Fabrication of Z-Scheme Heterojunction of SiC/Pt/Cds Nanorod for Efficient Photocatalytic H2 Evolution
Dan Cao , Hua An , Xiaoqing Yan , Yuxin Zhao , Guidong Yang ,*, Hui Mei
1 XJTU-Oxford International Joint Laboratory for Catalysis, School of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049, P. R. China.2 Science and Technology on Thermostructural Composite Materials Laboratory, School of Materials Science and Engineering,Northwestern Polytechnical University, Xi’an 710072, P. R. China.
Abstract: In this study, a novel silicon carbide/platinum/cadmium sulfide(SiC/Pt/CdS) Z-scheme heterojunction nanorod is constructed using a simple chemical reduction-assisted hydrothermal method, in which Pt nanoparticles are anchored at the interface of SiC nanorods and CdS nanoparticles to induce an electron-hole pair transfer along the Z-scheme transport path. Multiple characterization techniques are used to analyze the structure, morphology, and properties of these materials. X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) results show that the SiC/Pt/CdS materials with good crystal structure are successfully synthesized. Transmission electron microscopy reveals that Pt nanoparticles grow between the interfaces of SiC nanorods and CdS nanoparticles. UV-Vis diffuse reflectance spectroscopy shows that the as-prepared Zscheme heterojunction samples have a wider light absorption range in comparison with pristine CdS materials.Photoluminescence spectroscopy and the transient photocurrent response further demonstrate that the SiC/Pt/CdS nanorod sample with an optimal molar ratio possesses the highest electron-hole pair separation efficiency. The loading amount of CdS on the surface of SiC/Pt nanorods is effectively adjusted by controlling the molar ratio of SiC and CdS to achieve the optimal performance of the SiC/Pt/CdS nanorod photocatalysts. The optimal H2 evolution capacity is achieved at SiC : CdS = 5 : 1 (molar ratio) and the maximum H2 evolution rate reaches a high value of 122.3 μmol·h-1. In addition,scanning electron microscopy, XRD, and XPS analyses show that the morphology and crystal structure of the SiC/Pt/CdS photocatalyst remain unchanged after three cycles of activity testing, indicating that the SiC/Pt/CdS nanocomposite has a stable structure for H2 evolution under visible light. To prove the Z-scheme transfer mechanism of electron-hole pairs,selective photo-deposition technology is used to simultaneously carry out the photo-reduction deposition of Au nanoparticles and photo-oxidation deposition of Mn3O4 nanoparticles in the photoreaction. The experimental results indicate that during photocatalysis, the electrons in the conduction band of CdS participate mainly in the reduction reaction,and the holes in the valence band of SiC are more likely to undergo the oxidation reaction. The electrons in the conduction band of SiC combine with the holes in the valence band of CdS to form a Z-scheme transport path. Therefore, a possible Z-scheme charge migration path in SiC/Pt/CdS nanorods during photocatalytic H2 production is proposed to explain the enhancement in the activity. This study provides a new strategy for synthesizing a Z-scheme photocatalytic system based on SiC nanorods. Based on the characterization results, it is determined that SiC/Pt/CdS nanocomposites are highly efficient, inexpensive, easy to prepare, and are stable structures for H2 evolution under visible light with outstanding commercial application prospects.
