brain and Spine, Indianapolis, Indiana, USA
4 Department of Anatomy and Cell Biology, Indiana University School of Medicine, Indianapolis, IN, USA
Optogenetics and its application in neural degeneration and regeneration
Josue D. Ordaz1,2,3, Wei Wu1,2,3, Xiao-Ming Xu1,2,3,4,*
1 Spinal Cord and Brain Injury Research Group, Stark Neurosciences Research Institute, Indiana University School of Medicine, Indianapolis, IN, USA
2 Department of Neurological Surgery, Indiana University School of Medicine, Indianapolis, IN, USA
3 Goodman Campbell Brain and Spine, Indianapolis, Indiana, USA
4 Department of Anatomy and Cell Biology, Indiana University School of Medicine, Indianapolis, IN, USA
How to cite this article:Ordaz JD, Wu W, Xu XM (2017) Optogenetics and its application in neural degeneration and regeneration. Neural Regen Res 12(8):1197-1209.
Neural degeneration and regeneration are important topics in neurological diseases. There are limited options for therapeutic interventions in neurological diseases that provide simultaneous spatial and temporal control of neurons.is drawback increases side ef f ects due to non-specif i c targeting. Optogenetics is a technology that allows precise spatial and temporal control of cells.erefore, this technique has high potential as a therapeutic strategy for neurological diseases. Even though the application of optogenetics in understanding brain functional organization and complex behaviour states have been elaborated, reviews of its therapeutic potential especially in neurodegeneration and regeneration are still limited.is short review presents representative work in optogenetics in disease models such as spinal cord injury, multiple sclerosis, epilepsy, Alzheimer’s disease and Parkinson’s disease. It is aimed to provide a broader perspective on optogenetic therapeutic potential in neurodegeneration and neural regeneration.
light-activated proteins; neural plasticity; spinal cord injury; epilepsy; Parkinson’s disease; Alzheimer’s disease; multiple sclerosis; neural engineering; memory retrieval; neuron inhibition; neuron activation; neural regeneration
Introduction
Optogenetics is a technology that combines optics with genetics to induce a precise gain or loss-of-function in cells or tissue by applying light (Yizhar et al., 2011a). This biological technique involves: 1) engineering a gene that must be delivered in a cell specif i c manner and expressed at adequate levels, 2) developing a mode to deliver light forin vitroandin vivostudies, and 3) detecting the ef f ect of optogenetics (i.e., dendritic density, immunofluorescence, electrophysiology, behaviour studies) (Yizhar et al., 2011a).is light-sensitive technology has revolutionized the study of neuroscience with single-cell and millisecond precision control of neurons (Deisseroth et al., 2006; Deisseroth, 2011). Accurate spatial and temporal control is especially important to a system as complex as the nervous system, containing a network of billions of cells.
Current methods for neurological treatment involve targeting the nervous system with deep brain stimulation (DBS) and pharmacologic therapy (Oluigbo et al., 2012; Connolly and Lang, 2014). While DBS provides precise temporal control of neurons, it lacks spatial specif i city. On the other hand, pharmacologic therapy can give spatial specificity, but it lacks temporal control of cellular processes (Li et al., 2012). Consequently, it is challenging for the neuroscience field to create a technique with these dual features. In the early 1970s, Francis Crick postulated that the fi eld of neuroscience needed a tool that could be used to control neurons with cellular and temporal precision (Crick, 1979). Crick suggested light activation of neurons may be the solution to these drawbacks. It took nearly three decades to achieve what he had envisioned with the introduction of optogenetics to the study of the nervous system (Boyden et al., 2005).is review aims to brief l y, yet concisely, introduce optogenetics and summarize its use as a tool to understand pathological circuitry and a possible treatment for neurological diseases.
Major Components of Optogenetics
Optogenetics works by transducing light-stimulated electrical currents directly into specific cells (Terakita, 2005). To achieve this purpose, this technique is comprised of three major components: 1) light-activated proteins, 2) light, and 3) mode of delivery.
Light-activated proteins
Light-activated proteins were fi rst discovered approximately 40 years ago.ere was the discovery of bacteriorhodopsin (proton pump) in 1971, halorhodopsin (chloride pump) in 1979 (Oesterhelt and Stoeckenius, 1971; Matsuno-Yagi and Mukohata, 1977), and channelrhodopsin-1 (proton channel) and -2 (cation channel) in 2002 and 2003 (Nagel et al., 2002, 2003), respectively.ese channels and pumps called opsins are microbial 7-transmembrane domain proteins containing trans-retinal cofactors that are activated upon light exposure.In general, these microbial opsins have the common characteristic of directly inducing electrical currents into cells upon light activation. This feature is different from rhodopsin (opsin found in the retina of vertebrates), which indirectly transduces electrical currentviaintracellular G-proteins (Oesterhelt and Stoeckenius, 1971; Stryer, 1986).
Despite their common role as cellular electrical transducers, each of these prokaryotic opsins brings about dif f erent effects on membrane potential upon activation with light. Upon light-induced electrical transduction, some opsins such as halorhodopsin (HR) hyperpolarize the membrane potential whereas other opsins such as channelrhodopsin (ChR) depolarize the membrane potential (Figure 1) (Zhang et al., 2006). Halorhodopsin hyperpolarizes the membrane potential by pumping chloride ions into cells, resulting in spiking and neurotransmission inhibition, and ChR depolarizes cell membranes by allowing cations to dif f use into the cells by an electrochemical gradient, which could induce an action potential (Nagel et al., 2003; Kikukawa et al., 2015).is is especially applicable in neurons because of their electrophysiological properties of generating action potentials.
Initially, although the optogenetics method seemed revolutionary, there was a lot of scepticism about its application in neuroscience.ere were concerns about whether photocurrents, which are currents induced by photons, would be too weak and slow to activate and inactivate neurons with millisecond precision. Moreover, it was thought that opsins may be toxic or not expressed at high enough levels in neurons to mediate a desired ef f ect. Since opsins require trans-retinal cofactors for activation, it was postulated that optogenetics would require a multicomponent delivery similar to the novel molecular strategies, which were engineered at the time (Zemelman et al., 2002; Banghart et al., 2004; Deisseroth, 2011).
The challenges of optogenetics application in neuroscience were overcome by Boyden et al. in 2005 (Boyden et al., 2005). Boyden’s group delivered the fi rst opsin into neurons by transfecting cultured hippocampal cells with lentivirus containing channelrhodopsin-2 (ChR2). In their study, they showed activation of neurons within milliseconds of light stimulation, synaptic neurotransmission, and neuronal spike trains resembling normal neuron electrophysiology. A significant observation was that cell health and electrophysiology properties were not af f ected by ChR2 expression. Furthermore, since retinoids were found present in suf fi cient amounts in mature mammalian brains, this conferred optogenetics as a single-component strategy to control neuronal activity (Deisseroth et al., 2006; Zhang et al., 2006). Complementary tools that could inhibit neuronal activity were then introduced following the discovery of natronobacterium pharaonic halorhodopsin (NpHR) (Han and Boyden, 2007) and a new class of inhibitory opsins called archaerhodopsin, which are outward proton pumps (Chow et al., 2010).
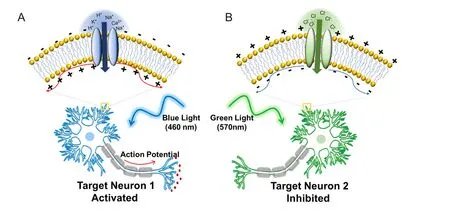
Figure 1 Optogenetic control of neural activities.
Since the serendipitous application of optogenetics to neural systems, the field has vastly expanded (Boyden, 2011). However, the introduction of optogenetics to neuroscience has not been without its challenges. For example, there were challenges in increasing the cell membrane transport of NpHR since it was observed to accumulate intracellularly at high expression levels (Gradinaru et al., 2007). To increase its cell surface expression, the C-terminal endoplasmic reticulum (ER) export peptide sequence from Kir2.1 channel was added, which resulted in the synthesis of the opsin enhanced natronobacterium pharaonic halorhodopsin (eNpHR) (Gradinaru et al., 2008). Moreover, NpHR was inefficient in inhibiting neurons since it pumped one chloride ion per photon. The finding of high-resolution crystal structure ofChR2 facilitated the reconstruction of its ion pore to conduct chloride ions instead of cations into the cell, which changed its properties to a neuron inhibitory channel and created a more ef ficient inhibitory opsin (Kato et al., 2012; Berndt et al., 2014; Wietek et al., 2014). NpHR also had a slow recovery rate from the inactivated state upon continuous stimulation, which was unfavourable to its application in neurons (Hegemann et al., 1985; Bamberg et al., 1993). High-throughput screening enabled the discovery of a new inhibitory opsin called archaerhodopsin-3 (outward proton pump) that mediates strong currents and spontaneously recovers from light-induced inactivation (Chow et al., 2010). With advanced understanding of opsin kinetics, structure, molecular traf fi cking, and optical properties, it has been possible to optimize their properties (e.g., increase membrane expression, light sensitivity, and expression safety) or modify them for diverse applications (e.g., slower or faster closing and/or opening kinetics, shifting wavelength of activation) (Zhang et al., 2008; Lin et al., 2009; Gradinaru et al., 2010; Gunaydin et al., 2010; Boyden, 2011).
