Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
Nawaphon Saelim,Khemjira Suksawaeng,Jutamat Chupan, Isariya Techatanawat
The Government Pharmaceutical Organization,Bangkok,Thailand
Biopharmaceutics classifcation system(BCS)-based biowaiver for immediate release solid oral dosage forms of moxifoxacin hydrochloride (Moxifox GPO)manufactured by the Government Pharmaceutical Organization(GPO)
Nawaphon Saelim*,Khemjira Suksawaeng,Jutamat Chupan, Isariya Techatanawat
The Government Pharmaceutical Organization,Bangkok,Thailand
A R T I C L E I N F O
Article history:
Available online 25 November 2015
Moxifoxacin hydrochloride
Biopharmaceutics classifcation system(BCS)
Permeability
Solubility
Biowaiver
Literature and experimental data relevant to the decision to allow a waiver of in vivo bioequivalence(BE)testing for approval of immediate release(IR)solid dosage forms containing moxifoxacin hydrochloride as the API manufactured by the Government Pharmaceutical Organization(GPO)are evaluated.The solubility of moxifoxacin hydrochloride determined by the shake fask method in six different pH mediums(1.2, 4.5,5.4,6.4,6.8 and 7.5)was 4.988±0.1962,27.012±0.4138, 21.668±0.5165, 47.200±0.8095, 73.438±1.7310 and 196.475±4.4624 mg/mL,respectively.The Dose/Solubility(D/ S)Ratio of the highest strength(400 mg)available in the market of moxifoxacin tablets was 80.192,14.808,18.460,8.475,5.447 and 2.036 mL,respectively.Therefore,the moxifoxacin hydrochloride can be classifed as highly soluble drug since highest dose strength of drug can solute in 250 mL or less of aqueous medium over the pH range of 1–7.5[1].Likewise,the absolute bioavailability of moxifoxacin is approximately 90%[2].Hence, moxifoxacin hydrochloride can be assigned to BCS Class I as high solubility and high permeability.Furthermore,moxifoxacin is not narrow therapeutic index drug according to the defnition of narrow therapeutic drugs by United States Code of Federal Regulations[3].Additionally,the excipients used are widely used in conventional formulation,have no unexpected infuence on the bioavailability of the product and thequantity of each excipient is within a maximum potency as per U.S.FDA inactive ingredient search for approved drug products.Moreover,dissolution profles of both the Moxifox GPO 400 mg tablets and the reference product(Avelox?400 mg tablets manufactured by Bayer PharmaAG)are similar as shown in Fig.1.Additionally,both products are considered as very rapidly dissolving since they release at least 85%of content in 15 minutes in three different buffers(pH 1.2,4.5 and 6.8) [1].Moreover,the Moxifox GPO is not designed for absorption in the oral cavity.Finally,risk assessment on biowaiverbased equivalence decision in terms of public health and individual patient risks was outweighed by the potential benefts of the biowaiver approach.Based on this evidence,the IR solid dosage forms containing moxifoxacin hydrochloride manufactured by GPO are eligible for biowaiver of in vivo bioequivalence(BE)testing.
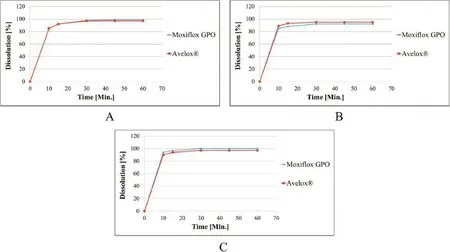
Fig.1–Dissolution profles of the Moxifox GPO 400 mg tablets and the reference product in(A)0.1 N HCl pH 1.2,(B)buffer pH 4.5 and(C)buffer pH 6.8.
R E F E R E N C E S
[1]U.S.Department of Health and Human Services,Food and Drug Administration.Guidance for industry:waiver of in vivo bioavailability and bioequivalence studies for immediaterelease solid dosage forms based on a biopharmaceutics classifcation system.2000.
[2]U.S.Department of Health and Human Services,Food and Drug Administration.Highlights of prescribing information: AVELOX.2013;14.
[3]U.S.Department of Health and Human Services,Food and Drug Administration.Center for Drug Evaluation and Research.Briefng information for the July 26,2011 meeting of the pharmaceutical science and clinical pharmacology advisory committee.2011.
*E-mail address:nawaphon.sae@gpo.or.th.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.016
1818-0876/?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年1期
Asian Journal of Pharmacentical Sciences2016年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- Determination of the antidepressant effect of mirtazapine augmented with caffeine using Swiss-albino mice
- Photosafety testing of dermally-applied chemicals based on photochemical and cassette-dosing pharmacokinetic data
- Bioequivalence study of abacavir/lamivudine (600/300-mg)tablets in healthy Thai volunteers under fasting conditions
- Evaluation of cytotoxic and infammatory properties of clove oil microemulsion in mice
- Analytical method development of pregabalin and related substances in extended release tablets containing polyethylene oxide
- Factors affecting formation of nanoemulsions containing modifed coconut oil and spearmint oil