Key Words: SiC; Nanorod; SiC/Pt/CdS; Photocatalyst; Z-scheme heterojunction
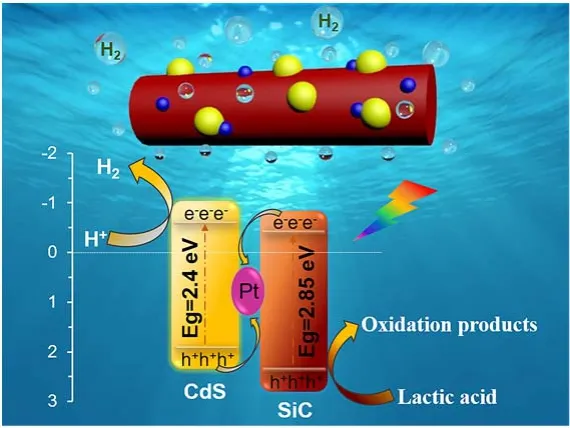
1 Introduction
Hydrogen is considered as one of the promising alternatives for the replacement of fossil fuel to solve the global energy and environmental crisis. To date, various approaches, such as gasification1, electrolysis2, and steam reforming of hydrocarbon fuels3, have been used to generate hydrogen on a large scale.However, for these technologies, hydrogen is generally produced under extreme conditions (high temperature, pressure and energy consumption), stimulating research into the development of sustainable way for hydrogen generation. In recent years, the development of photocatalytic water splitting has attracted widespread interests because this technology as a potential and green path could directly convert water into hydrogen under a driven force of solar energy at room temperature and atmospheric pressure4-6. To drive the photoreaction of water splitting, a well-performance photocatalyst is absolutely necessary, which must efficiently absorb solar light and produce an abundance of charge carriers to take part in the photoreaction of H2production. With this viewpoint, various semiconductors with suitable band structures,such as metal oxides7, sulfides8, nitrides9, and carbides10, have been synthesized. Among these semiconductor materials, SiC with excellent electronic property, high thermal conductivity and good mechanical strength has become a promising catalyst in the photocatalytic H2evolution. More importantly, it has suitable band gap and its bottom of conduction band (CB) is above the reduction potential of H+/H2(0 eV), which satisfy thermodynamic requirements set by the reduction potentials for water splitting11-13. Additionally, it has been reported that SiC semiconductor with different morphology and nanostructure,such as nanoparticle, nanorod, and nanofiber, can endow this materials somewhat special and novel properties in the process of H2production from water splitting13-16. With respect to the community of SiC, the SiC nanorod as a shining star, which has suitable aspect ratios can shorten the migration distance of carriers to achieve efficient migration and separation of electronhole pairs, meanwhile, the structure of nanorod has the advantage of being easier to recycle than the other structures,which can reduce losses as much as possible. Unfortunately, the pure SiC nanorod still exhibits an unsatisfactory photocatalytic activity due to its relatively low optical response region and the acute recombination of charge carriers12.
To overcome those shortcomings, significant efforts have been made to design and construct the SiC based heterostruction photocatalyst materials, which could effectively accelerate the separation of photogenerated charges and enhance the visiblelight-induced photocatalytic activity17-20. In particular, the fabrication of all-solid-state Z-scheme heterojunction system is considered to be a fascinating protocol for obtaining efficient SiC-based nanorod photocatalyst. In the Z-scheme heterojunction system, the electrons and holes can be directly transmitted through the interface contact, which could effectively shorten the transmission distance and reduce the incidence of side reactions, thereby improving the visible light utilization rate and photocatalytic efficiency. For example, the synthesis of ternary SiC/Pt/CdS Z-scheme heterojunction composite is deemed to be a promising approach to greatly enhance the photocatalytic activity of SiC nanorod, in which platinum as electron conductor is situated at the interface between SiC and CdS, and the holes on the valence band of CdS will quench together with the electrons on the conduction band of SiC under the aid of Pt “bridge”, leading to the efficient separation and migration of photogenerated electrons and holes.
According to the above-mentioned motivation, in this work,we for the first time design and construct a novel Z-scheme SiC/Pt/CdS nanorod with the high-speed charge transfer path achieved by platinum as an electron transfer bridge. The loading amount of CdS on the surface of SiC/Pt materials was effectively tuned by controlling the molar ratio of SiC and CdS, which has a significant effect on the recombination efficiency of photogenerated electron-holes and light absorption ability of the as-prepared SiC/Pt/CdS photocatalyst. The obtained SiC/Pt/CdS sample with SiC and CdS molar ratio of 5 : 1 exhibits the strongest separation efficiency of photogenerated electron-hole pairs, and thus leading to the best photocatalytic activity. In addition, a possible hydrogen evolution mechanism and the electrons migration path of the Z-scheme heterojunction system is proposed according to the characterization results.
2 Experimental
2.1 Material synthesis
Silicon carbide (SiC, Changsha Sinet Advanced Materials Co.Ltd. of China, 99%); cadmium chloride (CdCl2, 99.99%),thiourea (CH4N2S, 98%), chloroplatinic acid (H2PtCl6, 99.99%),manganese sulfate (MnSO4, 99%), chloroauric acid(HAuCl4·4H2O, 99.99%) were purchased from Sinopharm Chemical Reagent Co. Ltd. All chemicals were used as received without further purification.