Light
A major component in optogenetics is the light wavelength to activate a specif i c opsin. For example, ChR2 is activated by ~460 nm light and NpHR is activated by ~570 nm light.is allows the coexpression of these opsins into a neuron or more complex system to simultaneously activate and inactivate neurons (Matsuno-Yagi and Mukohata, 1977; Nagel et al., 2003). Since these two opsins work antagonistically (i.e., ChR2 activates neurons; NpHR inactivates neurons), this strategy would not be suitable if one is interested in synergistically activating two dif f erent groups of neurons. Zhang et al. addressed this issue by identifying and characterizing a cation-conducting channelrhodopsin (VChR1) with red-shied absorption spectrum and maximum absorption wavelength ~70 nm higher than ChR2 (531 nm) (Zhang et al., 2008). Since ChR2 and VChR1 both depolarize membrane potentials and are activated by dif f erent wavelengths, it is possible to simultaneously activate different groups of neurons within a neural circuit.
One of the challenges of optogenetics is targeting deeper brain structures while minimizing invasiveness of implanting optical fibers to deliver light. A strategy to overcome this is to develop opsins with red-shifted activation wavelengths. This will reduce light scattering and allow deeper light penetration (Figure 2B) (Pansare et al., 2012). Recent developments in opsin engineering to address this issue include the cation channels (activation wavelengths): red-activatable ChR (ReaChR) (~590–630) (Lin et al., 2013); Channelrhodopsin-1/Volvox Channelrhodopsin (C1V1; E122T mutation; ChR1/VChR1 chimera) (~600 nm) (Yizhar et al., 2011b); Chrimson (~660 nm) (Klapoetke et al., 2014); fast red-activatable Channelrhodopsin (bReaChES) (~590 nm) (Kim et al., 2016); Volvox Channelrhodopsin (VChR1; similar to channelrhodopsin-1) (~531 nm) (Zhang et al., 2008) and the chloride pump Jaws (~635 nm) (Chuong et al., 2014). Moreover, the ChR2 gene has been modif i ed to produce a “bistable” channel also known as step-function opsin (SFO).is channel is opened by one wavelength and closed by a different wavelength. This allows control of how long a channel can stay in the opened state (Berndt et al., 2009). Since these channels can be kept open for longer periods of time, their conduction ef fi ciency is higher, which reduces the light intensity necessary to activate a neuron or other cells.
Mode of delivery
Opsin delivery
Similar to the genetically encoded GFP, opsins can be specifically delivered to a subset of cells. One of the delivery methods used is transfection of lentivirus or adeno-associated virus (AAV) packaged with the opsin gene, a marker such as enhanced green fl uorescent protein (EGFP) or enhanced yellow fluorescent protein (EYFP) and a cell-specific promotor (e.g., calcium/calmoduluin-dependent protein kinase II alpha (CaMKIIα) promotor for excitatory neurons, glial fibrillary acidic protein (GFAP) promoter for astrocytes, and preprohypocretin/preproorexin (ppHcrt) promotor for hypocretin neurons). Another method includes using transgenic mice to create a uniform expression of the opsin in a specif i c group of cells (Zhang et al., 2010; Adamantidis et al., 2014). However, some cells (e.g., parvalbumin interneurons, dopaminergic neurons) contain promotors with weak transcriptional activity, which makes it dif fi cult to express opsins in a cell-specif i c manner at necessary levels on the cell membrane. To overcome this, conditional expression systems using Cre recombinase-locus of crossover in P1 (Cre-loxP) are used to increase the expression of opsins in these cells (Atasoy et al., 2008; Kuhlman and Huang, 2008; Yizhar et al., 2011a). Briefly, transgenic mice are engineered to express Cre recombinase in a cell-specif i c manner (e.g., in PV+GABAergic neurons). A vector containing an upstream ubiquitous promotor, a stop cassette flanked by unidirectional lox-P and a downstream opsin gene is then delivered to the region of interest. In this manner, cells that uptake the vector but do not express Cre recombinase will not be able to express the opsin because of the stop cassette. However, cells containing Cre recombinase will excise the stop cassette and allow high expression of opsin under the control of a ubiquitous transcription promoter (Zeng and Madisen, 2012).
Light delivery
Forin vitrostudies, a laser or light emitting diode (LED) can be directly coupled to the microscope light path (Zhang et al., 2010). Forin vivooptogenetic stimulation of superf i cial layers of the cortex (e.g., layer 5 of primary motor cortex), small LED bulbs can be implanted on thinned skull directly above the target region (Gradinaru et al., 2007; Huber et al., 2008). However, since light power density drops to as low as 1% upon 1 mm penetration of tissue, a fi ber-optic-based optic neural interface has been developed in which LED or laser diode systems can be coupled to lightweight flexible optic fi bers to deliver light to deeper brain tissue (Figure 2C) (Adamantidis et al., 2007; Aravanis et al., 2007).
One of the challenges in neuroscience research is to ob-serve how neural activity results in behaviour changes.is requires thein vivorecording of neural activity, which was previously done with conventional 1 or 2-photon imaging. However, previous methods required the stereotaxic stabilization of the head, which prevents studying freely moving animals and cannot image deep structures such as the hippocampus and amygdala (Doronina-Amitonova et al., 2013; Gunaydin et al., 2014). To overcome this issue, fiber-optic imaging/recording systems, which involve fl uorescence modalities such as: 2-photon, scanning confocal fluorescence, and epif l uorescence have been developed (Helmchen et al., 2001; Flusberg et al., 2005; Sawinski et al., 2009). Coupled with optogenetics technology, it is possible to simultaneously stimulate and record intracellular Ca2+, electrical activity, and/or fl uorescent proteins in specif i c groups of neurons in any brain structure and observe the resulting behaviour in freely moving animals (Gradinaru et al., 2007; Miyamoto and Murayama, 2016). Szabo et al. made this possible by coupling a microscope to a fiber bundle containing a micro-objective to activate neurons and simultaneously image and record their activity (Szabo et al., 2014).is technology, which is called a fi berscope, is capable of fl uorescence imaging with epifluorescence, structured illumination, or scanless multipoint confocal microscopy. Other developments have included the use of an optrode, which combines an optic fi ber with an electrode to simultaneously stimulate neurons and record their activity with single-neuron resolution (Arenkiel et al., 2007; Tamura et al., 2012). Recent advances in light delivery have implemented closed-loop circuits, which allow real-time control of neurons based on error of desired and measured output, and wireless light stimulation, which does not require restraining animals during placement of optical fi bers (Grosenick et al., 2015; Montgomery et al., 2015; Park et al., 2015). Similarly, a wireless optrode has been developed (Gagnon-Turcotte et al., 2017).ese technologies provide a more natural environment for optogenetic stimulation and neuron activity recording (Figure 2).
Application of Optogenetics in Neuroscience and Neurological Disorders
Optogenetics could be an invaluable tool in the field of neuroscience. It induces gain or loss of function in neurons and behavior with precise control for quick and specif i c outcomes. Since its introduction for neuroscience application in 2005 (Boyden et al., 2005), this light-sensitive technology has vastly improved our understanding of treatment mechanism, pathology of neurological disease, neural circuitry, and physiological processes. It has been used to advance our understanding of complex processes such as: learning (Schroll et al., 2006), sleep (Adamantidis et al., 2007), vision (Farah et al., 2007), addiction (Witten et al., 2010), and movement (Chen et al., 2014; Proville et al., 2014). Moreover, it has been used to fi nd new targets for therapy.us, optogenetics is a powerful tool in basic science research.
One of the challenges of optogenetics is its translatability to the clinic. Opsin gene delivery is invasive, since it would require injecting viruses into nervous tissue. Delivering light to deep brain regions would require optic fi ber implantation, which may cause direct trauma. Moreover, applying light to the brain may cause heat damage. However, once these problems are overcome, optogenetics can be another tool to treat neurological diseases. Applying optogenetics to several neurological disease models such as spinal cord injury, epilepsy, Alzheimer’s disease (AD) and Parkinson’s disease (PD) may provide an alternative treatment with less side ef f ects than current therapies. The nervous system is made up of complex neural networks that work with spatial and temporal precision.erefore, the ideal treatment would restore normal physiology, and optogenetics is a capable tool. In the following session, we will focus on the application of optogenetics in these diseases and discuss the challenges of translating this technique to the clinic.
Spinal cord injury
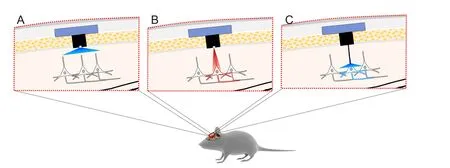
Figure 2 Improvements in optogenetics.
Spinal cord injury (SCI) is a devastating traumatic event disconnecting the brain to the peripheral nervous system (PNS). Patients may suffer from urinary and bowel incontinence,sexual dysfunction, and sensorimotor paralysis (Furlan et al., 2011; Simpson et al., 2012). It is a global health problem with an estimated prevalence in the range of 236–1,009 per million and costing a patient up to $1 million in the first year and $185 thousand every year thereaer (Cripps et al., 2011; NSCISC, 2016). Although many research ef f orts have advanced our understanding of pathophysiological mechanisms, new treatments have been inadequate. Unlike the PNS, the adult mammalian central nervous system (CNS) is unable to regenerate. Two mechanisms are recognized that prevent axonal regeneration in the CNS: 1) the environment following injury is inhibitory for axonal regeneration (Yiu and He, 2006; Fitch and Silver, 2008; Hackett and Lee, 2016), and 2) neurons do not have sufficient intrinsic capacity to regenerate (He and Jin, 2016).