2.1.1 Synthesis of pure CdS
The pure CdS was prepared by a hydrothermal method. 0.1 g of trisodium citrate was well dissolved in 50 mL deionized water solution with magnetically stirring for 0.5 h, and then, 0.171 g of CdCl2was added to above solution with vigorous stirring for 5 h at room temperature. Therefore, 0.171 g of thiourea as precursor of sulfur source was added. Finally, the above mixture solution was transferred to a 100 mL Teflon-lined stainless steel autoclave and heated at 160 °C for 2 h. After cooling down to room temperature, collect the sample by the centrifugation and rinse several times with deionized water and ethanol, and then dry in a drying over at 60 °C for 12 h, resulting in pure CdS.
2.1.2 Synthesis of SiC/Pt
The SiC/Pt was prepared by a chemical reduction method.Typically, 0.03 g of SiC was dispersed in 50 mL of deionized water with ultrasound treatment until uniformly. 0.1 mL(corresponding to 1.5% (w, mass fraction) Pt) of chloroplatinic acid aqueous solution was added in the above dispersion solution with magnetically stirred for 3 h at room temperature. Thereafter,another 1 mL of NaBH4solution (0.053 mol·L-1) was dropwise added into the mixture above and continued agitating for 1 h,centrifuged and washed with deionized water until the pH value reached 7. The precipitate was dried at 60 °C, resulting in SiC/Pt composite.
2.1.3 The synthesis of SiC/Pt/CdS
The SiC/Pt/CdS was prepared by a chemical reduction assisted hydrothermal method. The above SiC/Pt sample was dispersed in 30 mL deionized water. 0.1 g of trisodium citrate was well dissolved in solution above with magnetically stirring for 0.5 h, and then, 0.0343 g of CdCl2was added to the above dispersion solution with vigorous stirring for 5 h at room temperature, in order to ensure that Cd2+adsorbed in large quantities on SiC nanorods. Therefore, 0.0343 g of thiourea as precursor of sulfur source was added. Finally, the above mixture solution was transferred to a 100 mL Teflon-lined stainless steel autoclave and heated at 160 °C for 2 h. After cooling down to room temperature, collect the sample by the centrifugation and rinse several times with deionized water and ethanol, and then dry in a drying over at 60 °C for 12 h, resulting in SiC/Pt/CdS (5 :1) (molar ratio) ternary composite, was denoted as SPC-5.
Other molar ratios of 3 : 1, 7 : 1 ternary composites were fabricated by adjusting the amount of CdCl2and thiourea, were denoted as SPC-3, SPC-7, respectively.
2.1.4 Synthesis of SiC/CdS and CdS/Pt
SiC/CdS (molar ratio 5 : 1) was prepared by a similar hydrothermal method but without H2PtCl6, was denoted as SC.CdS/Pt was prepared by a chemical reduction method.
2.2 Material characterization
The crystal structure of products was carried out using a powder X-ray diffraction (XRD) (SHIMADZU, Lab X XRD-6100). The microstructures and morphologies of the obtained samples were observed with a field-emission scanning electron microscope (FESEM) (JEOL, JSM-6700F). The geometry and surface states of samples were further investigated by a transmission electron microscopy (TEM) (JEOL, JEM-2100).The elementary composition was analyzed by the affiliated energy dispersive X-ray spectroscopy (EDX) instrument belongs to the above SEM. Ultraviolet-visible (UV-Vis) diffuse reflectance spectra were obtained using a UV-Vis spectrophotometer (UV-2600, SHIMADZU). The photoluminescence mission spectra were tested using a fluorescence spectrophotometer (HORIBA, JY Fluorolog-3)with 450 nm excitation wavelength. Additionally, the valence band (EVB) and conduction band (ECB) edge potentials of samples can be determined by the following two formulas:


whereEerepresents the free electron energy on the hydrogen scale (4.5 eV), andXrepresents the absolute electronegativity of semiconductor materials. TheXvalues for CdS and SiC are 5.19 and 5.47 eV, respectively18,21.