Optogenetics is an intriguing treatment for SCI, and applying optogenetics in SCI regeneration studies has just been emerging. Upon axonal injury, there is an increase in intracellular calcium (Ziv and Spira, 1995, 1997). Calcium serves a multitude of purposes following injury including: regulating gene expression through mitogen-activated protein kinase (MAPK) and Ca2+/calmodulin-dependent protein kinase (CaMK) phosphorylation of cAMP response element binding protein (CREB); activating proteases to cleave spectrin to reduce membrane tension; promoting vesicle fusion to the plasmalemmaviaCa2+dependent SNARE protein association; activating delta-like 1 homolog (DLK1) to promote retrograde signalling to the soma; and stimulating growth cone formation to promote neural regeneration (Figure 3) (Ziv and Spira, 1997; Deisseroth et al., 1998; Wu et al., 2001; Kamber et al., 2009; Ghosh-Roy et al., 2010; Watkins et al., 2013; Hendricks and Shi, 2014). Further increase in intracellular calcium occurs through action potentials, which are induced by retrograde membrane depolarization following axon transectionviavoltage-gated sodium channels (Mandolesi et al., 2004). This depolarization is crucial for axon regeneration since studies have shown that blockade with tetrodotoxin (i.e., voltage-gated sodium channel blocker) prevents neurite regeneration (Mandolesi et al., 2004). Furthermore, other studies have shown that electrical stimulation of peripheral neurons enhances neurite elongation (Brushart et al., 2002; Udina et al., 2008). However, electrical stimulation of axotomized rubrospinal neurons (i.e., CNS neurons) has failed to show enhancement of neurite outgrowth (Harvey et al., 2005).is may be due to the counteracting molecules in the CNS such as myelin-associated inhibitors and chondroitin sulfate proteoglycans (CSPGs), which inhibit regeneration (Yiu and He, 2006). Interestingly, expression of constitutively active CREB in DRG neurons was sufficient to overcome myelin-associated inhibitors of axon regeneration in spinal cord axons (Gao et al., 2004). However, calcium levels are likely inadequate to alter gene expression into a regenerative state through CREB following CNS injury, since intracellular calcium elevation is transient and not significantly different than those observed during physiological burst activities (Friel and Chiel, 2008; Rishal and Fainzilber, 2010). Therefore, optogenetics using ChR2 for induction of membrane depolarization and action potentials to enhance the elevation of intracellular calcium following injury is a possible treatment option for SCI. Moreover, ChR2 is permeable to calcium ions, so it would also directly increase intracellular calcium (Nagel et al., 2003).
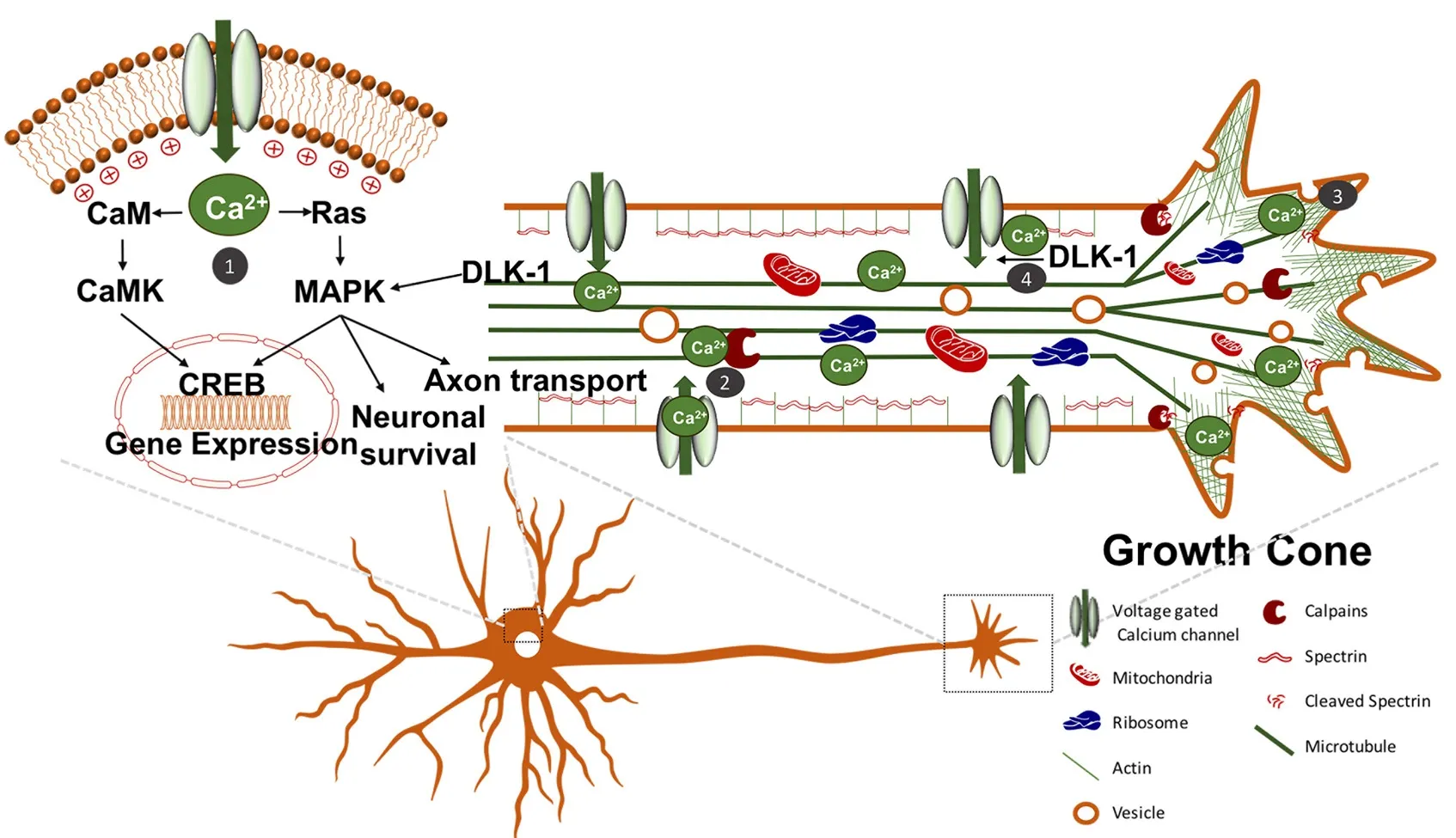
Figure 3 Augmenting intracellular calcium signalling to induce axon regeneration.
Optogenetics has been used to treat respiratory dysfunction in Sprague-Dawley rats following cervical SCI by transfecting spared motor neurons that have lost their presynaptic input. In this study, intermittent optogenetic stimulation of the phrenic motor neuron pool following cervical 2 (C2) spinal hemisection resulted in return to normal hemidiaphragm electromyography (EMG) activity in synchrony with the non-lesioned side (Alilain et al., 2008). Interestingly, long periods of intermittent stimulation caused long-lasting return of rhythmic diaphragm activity even aer stimulation ceased.is was not observed in control animals, indicating optogenetic stimulation induced neuroplasticity. To support this, optogenetics has been shown to induce synaptic plasticity by long-term potentiation (LTP) of neurons in the hippocampus (Zhang and Oertner, 2007). Neuroplasticity is of paramount importance for recovery following SCI as spared pathways can be recruited to replace damaged axons (Sandrow-Feinberg and Houlé, 2015). Furthermore, neuron electrical activity, which can be augmented with optogenetics, has been shown to increase proliferation and dif f erentiation of oligodendrocyte and neuron precursor cells, increase myelin thickness, and enhance motor skill in mice (Gibson et al., 2014).
Optogenetics has also been studied in another type of CNS injury, stroke, which is similar to SCI in which there is neuronal death and scar tissue formation (Choudhury and Ding, 2016). In a study by Cheng et al. (2014), stimulation of layer 5 primary cortex with ChR2 in the region immediately adjacent to the stroke lesion resulted in signif i cant increase in neuroplasticity markers, cerebral blood fl ow, rotating beam test performance and weight gain (Figure 4). Similarly, a different study showed optogenetic stimulation of transplanted neural stem cells containing ChR2 promoted motor function recovery, increased expression of neural plasticity markers, and downregulated transcription of pro-inf l ammatory genes in a stroke model (Daadi et al., 2016). More recently, optogenetic stimulation of the lateral cerebellar nucleus (LCN) in a middle cerebral artery (MCA) stroke model improved mouse performance in rotator beam.is study also showed an increase in the neuroplasticity marker growth-associated protein 43 (GAP43) in the ipsilesional cortex (Shah et al., 2017).e successful application of optogenetics for stroke recovery suggests its therapeutic potential for SCI. Moreover, optogenetics has been combined with a fl exible electrophysiology recording probe to interrogate the normal neural circuitry in spinal cord for movement of hindlimbs (Lu et al., 2017).is technology demonstrated feasible fi ber probe integration into the spinal cord with minimal trauma, which shows its potential application for circuitry restoration in SCI. In summary, optogenetics has a potential to improve SCI through: 1) increasing intracellular calcium leading to activation of intrinsic regeneration mechanisms, 2) enhancing neuroplasticity of spared axons, 3) promoting axon myelination, and 4) restoration of normal circuitry. Whether optogenetics can promote regeneration of severed axons and subsequently functional recovery in SCI animal models remains to be determined.