2.3 Photocatalytic H2 evolution
The photocatalytic H2evolution reaction was carried out in a Pyrexreaction cell. Typically, 0.005 g of sample was dispersed in 100 mL aqueous solutions containing 10 mL lactic acid as sacrificial agent to capture holes. The suspension was evacuated with N2for 3 min to remove the O2in the solution and then irradiated with a Xe lamp (300 W) having an optical cut-off filter(λ> 420 nm) to erase ultraviolet light. The amount of H2production was analyzed by an online gas chromatography (GC).The photocatalytic activity of the different samples was compared by calculating the average of the hydrogen production rates for the first 3 h.
A recycling experiment of photocatalytic H2evolution reaction was carried out under the same conditions to assess the stability of the prepared photocatalyst in long-term operation.After every 3 h photocatalytic H2evolution reaction, the suspensions were centrifuged and washed several times with ethanol and deionized water. And then, the collected photocatalysts was redispersed into the aqueous solution containing 10 mL lactic acid and 90 mL deionized water.Afterwards, the renewed Pyrex reaction cell system was exhausted by pure N2flow to remove residual air before the next cyclic experiment.
The apparent quantum efficiency (AQE) of photocatalytic H2production was measured under the similar conditions of photocatalytic H2evolution reaction. A Xe lamp (300 W)equipped with a band-pass filter at different light wavelengths was used as a monochromatic light source. The light density of incident light was detected by using an irradiatometer (FZ-A,Beijing Normal University Optical Instrument). The illuminated area on the Pyrex reaction cell was about 12.56 cm2. The amount of H2evolution was investigated after irradiated for 2 h.Afterwards, the AQE for H2evolution was calculated according to the following equation:

2.4 Photoelectrochemical measurements
The electrochemical measurements were implemented on a CHI760D (Shanghai Chenhua Ltd.) electrochemical work station in a three-electrode system with a platinum wire counter electrode and an Ag/AgCl (saturated KCl) reference electrode,using a concentration of 1 mol·L-1KNO3aqueous electrolyte under ambient temperature (25 °C). The suspension was prepared by mixing 2 mL of acetone and 50μL of naphthol as well as 3 mg of the as-prepared photocatalyst. The homogeneous slurry was deposited onto the FTO glass (effective area: 0.64 cm2) and dried at room temperature overnight as the working electrodes. The transient photocurrent response was performed on the electrochemical analyzer using 0.3 V bias voltage under visible light (λ> 420 nm) irradiation.
3 Results and discussion
3.1 Schematic diagram for synthesis mechanism of SiC/Pt/CdS heterojunction photocatalyst
The general synthetic route for SiC/Pt/CdS is described in Scheme 1. First of all, the Pt nanoparticles can be easily loaded on the SiC nanorods by chemical reduction method. Typically,chloroplatinic acid aqueous solution was added into the aqueous suspension containing SiC to enable Pt4+to adsorb on the surface of SiC nanorods, thereafter, NaBH4solution was used to reduce Pt4+to form Pt nanoparticles on the surface of SiC nanorods.Further, the trisodium citrate could capture metal ions and enable a large amount of Cd2+to adsorb on the surface of the nanorods.And the thiourea was then added to slowly release sulfur ions,which can combine with Cd2+by hydrothermal condition under 160 °C. Finally, the CdS nanoparticles were successfully grown on the nanorods. So that, the Pt nanoparticles were located at the interface between CdS nanoparticles and SiC nanorods as the section image shown, obtaining SiC/Pt/CdS ternary composite samples with different molar ratios. In addition, SiC/CdS binary composite samples were also synthesized in the same process for comparison.
3.2 Crystal structure analysis of samples
The crystal structure of pure CdS, binary SC nanomaterial and ternary SPC-3, SPC-5, SPC-7 nanocomposites were performed using X-ray diffraction (XRD) patterns. As shown in Fig. 1, the XRD pattern of pure CdS displays distinct diffraction peaks at 2θof 24.8°, 26.5°, 28.2°, 36.6°, 43.7°, 47.8°, 51.8°,corresponding to the (100), (002), (101), (102), (110), (103) and(112) crystal planes of hexagonal phase CdS (JCPDS No.41-1049), respectively. Meanwhile, pure SiC displays distinct diffraction peaks at 2θof 34.2°, 35.7°, 41.5°, 60.2°, 71.9°,corresponding to the (101), (102), (104), (110) and (116) crystal planes of hexagonal phase SiC (JCPDS No.29-1131),respectively. These indicate that both SC and SPC series photocatalysts contain hexagonal phase CdS and hexagonal phase SiC with good crystallinity. However, no obvious characteristic diffraction peaks for the metal Pt nanoparticles are observed, which can be attributed to the small Pt particle size and the little content, resulting in a low diffraction intensity. The XRD results indicate that the binary SC and ternary SPC photocatalysts have been successfully prepared by a simple chemical reduction assisted hydrothermal method.
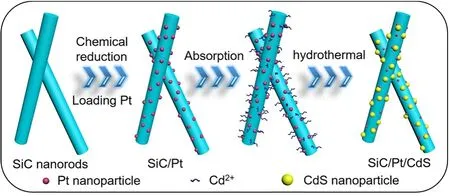
Scheme 1 Illustration of the synthesis of SiC/Pt/CdS composites by the chemical reduction assisted hydrothermal method.
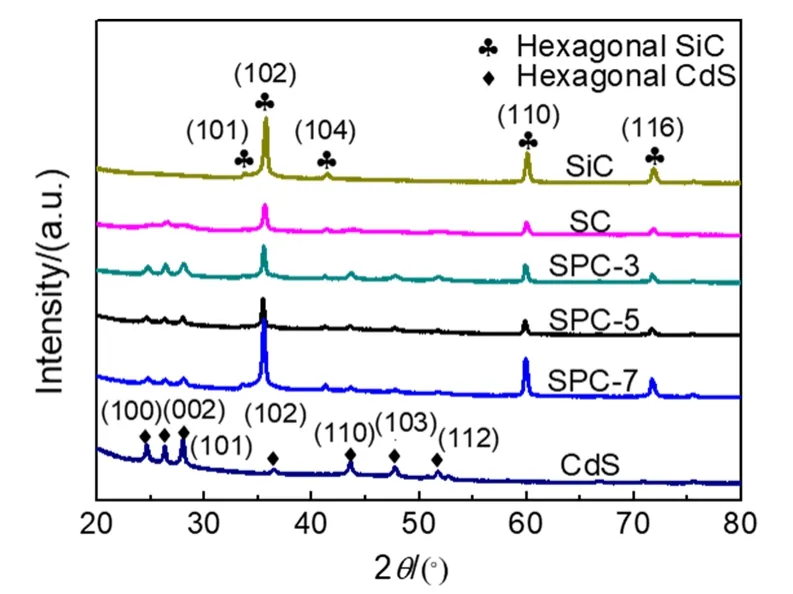
Fig. 1 XRD patterns of SiC, pure CdS, SC, SPC-3, SPC-5 and SPC-7.
3.3 Morphology and structure analysis of the samples
The microstructure and morphology of the obtained samples were carefully investigated by scanning electron microscopy(SEM). A general view of the as-prepared samples was shown in the Fig. 2a-f. As can be seen from Fig. 2a, the pure SiC has a rod-like structure with a diameter ranging from 100 nm to 500 nm, and the pure CdS is granule structures with the particle size in the range of 100-700 nm (Fig. 2b). Fig. 2c is the SEM image of SC sample, which can clearly see that the CdS particles are more evenly distributed on the SiC nanorods.
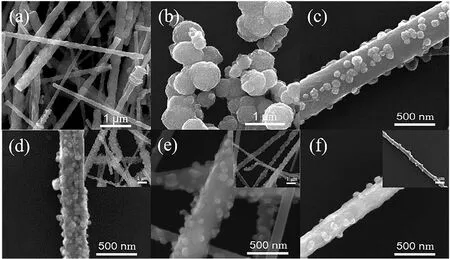
Fig. 2 SEM images of (a) pure SiC, (b) pure CdS, (c) SC,(d) SPC-3, (e) SPC-5, (f) SPC-7.