Multiple sclerosis
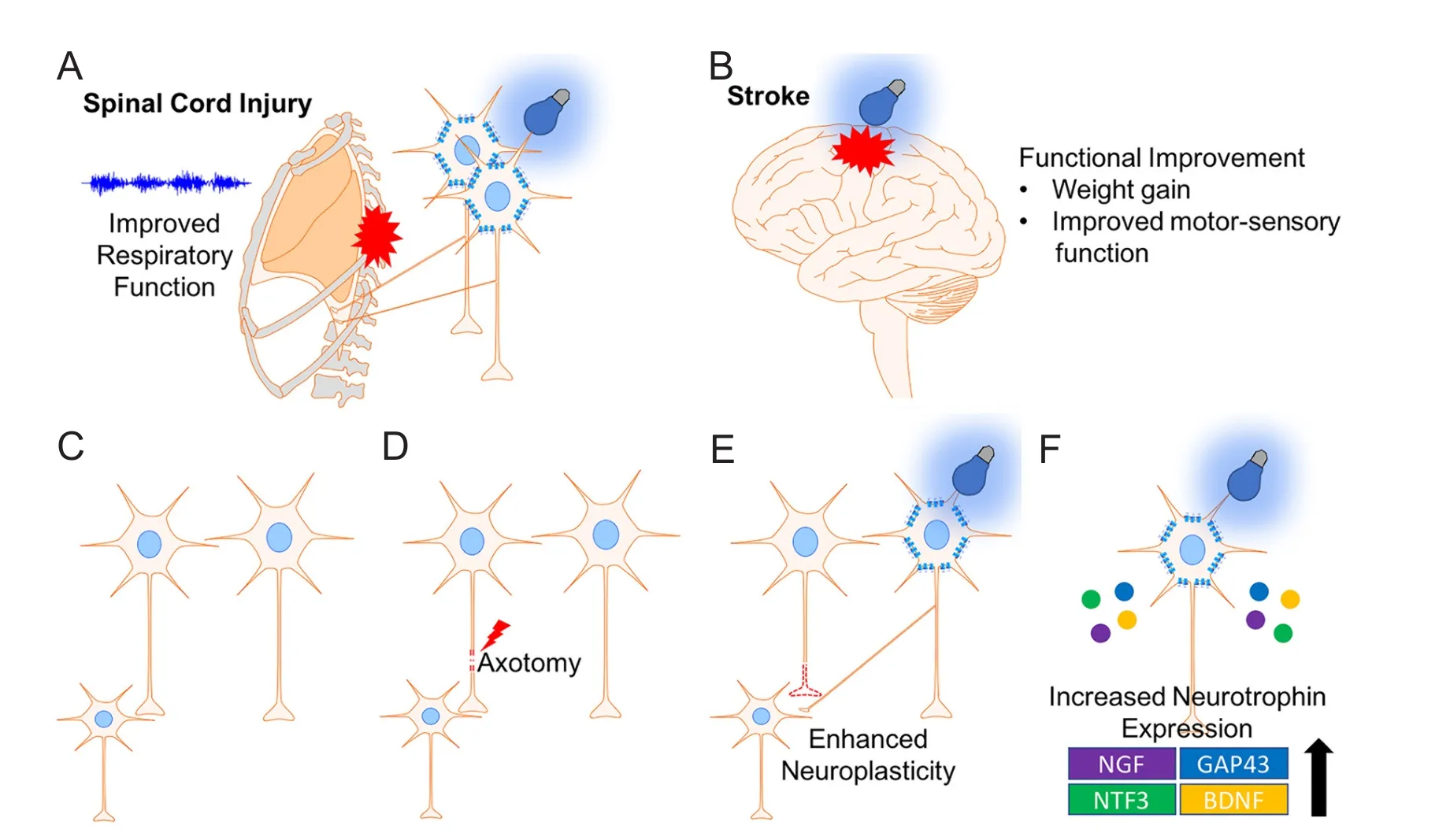
Figure 4 Optogenetics promotes recovery following central nervous system injury.
Multiple sclerosis (MS) is a progressive inflammatory demyelination disease af f ecting several tract fi bers of the CNS (Hauser and Oksenberg, 2006). Patients may present witha wide range of symptoms including, but not limited to, optic neuritis, paraesthesia, motor weakness, and ocular movement dysfunction (e.g., internuclear opthalmoplegia). Treatments have focussed on anti-inflammatory agents to reduce symptomatic episode duration, frequency, and severity (Goldenberg, 2012). However, current therapy is only partially ef f ective and contains several side ef f ects. An intriguing therapy for MS is to increase neuronal activity, which has been shown to increase myelination and oligodendrocyte proliferation with electrical stimulation (Li et al., 2010). However, electrical stimulation is non-specific and can cause several side ef f ects. To overcome this, a previous study demonstrated the potential of optogenetics for MS treatment. Using ChR2 optogenetic stimulation of the premotor cortex in mouse models, Gibson et al. reported that enhanced neuronal activity increased axon myelin thickness and oligodendrocyte proliferation (Figure 5A) (Gibson et al., 2014). Optogenetics was mainly used in this study as proof-of-concept (i.e., enhancing neural activity increases myelination), establishing a target for MS treatment. Since MS is a disease of demyelination, electrical stimulation and optogenetics are potential techniques to use in this disease.
Epilepsy
Epilepsy is a disease characterized by neuron hyperexcitability causing recurrent seizures and af f ecting about 50 million people worldwide (Banerjee et al., 2009). Current treatments include pharmacologic targeting, vagus nerve stimulation, and DBS (Perucca and Tomson, 2011; Fisher, 2012; Orosz et al., 2014; Schmidt and Schachter, 2014; Ben-Menachem et al., 2015). However, these treatments contain several side effects including neurocognitive side ef f ects and hypersensitivity reaction from pharmacotherapy and hallucinations and memory impairment from DBS (Walia et al., 2004; Andrade et al., 2006; Girardeau et al., 2009; Hessen et al., 2009; Yang et al., 2011). Moreover, resistance to pharmacotherapy is a major concern (Picot et al., 2008). Another challenge is that traditional DBS requires continuous stimulation of internal brain structures. Since seizures occur erratically with potentially long interictal periods, there is unnecessary DBS that may contribute to the side ef f ects observed (Hartikainen et al., 2014). To overcome the continuous DBS, the closed-loop device NeuroPace RNS?System has been developed and approved for use in medication-resistant partial onset seizures (Morrell, 2011; Morrell and Halpern, 2016). This system detects seizure activity and ablates the seizure focus on-demand and has been shown to improve quality of life and to have no ef f ect on mood and cognition (Morrell and Halpern, 2016). Although this device has temporal specif i city, it lacks spatial specif i city, which may alter normal brain regions (Li et al., 2012). Optogenetics may be benef i cial to circumvent this problem with its high temporal and spatial resolution.
Optogenetics has been shown to be ef f ectivein vitroandin vivo. In a study by T?nnesen et al.,in vitrooptogenetic inhibition of hippocampal principal cells of regions CA1 and CA3 in a pharmacoresistant model was suf ficient to reduce seizure activity (T?nnesen et al., 2009). Furthermore, in aninvivomodel of neocortical epilepsy, which is commonly pharmacoresistant (Schuele and Luders, 2008), transfection and inhibition of hyperexcitable cortical pyramidal neurons with halorhodopsin reduced seizure activity (Figure 5B)(Wykes et al., 2012). Optogenetics has also shown ef fi cacy in thalamocortical seizures following stroke using halorhodopsin inhibition of neurons (Paz et al., 2013), temporal lobe epilepsy by activation of parvalbumin positive gamma-aminobutyric acid (GABAergic) interneurons with channelrhodopsin-2 (Krook-Magnuson et al., 2013), and for penicillin induced absence seizures by inhibition of thalamic neurons with enhanced Natronomonas pharaonis halorhodopsin (eNpHR) (Figure 5B) (Han et al., 2015). Moreover, optogenetics has opened insight into the role of the cerebellum in modulating temporal lobe epilepsy. In a study by Krook-Magnuson et al., a closed-loop seizure device was used to detect temporal lobe seizure activity and deliver light to lateral or midline cerebellar regions containing ChR2 or NpHR expressing Purkinje cells (Krook-Magnuon et al., 2014).is study showed that cerebellar-directed intervention can inhibit seizure activity and increase interictal period. Although optogenetics has been shown ef f ective for treating epilepsy in animal models, translating this into the clinic is challenging, considering not all epilepsies are the same. To overcome this challenge, a study showed optogenetic activation of the deep/intermediate layers of the superior colliculus suppressed seizures originating from diverse networks including thalamocortical (absence), brainstem, forebrain (complex partial), and forebrain plus brainstem (Soper et al., 2016).erefore, this is an intriguing target for clinical application.
Previous methods to decipher pathological neural circuitry in epilepsy include pharmacological stimulation and glutamate photo-uncaging. However, these methods are limited by long latency in the former and long-lasting ef f ect and requirement of glutamate replenishing in the latter (Zemelman et al., 2003; Shepherd and Svoboda, 2005; Jin et al., 2006; Zhang et al., 2012). Optogenetics overcomes these limitations by its short evoked-potential latency period and fast“on-off” kinetics allowing for effective neuron stimulation with millisecond interpulse intervals (Boyden et al., 2005). In studies by Meeren et al. and Sorokin et al., optogenetics was used to understand the pathway responsible for generalized absence seizures, which was previously questioned (Meeren et al., 2005; Sorokin et al., 2017).ey found that rhythmic synchronized spiking or phasic firing of thalamacortical neurons was necessary and suf fi cient to induce synchronous spike wave discharge (SWD) and behaviour activity indicative of absence seizures. Moreover, optogenetics was used to identify the mechanism of low-frequency DBS for the treatment of temporal lobe epilepsy.e ef ficacy treatment was found to be through activation of glutamatergic CaMKIIα-positive neurons in the entorhinal cortex, which then activate GABAergic principal cells in the hippocampus to ablate seizure activity (Xu et al., 2016). In summary, optogenetics can be applied in epilepsy research, especially as a tool to improve our understanding of pathophysiology of dif f erent types of epilepsy.
Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by progressive cognitive decline (Qiu et al., 2009) and the pathological markers: extracellular senile plaques made up of beta-amyloid protein and intracellular neurofibrillary tangles (Kumar et al., 2015). Most of the research has focussed on these pathological markers and memory dysfunction in later stages of AD (Selkoe, 2001, 2002; West and Bhugra, 2015). However, studies have shown aberrant synaptic phenotypes (Terry et al., 1991; Jacobsen et al., 2006), decreased dendritic spine density in the dentate gyrus, LTP impairment, and dysfunctional perforant pathway input from the entorhinal cortex to the dentate gyrus in mouse models of memory impairment prior to formation of senile plaques (Jacobsen et al., 2006).ese fi ndings have increased interest in earlier stages of AD.
A recent study by Roy et al. showed that early memory deficits in AD are a result of aberrant memory retrieval and not memory consolidation (Roy et al., 2016). In their study, early AD mouse models were used by overexpressing the delta-9 variant of presinillin-1 in combination with the Swedish mutation of amyloid precursor protein. To deduce the mechanism of memory impairment, contextual fear conditioning (CFC) was used. In their model, the unconditioned stimulus electric shock was paired with the chamber “Context A”.is caused the conditioned response “freezing”. Placing control mice in “Context A” elicited freezing response, but early AD mice did not freeze after placement in “Context A” because of memory impairment. However, following optogenetic activation of engram cells (i.e., neurons holding traces of specif i c memory) in the dentate gyrus with ChR2, mice responded with freezing even in a different environment “Context B”.is showed that memory impairment in early AD is not the result of impaired memory storage but dysfunctional memory retrieval. Moreover, this study also showed the potential of neuron stimulation in the treatment of AD. As mentioned above, reduced dendritic spine density in the dentate gyrus is observed in early AD. Remarkably, optogenetic induction of LTP between the perforant path synapse and dentate gyrus engram cells recovered spine density to control levels, and this correlated with improved longterm memory (Figure 5C) (Roy et al., 2016).
Optogenetics has been used to clarify the causal relationship between synaptic plasticity and memory encoding, which was previously challenging to elucidate (Stevens, 1998). In a study by Nabavi et al. (2014), memory from cued-fear conditioning was inactivated and reactivated by long-term depression (LTD) and LTP, respectively.is was observed by conditioning mice to reduce lever pressing (conditioned response; CR) by pairing the unconditioned stimulus (US) “foot shock” with blue light stimulation (conditioned stimulus; CS) of oChIEF-(mammalian-codon optimized variant of ChIEF; ChIEF is a variant of ChEF with isoleucine 170 mutated to valine); ChEF is a chimera of channelrhodopsin 1 and 2) expressing neurons in the medial geniculate nucleus projecting to the lateral amygdala, which is an essential pathway for fear conditioning (Repa et al., 2001). Aer the training phase, mice showed reduced lever pressing upon blue light stimulation, which indicated successful conditioning. Interestingly, pairing light stimulus with the foot shock resulted in LTP, which was detected by measuring the ratio of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) to N-methyl-D-aspartate (NMDA) receptor in fl uence in optogenetically driven synaptic responses in amygdala slices. Furthermore, optical induction of LTD caused the loss of conditioned response to blue light stimulation, and this response was reactivated by inducing optical LTP.erefore, this study showed that memory can be inactivated and reactivated using LTD and LTP, respectively, and clarifi ed the causal relationship between these synaptic processes and memory. Collectively, optogenetics was mainly used as a tool to elucidate memory encoding in animal models. In addition, it was used as a proof-of-concept to show that stimulation of dentate gyrus engram cells in AD mice restores spine density, which correlated with memory retrieval.
Parkinson’s disease
Parkinson’s disease (PD) is a neurodegenerative disease af f ecting 1–2% of the population above 60 years. It is characterized pathologically by loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) and clinically by bradykinesia, cogwheel rigidity, resting tremor, and shuffl ing gait (Lang and Lozano, 1998; Tanner and Aston, 2000; Dauer and Przedborski, 2003). Current therapies have been focussing on improving symptoms and quality of life but have not been successful in reversing the disease progression (Connolly and Lang, 2014). The two main therapies used for PD are pharmacotherapy and DBS. However, these therapies have many potential side ef f ects such as: nausea, constipation, orthostasis, psychosis, impulse control disorders, depression, and mania (Weintraub et al., 2006; Temel, 2010; Connolly and Lang, 2014). Moreover, medication-associated resistance and dyskinesia may develop following long-term therapy with levodopa, which is one of the most effective treatments for PD (Fahn, 2000; Aquino and Fox, 2015). DBS is an ef f ective treatment option for patients with insuf fi cient response to pharmacotherapy and/or develop dyskinesia. However, this treatment is invasive since it requires a surgical procedure to implant electrodes into deep brain nuclei (Okun, 2012). Moreover, since DBS requires electrical stimulation, it is a non-specif i c targeting treatment (Li et al., 2012).
Optogenetics provides more specif i c neuron targeting with high temporal resolution.is light-guided technology has been used in the PD fi eld to study movement, dopaminergic neuron transplantation and pathological circuitry. To understand the role of dopamine in movement, Howe and Dombeck used 2-photon intracellular calcium (by expressig green fl uorescent protein (GFP)-Calmodulin fusion protein 6f (GCaMP6f) in dopaminergic neurons) recording of dopaminergic neurons (DA) projecting to the dorsal striatum during initiation of movement, acceleration, and resting on cylindrical treadmill (Howe and Dombeck, 2016).e pattern recorded was then simulated with ChR2 optogeneticstimulation of the dopaminergic neurons. Their findings suggested DA neurons may not be all that are required to initiate or accelerate movement. However, DA neuron input to the dorsal striatum may be required for motivation and modulation of movement.is study suggests application of time-precision fi ring in the transplanted DA neurons for PD treatment may increase its ef fi cacy.
Dopaminergic neurons derived from human embryonic stem cells have been successfully transplanted in rodent and non-human primate PD models (Kriks et al., 2011). Using optogenetics with eNpHR3.0 to interrogate the mechanism of efficacy, transplanted DA neurons were found to release dopamine to the striatum and enhance medium spiny neuron excitatory post-synaptic potentials (EPSPs) through modulation of glutamatergic transmission.is is similar to physiologic dopaminergic neuron function (Steinbeck et al., 2015). A more recent study using Drosophila larvae demonstrated that enhancing DA neuron activity promoted recovery of PD symptoms (Qi et al., 2017).erefore, utilization of an excitatory opsin (i.e., ChR2) to activate transplanted DA neurons may enhance this treatment’s ef ficacy through increasing the release of dopamine (Figure 5D).
Optogenetics has also been used to understand the therapeutic mechanism of DBS for PD. In a study using transgenic mice expressing ChR2 under Thy1 promotor, the mechanism of DBS was determined to be through activation of af f erent neurons projecting from layer 5 of the cortex to the subthalamic nucleus (Gradinaru et al., 2009). One caveat to this study was that all layer 5 neurons contained ChR2, and since not all layer 5 neurons project to the subthalamic nucleus, it was not clear whether the behavior improvement was exclusively through the subthalamus projecting fibers or fibers projecting elsewhere. Moreover, the region of the cortex providing therapeutic benef i t was unclear. To clarify this role, Sanders and Jaeger (2016) injected adeno-associated virus constructs containing wheat germ agglutinin-Cre recombinase (WGA-Cre) in the subthalamic nucleus to retrogradely express Cre recombinase (Cre) in the projections to the subthalamic nucleus followed by injecting a Cre-dependent hChR2-EYFP construct in layer 5 of primary motor cortex (M1).is resulted in hChR2 expression in M1 layer 5 neurons projecting to the subthalamic nucleus. Optogenetic stimulation of afferent STN in M1 resulted in improved bradykinesia and hypokinesia, which clarified that these neurons mediate DBS ef fi cacy in PD.
Optogenetics has also been used to study the neural circuitry involved in PD and levodopa-induced dyskinesia (LID). In a study by Kravitz et al., ChR2 was used to either activate the direct (D1) or indirect (D2) pathway using ChR2 (Kravitz et al., 2010).ey found stimulation of the indirect pathway with ChR2 in mice causes bradykinesia, freezing, and decreased locomotion whereas stimulation of the direct pathway rescued these deficits. Optogenetics has also been used to study dyskinesia, which is an undesirable side effect from levodopa therapy. Hernandez et al. showed simultaneous stimulation of the medium spiny neurons of the direct and indirect pathway induced dyskinesia with levodopa (Hernandez et al., 2017). Moreover, a different study showed striatal cholinergic neurons also play an important role in regulating LID (Bordia et al., 2016). Interestingly, optogenetic inhibition of subthalamic nucleus was shown to be ef f ective in treating LID in rat PD models (Fahn, 2000; Yoon et al., 2016). Collectively, these studies demonstrate optogenetics application to understandcurrent therapies in PD and to dissect pathological and physiological movement circuitry. Moreover, optogenetics has potential application for transplanted DA neurons to stimulate their release of dopamine.