The SiC/Pt/CdS was prepared by a simple chemical reduction-assisted hydrothermal method, and its hydrogen evolution activity is closely related to the loading amount of CdS on the SiC surface. When the molar ratio of SiC to CdS is 3 : 1,as shown in Fig. 2d, a large number of CdS nanoparticles are tightly coated on the surface of the SiC, to a certain extent, that affect the light absorption capacity of SiC, and it is not conducive to the redox reaction. Nevertheless, when the molar ratio of SiC to CdS is 5 : 1, as shown in Fig. 2e, the moderate CdS nanoparticles uniformly loaded on the surface of the SiC nanorods to form a ternary heterojunction structure together with the Pt between the SiC and CdS interfaces. This structure not only in favor of the light absorption, but also facilitates the separation and migration of photogenerated electrons and holes,and effectively enhances photocatalytic activity. When the molar ratio of SiC to CdS is 7 : 1, it can be seen from Fig. 2f, several CdS nanoparticles are scattered on the surface of the SiC nanorods. At this point, a large number of SiC nanorods surface arebare, which severely weakens the role of the heterojunction system. On the one hand, the amount of charge carriers can be significantly decreased, on the other hand, their transfer path would be prolonged, all of which cause an easy recombination of electrons and holes, as a result of reducing the hydrogen evolution efficiency.
To further demonstrate the detailed nanostructure of the SiC/Pt/CdS heterojunction, the typical TEM and high-resolution TEM (HRTEM) were performed. In Fig. 3a, b, the SiC/Pt sample reveals that lots of tiny Pt is uniformly loaded on SiC nanorod,the ordered interplanar space of 0.235 nm corresponds to (102)crystal plane of SiC and the lattice space of 0.226 nm corresponds to (111) crystal plane of Pt, indicating that Pt has been effectively loaded on the SiC nanorods by a chemical reduction method. In Fig. 3c, the CdS nanoparticles with uniform particle size are uniformly dispersed on the SiC nanorods, in accordance with the data of SEM images. Fig. 3d shows the HRTEM image of SPC-5 sample. The clear lattice spaces of 0.359 nm and 0.235 nm can be matched to (100) plane of CdS and (102) plane of SiC, respectively. More importantly,the (111) plane of platinum with lattice space of 0.226 nm can be explicitly observed at the interface between CdS nanoparticle and SiC nanorod, which are in good accordance with the XRD data, indicating that the Z-scheme SiC/Pt/CdS heterojunction composites are successfully constructed.
The chemical composition of the constructed SiC/Pt/CdS nanocomposite was detected by the elemental mapping spectra.As shown in Fig. 4, the elemental mapping images unambiguously show that an individual rod-like nanostructure was predominantly composed of Si and C. And the Cd and S elements can be clearly observed, which present a granule nanostructure on the surface of SiC nanorod. However, due to the low content of Pt loading in ternary composites, it is not well shown in the elemental mapping spectra. As a supplement, the energy dispersive X-ray analysis in Fig. 4f clearly shows the presence of a small amount of Pt, which is in good accordance with the HRTEM image.
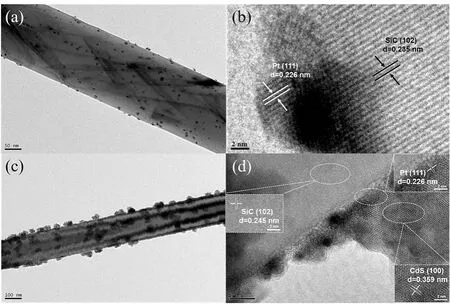
Fig. 3 (a) TEM images of SiC/Pt, (b) the high-resolution TEM image of SiC/Pt, (c) the TEM image of SPC-5,(d) the high-resolution TEM image of SPC-5.
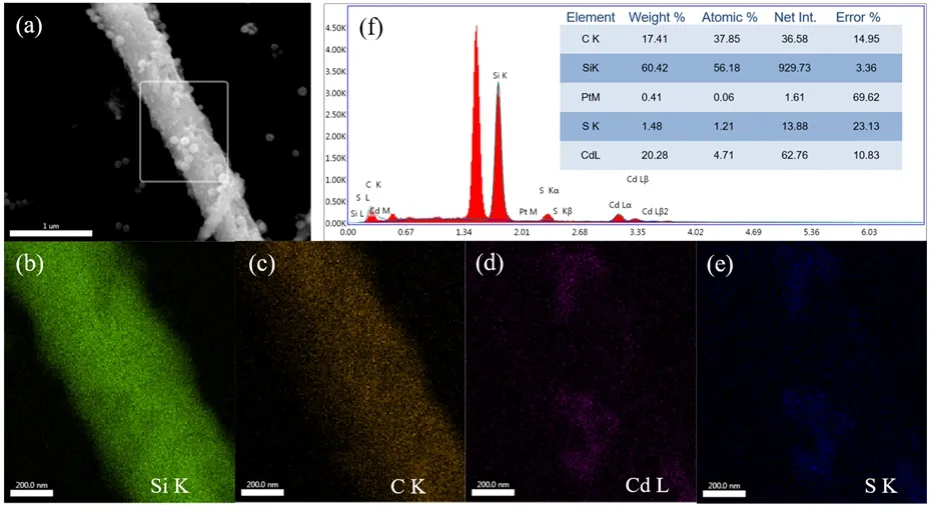
Fig. 4 SEM image of (a) SPC-5 nanocomposite, the corresponding EDX elemental mapping images of Si (b), C (c), Cd (d) and S (e) in the selected area of SEM image (a) and energy-dispersive X-ray pattern (f).