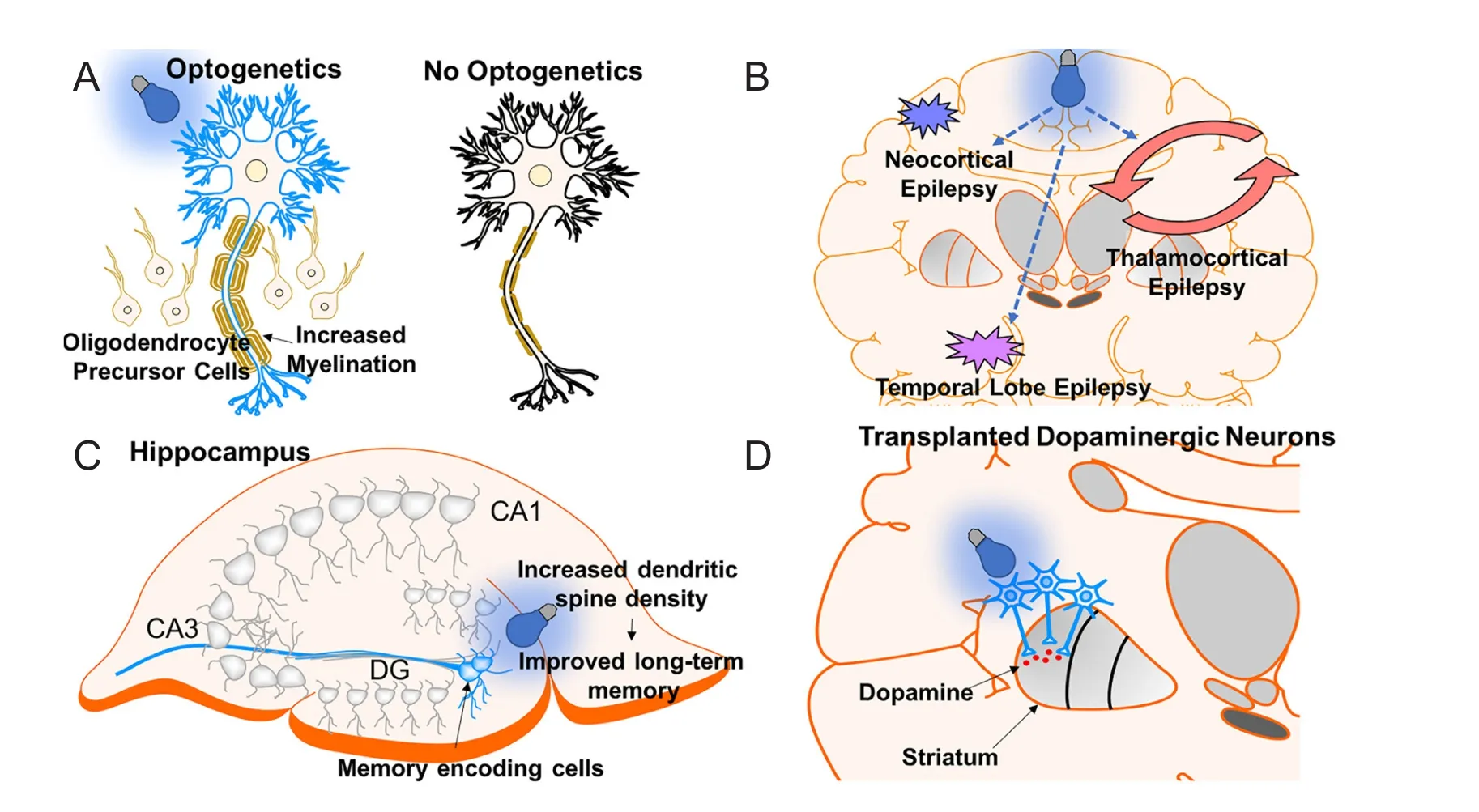
Figure 5 Application of optogenetics in other neurological disorders.
Challenges and Future Direction
A major obstacle in the application of optogenetics is the translation from bench to patient, specifically the delivery of optogenetic tools in patients. It is an invasive treatment, requiring implantation of an optic fi ber into brain tissue to supply light to activate opsins in deep regions.is implantation poses two potential risks: 1) overheating from light can induce damage to tissue, and 2) direct introduction of foreign objects into brain may cause tissue scarring and can become the source of infection by introducing microbes into the CNS.
To ensure successful clinical optogenetics application one must not overlook the opsin expression level in the targeted neuron. Several factors must be taken into consideration: 1) a non-invasive method to express opsins in specif i c targeted neurons, 2) a method to evaluate opsin expression level in specif i c targeted neurons, and 3) the potential risk of opsin, a foreign protein antigen, as autoimmune agent in patients. Currently, animal studies use viral transfection and transgenic mice. However, these techniques may raise technical and ethical issues for human application.
Despite the abovementioned challenges, progress has been made in optogenetics translational studies. Optogenetics has been safely and ef f ectively applied to awake non-human primate rhesus macaques (Han et al., 2009, 2011). Clinical trials in phases I and II using adeno-associated virus injection have successfully demonstrated its safety in patients (Bartus et al., 2013; Murphy and Rabkin, 2013; Simonato et al., 2013). Another remarkable translational ef f ort was the fi rst patient dosed with optogenetics for retinitis pigmentosa in a clinical trial conducted by RetroSense Therapeutics, which was reported in 2016. Similar to retinitis pigmentosa, there are many other diseases which do not have effective treatments or have treatments that are intolerable. Optogenetics has not only helped us discover the pathophysiology of many neurological diseases, but has also opened the door to use this as a treatment option.
Author contributions:JDO wrote the paper. JDO and WW were responsible for making figures and edited the paper. XMX reviewed and edited the paper. All authors participated in the conception of this study and approved the fi nal version of this paper.
Conf l icts of interest:None declared.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Adamantidis AR, Zhang F, de Lecea L, Deisseroth K (2014) Optogenetics: opsins and optical interfaces in neuroscience. Cold Spring Harb Protoc 2014:815-822.
Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L (2007) Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450:420-424.
Alilain W, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J (2008) Light induced rescue of breathing aer spinal cord injury. J Neurosci 28:11862-11870.
Andrade DM, Zumsteg D, Hamani C, Hodaie M, Sarkissian S, Lozano AM, Wennberg RA (2006) Long-term follow-up of patients with thalamic deep brain stimulation for epilepsy. Neurology 66:1571-1573.
Aquino CC, Fox SH (2015) Clinical spectrum of levodopa-induced complications. Mov Disord 30:80-89.
Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K (2007) An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4:S143-156.
Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G (2007) In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54:205-218.
Atasoy D, Aponte Y, Su HH, Sternson SM (2008) A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and longrange circuit mapping. J Neurosci 28:7025-7030.
Bamberg E, Tittor J, Oesterhelt D (1993) Light-driven proton or chloride pumping by halorhodopsin. Proc Natl Acad Sci U S A 90:639-643.
Banerjee PN, Filippi D, Hauser WA (2009)e descriptive epidemiology of epilepsy-a review. Epilepsy Res 85:31-45.
Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH (2004) Light-activated ion channels for remote control of neuronal fi ring. Nat Neurosci 7:1381-1386.
Bartus RT, Baumann TL, Sif f ert J, Herzog CD, Alterman R, Boulis N, Turner DA, Stacy M, Lang AE, Lozano AM, Olanow CW (2013) Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology 80:1698-1701.
Ben-Menachem E, Revesz D, Simon BJ, Silberstein S (2015) Surgically implanted and non-invasive vagus nerve stimulation: a review of ef ficacy, safety and tolerability. Eur J Neurol 22:1260-1268.
Berndt A, Lee SY, Ramakrishnan C, Deisseroth K (2014) Structure-Guided Transformation of Channelrhodopsin into a Light-Activated Chloride Channel. Science 344:420-424.
Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K (2009) Bi-stable neural state switches. Nat Neurosci 12:229-234.
Bordia T, Perez XA, Heiss JE, Zhang D, Quik M (2016) Optogenetic activation of striatal cholinergic interneurons regulates L-dopa-induced dyskinesias. Neurobiol Dis 91:47-58.
Boyden ES (2011) A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 Biol Rep 3:11.
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8:1263-1268.
Brushart TM, Hof f man PN, Royall RM, Murinson BB, Witzel C, Gordon T (2002) Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci 22:6631-6638.
Chen CH, Fremont R, Arteaga-Bracho EE, Khodakhah K (2014) Short latency cerebellar modulation of the basal ganglia. Nat Neurosci 17:1767-1775.
Cheng MY, Wang EH, Woodson WJ, Wang S, Sun G, Lee AG, Arac A, Fenno LE, Deisseroth K, Steinberg GK (2014) Optogenetic neuronal stimulation promotes functional recovery aer stroke. Natl Acad Sci U S A 111:12913-12918.
Choudhury GR, Ding S (2016) Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol Dis 85:234-244.
Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES (2010) High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463:98-102.
Chuong AS et al. (2014) Noninvasive optical inhibition with a red-shi-ed microbial rhodopsin. Nat Neurosci 17:1123-1129.
Connolly BS, Lang AE (2014) Pharmacological treatment of Parkinson disease: a review. JAMA 311:1670-1683.
Cripps RA, Lee BB, Wing P, Weerts E, Mackay J, Brown D (2011) A global map for traumatic spinal cord injury epidemiology: towards a living data repository for injury prevention. Spinal Cord 49:493-501.
Daadi MM, Klausner JQ, Bajar B, Goshen I, Lee-Messer C, Lee SY, Winge MCG, Ramakrishnan C, Lo M, Sun G, Deisseroth K, Steinberg GK (2016) Optogenetic stimulation of neural gras enhances neurotransmission and downregulates the inf l ammatory response in experimental stroke model. Cell Transplant 25:1371-1380.
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889-909.
Deisseroth K (2011) Optogenetics. Nat Meth 8:26-29.
Deisseroth K, Heist EK, Tsien RW (1998) Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 392:198-202.
Deisseroth K, Feng G, Majewska AK, Miesenb?ck G, Ting A, Schnitzer MJ (2006) Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci 26:10380.
Doronina-Amitonova LV, Fedotov IV, Ivashkina OI, Zots MA, Fedotov AB, Anokhin KV, Zheltikov AM (2013) Implantable fi ber-optic interface for parallel multisite long-term optical dynamic brain interrogation in freely moving mice. Sci Rep 3:3265.
Fahn S (2000) The spectrum of levodopa-induced dyskinesias. Ann Neurol 47:S2-9; discussion S9-11.
Farah N, Reutsky I, Shoham S (2007) Patterned optical activation of retinal ganglion cells. Conf Proc IEEE Eng Med Biol Soc 2007:6368-6370.
Fitch MT, Silver J (2008) CNS injury, glial scars, and inf l ammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 209:294-301.