The surface chemical compositions and elemental valence state in the as-prepared samples were further examined by X-ray photoelectron spectroscopy (XPS). Fig. 5a shows the XPS survey spectra of the SPC5 samples, indicating the existence of C, Si, O, Pt, S and Cd in the samples. Herein, O 1ssignal was also detected in the products and possibly originate from oxygen containing species adsorbed or slight oxidation of Si species on sample surface. The XPS spectrum of C 1s(Fig. 5b) shows four binding energy peaks at 282.01 eV, 284.5 eV, 286.3eV and 288.17 eV, attributable to the functional groups of C―Si, C―C,C―O and O―C=O, respectively. The C―O and O―C=O bonds can be referred to the mixture of oxidized forms of carbon,i.e., other carbon-oxygen species11,22. An XPS signal of Si 2pobserved at a binding energy around 100.43 eV can be assigned to the Si―C bond in SiC (Fig. 5c). The peak at 101.7 eV can be assigned to SiOx-Cy, which resulted from the surface adsorption O2of the SiC sample23,24. The peaks at binding energy values of 71.17 eV and 74.37 eV for Pt 4f7/2and Pt 4f5/2were observed in the Pt 4fspectra spectrum of Fig. 5d, corresponding to metallic Pt25. The corresponding high-resolution S 2p(Fig. 5e) can be well fitted into two peaks at 161.2 and 162.36 eV, corresponding to S 2p3/2and S 2p1/2in the form of S2-, respectively. The high resolution spectrum of Cd 3d(Fig. 5f) displays two peaks at 404.49 and 411.31 eV, which are assigned to the characteristic binding energies of Cd2+3d5/2and Cd2+3d3/2in CdS, respectively26.The XPS analysis together with the characterization results of SEM, TEM, and XRD corroborate the successful synthesis of SiC/Pt/CdS.
3.4 Visible light catalytic performance test of the samples
The photocatalytic activities of the as-synthesized photocatalysts were thoroughly investigated by photocatalytic hydrogen production under visible-light illumination (λ≥ 420 nm) using 10% (volume fraction) aqueous solution of lactic acid as scavenger. As depicted in Fig. 6a, b, under visible light irradiation for 3 h, pure CdS nanoparticles exhibit the extremely lowest H2evolution performance with 0.323 μmol. In contrast,the binary SiC/CdS composite material (SC) was slightly increased, but it was only 12.52 μmol. However, after loading Pt with a mass fraction of 1.5% (w) on pure CdS nanoparticles(CdS/Pt), its hydrogen evolution activity is significantly improved, reaching 159.5 μmol. The above experimental results show that the addition of Pt can promote the migration and separation of photogenerated charges to significantly improve the photocatalytic activity. When the molar ratio of SiC to CdS is 5 : 1, the hydrogen evolution activity of SPC-5 is as high as 367.0 μmol after 3 h visible-light irradiation, which is 1153,29.7, and 2.3 times of pure CdS, binary SiC/CdS, and CdS/Pt nanocomposites, respectively, indicative of the important role of the “bridge” of Pt between the SiC and CdS interfaces. This phenomenon is due to the fact that the photogenerated electrons on the conduction band of SiC are transferred to the metal particles Pt and simultaneously combined with the holes on the valence band of CdS, so that the number of electrons participating in the reduction reaction on the conduction band of CdS is increased. In addition, when the molar ratio of SiC to CdS was reduced to 3 : 1 or further increased to 7 : 1, the photocatalytic hydrogen evolution activity of SiC/Pt/CdS composites is significantly changed, and the corresponding amounts of H2evolution are 277.7 and 234.3 μmol, respectively.