Flusberg BA, Cocker ED, Piyawattanametha W, Jung JC, Cheung ELM, Schnitzer MJ (2005) Fiber-optic fluorescence imaging. Nat Meth 2:941-950.
Friel DD, Chiel HJ (2008) Calcium dynamics: analyzing the Ca2+regulatory network in intact cells. Trends Neurosci 31:8-19.
Furlan JC, Noonan V, Singh A, Fehlings MG (2011) Assessment of impairment in patients with acute traumatic spinal cord injury: a systematic review of the literature. J Neurotrauma 28:1445-1477.
Gagnon-Turcotte G, LeChasseur Y, Bories C, Messaddeq Y, De Koninck Y, Gosselin B (2017) A wireless headstage for combined optogenetics and multichannel electrophysiological recording. IEEE Trans Biomed Circuits Syst 11:1-14.
Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT (2004) Activated CREB is suf fi cient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron 44:609-621.
Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD (2010) Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci 30:3175-3183.
Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304.
Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB (2009) Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12:1222-1223.
Goldenberg MM (2012) Multiple Sclerosis Review. P T 37:175-184.
Gradinaru V, Mogri M,ompson KR, Henderson JM, Deisseroth K (2009) Optical deconstruction of Parkinsonian neural circuitry. Science 324:354-359.
Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I,ompson KR, Deisseroth K (2010) Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141:154-165.
Grosenick L, Marshel JH, Deisseroth K (2015) Closed-loop and activity-guided optogenetic control. Neuron 86:106-139.
Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P (2010) Ultrafast optogenetic control. Nat Neurosci 13:387-392.
Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K (2014) Natural neural projection dynamics underlying social behavior. Cell 157:1535-1551.
Hackett AR, Lee JK (2016) Understanding the NG2 glial scar aer spinal cord injury. Front Neurol 7:199.
Han X, Boyden ES (2007) Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One 2:e299.
Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES (2009) Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron 62:191-198.
Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, Boyden ES (2011) A highlight sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci 5:18.
Han Y, Ma F, Li H, Wang Y, Xu K (2015) Optogenetic control of thalamus as a tool for interrupting penicillin induced seizures. Conf Proc IEEE Eng Med Biol Soc 2015:6606-6609.
Hartikainen KM, Sun L, Polvaara M, Brause M, Lehtim?ki K, Haapasalo J, M?tt?nen T, V?yrynen K, Ogawa KH, ?hman J, Peltola J (2014) Immediate effects of deep brain stimulation of anterior thalamic nuclei on executive functions and emotion-attention interaction in humans. J Clin Exp Neuropsychol 36:540-550.
Harvey PJ, Grochmal J, Tetzlaf f W, Gordon T, Bennett DJ (2005) An investigation into the potential for activity-dependent regeneration of the rubrospinal tract aer spinal cord injury. Eur J Neurosci 22:3025-3035.
Hauser SL, Oksenberg JR (2006)e neurobiology of multiple sclerosis: genes, inf l ammation, and neurodegeneration. Neuron 52:61-76.
He Z, Jin Y (2016) Intrinsic control of axon regeneration. Neuron 90:437-451.
Hegemann P, Oesterbelt D, Steiner M (1985) The photocycle of the chloride pump halorhodopsin. I: Azide-catalyzed deprotonation of the chromophore is a side reaction of photocycle intermediates inactivating the pump. EMBO J 4:2347-2350.
Helmchen F, Fee MS, Tank DW, Denk W (2001) A miniature head-mounted two-photon microscope: high-resolution brain imaging in freely moving animals. Neuron 31:903-912.
Hendricks BK, Shi R (2014) Mechanisms of neuronal membrane sealing following mechanical trauma. Neurosci Bull 30:627-644.
Hernandez F, Castela I, Ruiz-DeDiego I, Obeso JA, Moratalla R (2017) Striatal activation by optogenetics induces dyskinesias in the 6-hydroxydopamine rat model of Parkinson disease. Mov Disord 32:530-537.
Hessen E, Lossius MI, Gjerstad L (2009) Antiepileptic monotherapy signif i cantly impairs normative scores on common tests of executive functions. Acta Neurol Scand 119:194-198.
Howe MW, Dombeck DA (2016) Rapid signaling in distinct dopaminergic axons during locomotion and reward. Nature 535:505-510.
Huber D, Petreanu L, Ghitani N, Ranade S, Hromádka T, Mainen Z, Svoboda K (2008) Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451:61-64.
Jacobsen JS, Wu C-C, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE (2006) Early-onset behavioral and synaptic def i cits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 103:5161-5166.
Jin X, Prince DA, Huguenard JR (2006) Enhanced excitatory synaptic connectivity in layer v pyramidal neurons of chronically injured epileptogenic neocortex in rats. J Neurosci 26:4891-4900.
Kamber D, Erez H, Spira ME (2009) Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone. Exp Neurol 219:112-125.
Kato HE, Zhang F, Yizhar O, Ramakrishnan C, Nishizawa T, Hirata K, Ito J, Aita Y, Tsukazaki T, Hayashi S, Hegemann P, Maturana AD, Ishitani R, Deisseroth K, Nureki O (2012) Crystal structure of the channelrhodopsin light-gated cation channel. Nature 482:369-374.
Kikukawa T, Kamo N, Demura M (2015) Photochemistry of Halorhodopsin. In: Optogenetics: Light-Sensing Proteins and Their Applications (Yawo H, Kandori H, Koizumi A, eds), pp 47-62. Tokyo: Springer Japan.
Kim CK, Yang SJ, Pichamoorthy N, Young NP, Kauvar I, Jennings JH, Lerner TN, Berndt A, Lee SY, Ramakrishnan C, Davidson TJ, Inoue M, Bito H, Deisseroth K (2016) Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat Meth 13:325-328.
Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine-Paton M, Wong GK, et al. (2014) Independent optical excitation of distinct neural populations. Nat Methods 11:338-346.
Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC (2010) Regulation of parkinsonian motor behaviors by optogenetic control of basal ganglia circuitry. Nature 466:622-626.
Kriks S, Shim J-W, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L (2011) Floor plate-derived dopamine neurons from hESCs ef fi ciently engrain animal models of PD. Nature 480:547-551.
Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I (2013) On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun 4:1376.
Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I (2014) Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro 1. pii: e.2014.
Kuhlman SJ, Huang ZJ (2008) High-resolution labeling and functional manipulation of specif i c neuron types in mouse brain by Cre-activated viral gene expression. PLoS One 3:e2005.
Kumar A, Singh A, Ekavali (2015) A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep 67:195-203.
Lang AE, Lozano AM (1998) Parkinson’s disease. First of two parts. N Engl J Med 339:1044-1053.
Li Q, Brus-Ramer M, Martin JH, McDonald JW (2010) Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci Lett 479:128-133.
Li Q, Ke Y, Chan Danny CW, Qian ZM, Yung Ken KL, Ko H, Arbuthnott Gordon W, Yung WH (2012)erapeutic deep brain stimulation in Parkinsonian rats directly inf l uences motor cortex. Neuron 76:1030-1041.
Lin JY, Lin MZ, Steinbach P, Tsien RY (2009) Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J 96:1803-1814.
Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY (2013) ReaChR: A red-shied variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci 16:1499-1508.
Lu C, Park S, Richner TJ, Derry A, Brown I, Hou C, Rao S, Kang J, Moritz CT, Fink Y, Anikeeva P (2017) Flexible and stretchable nanowire-coated fi bers for optoelectronic probing of spinal cord circuits. Sci Adv 3:e1600955.
Mandolesi G, Madeddu F, Bozzi Y, Maf f ei L, Ratto GM (2004) Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J 18:1934-1936.
Matsuno-Yagi A, Mukohata Y (1977) Two possible roles of bacteriorhodopsin; a comparative study of strains of Halobacterium halobium dif f ering in pigmentation. Biochem Biophys Res Commun 78:237-243.
Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A (2005) Evolving concepts on the pathophysiology of absence seizures:e cortical focus theory. Arch Neurol 62:371-376.
Miyamoto D, Murayama M (2016) The fiber-optic imaging and manipulation of neural activity during animal behavior. Neurosci Res 103:1-9.
Montgomery KL, Yeh AJ, Ho JS, Tsao V, Mohan Iyer S, Grosenick L, Ferenczi EA, Tanabe Y, Deisseroth K, Delp SL, Poon AS (2015) Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat Methods 12:969-974.
Morrell MJ (2011) Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77:1295-1304.
Morrell MJ, Halpern C (2016) Responsive direct brain stimulation for epilepsy. Neurosurg Clin N Am 27:111-121.
Murphy AM, Rabkin SD (2013) Current status of gene therapy for brain tumors. Transl Res 161:339-354.
Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R (2014) Engineering a memory with LTD and LTP. Nature 511:348-352.
Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P (2002) Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296:2395-2398.
Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E (2003) Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A 100:13940-13945.
NSCISC (2016) Facts and Figures at a Glance. In: Birmingham, AL: National Spinal Cord Injury Statistical Center.
Oesterhelt D, Stoeckenius W (1971) Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol 233:149-152.
Okun MS (2012) Deep-brain stimulation for Parkinson’s disease. New Engl J Med 367:1529-1538.
Oluigbo CO, Salma A, Rezai AR (2012) Deep brain stimulation for neurological disorders. IEEE Rev Biomed Eng 5:88-99.
Orosz I, McCormick D, Zamponi N, Varadkar S, Feucht M, Parain D, Griens R, Vallee L, Boon P, Rittey C, Jayewardene AK, Bunker M, Arzimanoglou A, Lagae L (2014) Vagus nerve stimulation for drug-resistant epilepsy: a European long-term study up to 24 months in 347 children. Epilepsia 55:1576-1584.
Pansare V, Hejazi S, Faenza W, Prud’homme RK (2012) Review of long-wavelength optical and NIR imaging materials: contrast agents, fl uorophores and multifunctional nano carriers. Chem Mater 24:812-827.
Park SI, Brenner DS, Shin G, Morgan CD, Copits BA, Chung HU, Pullen MY, Noh KN, Davidson S, Oh SJ, Yoon J, Jang KI, Samineni VK, Norman M, Grajales-Reyes JG, Vogt SK, Sundaram SS, Wilson KM, Ha JS, Xu R, et al. (2015) Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat Biotechnol 33:1280-1286.
Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR (2013) Closed-loop optogenetic control of thalamus as a new tool to interrupt seizures aer cortical injury. Nat Neurosci 16:64-70.
Perucca E, Tomson T (2011)e pharmacological treatment of epilepsy in adults. Lancet Neurol 10:446-456.
Picot M-C, Baldy-Moulinier M, Daurès J-P, Dujols P, Crespel A (2008)e prevalence of epilepsy and pharmacoresistant epilepsy in adults: A population-based study in a Western European country. Epilepsia 49:1230-1238.
Proville RD, Spolidoro M, Guyon N, Dugue GP, Selimi F, Isope P, Popa D, Lena C (2014) Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nat Neurosci 17:1233-1239.
Qi C, Varga S, Oh SJ, Lee CJ, Lee D (2017) Optogenetic rescue of locomotor dysfunction and dopaminergic degeneration caused by alpha-synuclein and EKO genes. Exp Neurobiol 26:97-103.
Qiu C, Kivipelto M, von Strauss E (2009) Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci 11:111-128.
Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE (2001) Two dif f erent lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci 4:724-731.
Rishal I, Fainzilber M (2010) Retrograde signaling in axonal regeneration. Exp Neurol 223:5-10.
Roy DS, Arons A, Mitchell TI, Pignatelli M, Ryan TJ, Tonegawa S (2016) Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531:508-512.
Sanders TH, Jaeger D (2016) Optogenetic stimulation of cortico-subthalamic projections is suf fi cient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiol Dis 95:225-237.
Sandrow-Feinberg HR, Houlé JD (2015) Exercise aer spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res 1619:12-21.
Sawinski J, Wallace DJ, Greenberg DS, Grossmann S, Denk W, Kerr JN (2009) Visually evoked activity in cortical cells imaged in freely moving animals. Proc Natl Acad Sci U S A 106:19557-19562.
Schmidt D, Schachter SC (2014) Drug treatment of epilepsy in adults. BMJ 348:g254.
Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A (2006) Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol 16:1741-1747.
Schuele SU, Luders HO (2008) Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol 7:514-524.
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81:741-766.
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789-791.
Shah AM, Ishizaka S, Cheng MY, Wang EH, Bautista AR, Levy S, Smerin D, Sun G, Steinberg GK (2017) Optogenetic neuronal stimulation of the lateral cerebellar nucleus promotes persistent functional recovery aer stroke. Sci Rep 7:46612.
Shepherd GM, Svoboda K (2005) Laminar and columnar organization of ascending excitatory projections to layer 2/3 pyramidal neurons in rat barrel cortex. J Neurosci 25:5670-5679.
Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, Gray SJ, Lowenstein PR, Vandenberghe LH, Wilson TJ, Wolfe JH, Glorioso JC (2013) Progress in gene therapy for neurological disorders. Nat Rev Neurol 9:277-291.
Simpson LA, Eng JJ, Hsieh JTC, Wolfe DL, the SRT (2012)e health and life priorities of individuals with spinal cord injury: A systematic review. J Neurotrauma 29:1548-1555.
Soper C, Wicker E, Kulick CV, N’Gouemo P, Forcelli PA (2016) Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiol Dis 87:102-115.
Sorokin JM, Davidson TJ, Frechette E, Abramian AM, Deisseroth K, Huguenard JR, Paz JT (2017) Bidirectional control of generalized epilepsy networks via rapid real-time switching of fi ring mode. Neuron 93:194-210.
Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, Mosharov EV, Studer L (2015) Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat Biotech 33:204-209.
Stevens CF (1998) A million dollar question: does LTP = memory? Neuron 20:1-2.
Stryer L (1986) Cyclic GMP cascade of vision. Annu Rev Neurosci 9:87-119.
Szabo V, Ventalon C, De Sars V, Bradley J, Emiliani V (2014) Spatially selective holographic photoactivation and functional fluorescence imaging in freely behaving mice with a fi berscope. Urology 84:1157-1169.
Tamura K, Ohashi Y, Tsubota T, Takeuchi D, Hirabayashi T, Yaguchi M, Matsuyama M, Sekine T, Miyashita Y (2012) A glass-coated tungsten microelectrode enclosing optical fibers for optogenetic exploration in primate deep brain structures. J Neurosci Methods 211:49-57.
Tanner CM, Aston DA (2000) Epidemiology of Parkinson’s disease and akinetic syndromes. Curr Opin Neurol 13:427-430.
Temel Y (2010) Chapter 3 - Limbic Ef f ects of High-Frequency Stimulation of the Subthalamic Nucleus. In: Vitamins & Hormones (Gerald L, ed), pp 47-63: Academic Press.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30:572-580.
T?nnesen J, S?rensen AT, Deisseroth K, Lundberg C, Kokaia M (2009) Optogenetic control of epileptiform activity. Proc Natl Acad Sci U S A 106:12162-12167.
Udina E, Furey M, Busch S, Silver J, Gordon T, Fouad K (2008) Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol 210:238-247.
Walia KS, Khan EA, Ko DH, Raza SS, Khan YN (2004) Side ef f ects of antiepileptics--a review. Pain Pract 4:194-203.
Watkins TA, Wang B, Huntwork-Rodriguez S, Yang J, Jiang Z, Eastham-Anderson J, Modrusan Z, Kaminker JS, Tessier-Lavigne M, Lewcock JW (2013) DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci U S A 110:4039-4044.
Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, Moberg PJ, Stern MB (2006) Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol 63:969-973.
West S, Bhugra P (2015) Emerging drug targets for Abeta and tau in Alzheimer’s disease: a systematic review. Br J Clin Pharmacol 80:221-234.
Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, Hegemann P (2014) Conversion of channelrhodopsin into a light-gated chloride channel. Science 344:409-412.
Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K (2010) Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330:1677-1681.
Wu G-Y, Deisseroth K, Tsien RW (2001) Activity-dependent CREB phosphorylation: Convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A 98:2808-2813.
Wykes RC, Heeroma JH, Mantoan L, Zheng K, MacDonald DC, Deisseroth K, Hashemi KS, Walker MC, Schorge S, Kullmann DM (2012) Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci Transl Med 4:161ra152.
Xu Z, Wang Y, Chen B, Xu C, Wu X, Wang Y, Zhang S, Hu W, Wang S, Guo Y, Zhang X, Luo J, Duan S, Chen Z (2016) Entorhinal principal neurons mediate brain-stimulation treatments for epilepsy. EBio-Medicine 14:148-160.
Yang CY, Dao RL, Lee TJ, Lu CW, Yang CH, Hung SI, Chung WH (2011) Severe cutaneous adverse reactions to antiepileptic drugs in Asians. Neurology 77:2025-2033.
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617-627.
Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K (2011a) Optogenetics in neural systems. Neuron 71:9-34.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K (2011b) Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477:171-178.
Yoon HH, Min J, Hwang E, Lee CJ, Suh JK, Hwang O, Jeon SR (2016) Optogenetic inhibition of the subthalamic nucleus reduces levodopa-induced dyskinesias in a rat model of Parkinson’s disease. Stereotact Funct Neurosurg 94:41-53.
Zemelman BV, Lee GA, Ng M, Miesenbock G (2002) Selective photostimulation of genetically chARGed neurons. Neuron 33:15-22.
Zemelman BV, Nesnas N, Lee GA, Miesenb?ck G (2003) Photochemical gating of heterologous ion channels: Remote control over genetically designated populations of neurons. Proc Natl Acad Sci U S A 100:1352-1357.
Zeng H, Madisen L (2012) Mouse transgenic approaches in optogenetics. Prog Brain Res 196:193-213.
Zhang F, Wang L-P, Boyden ES, Deisseroth K (2006) Channelrhodopsin-2 and optical control of excitable cells. Nat Meth 3:785-792.
Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K (2010) Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc 5:439-456.
Zhang F, Prigge M, Beyrière F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K (2008) Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci 11:631-633.
Ziv NE, Spira ME (1995) Axotomy induces a transient and localized elevation of the free intracellular calcium concentration to the millimolar range. J Neurophysiol 74:2625-2637.
Ziv NE, Spira ME (1997) Localized and transient elevations of intracellular Ca2+induce the dedifferentiation of axonal segments into growth cones. J Neurosci 17:3568-3579.
*< class="emphasis_italic">Correspondence to: Xiao-Ming Xu, M.D., Ph.D., xu26@iupui.edu.
Xiao-Ming Xu, M.D., Ph.D., xu26@iupui.edu.
orcid: 0000-0002-7229-0081 (Xiao-Ming Xu)
10.4103/1673-5374.213532
Accepted: 2017-07-11
- 中國神經(jīng)再生研究(英文版)的其它文章
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- A novel triple immunoenzyme staining enables simultaneous identif i cation of all muscle fi ber types on a single skeletal muscle cryosection from normal, denervated or reinnervated rats
- Optical coherence tomography and T cell gene expression analysis in patients with benign multiple sclerosis
- Impact of Pitx3 gene knockdown on glial cell linederived neurotrophic factor transcriptional activity in dopaminergic neurons
- Dried Rehmannia root protects against glutamateinduced cytotoxity to PC12 cells through energy metabolism-related pathways
- Dexmedetomidine mitigates isof l urane-induced neurodegeneration in fetal rats during the second trimester of pregnancy