Fig. 5 (a) XPS survey spectrum and (b-f) high-resolution XPS spectra of C (b), Si (c), Pt (d), S (e) and Cd (f) for SPC5.
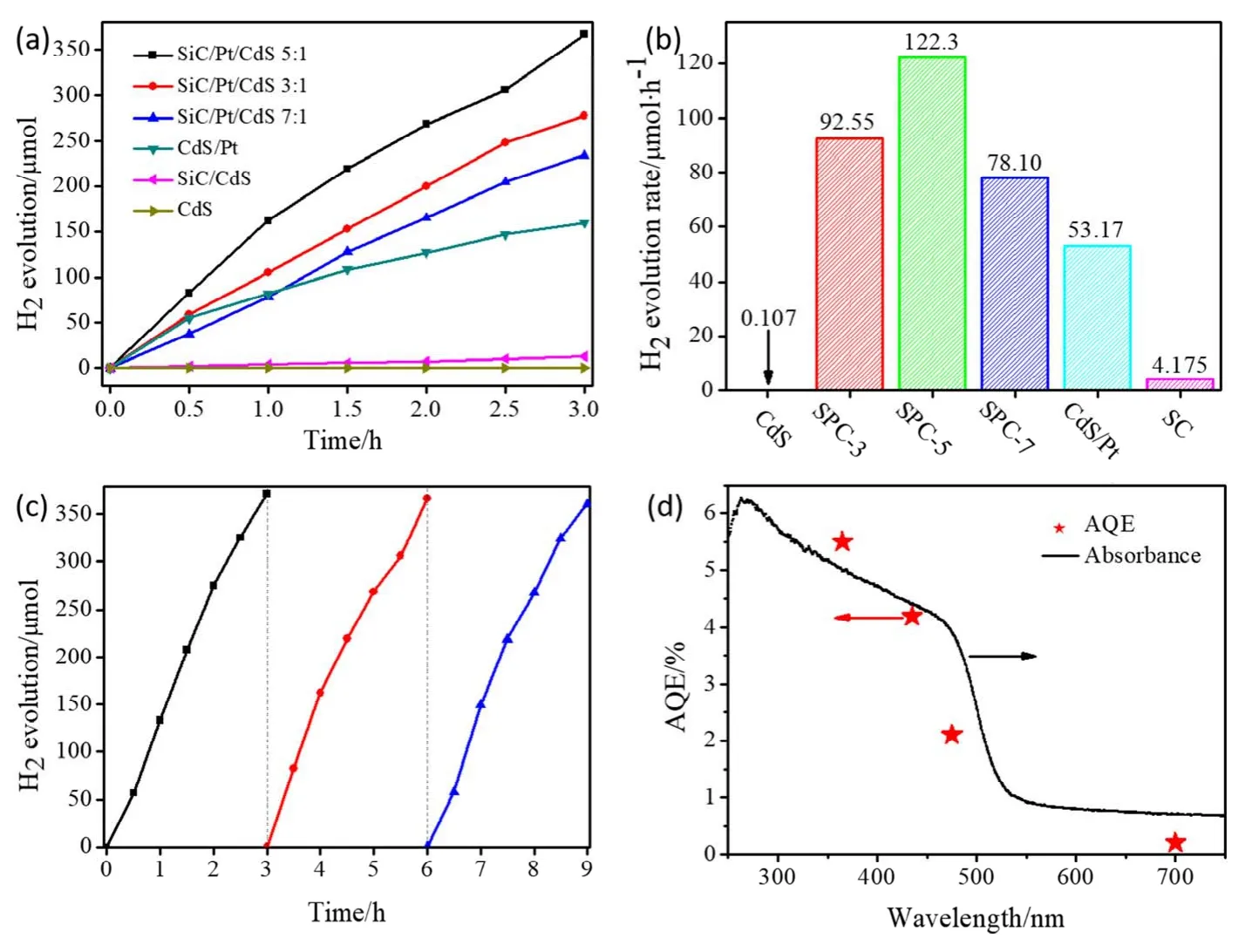
Fig. 6 (a) Plots of photocatalytic H2 evolution amount vs irradiation time and (b) comparison of H2 generation rates over different photocatalysts; (c) recycling test of photocatalytic H2 production over SPC-5 nanocomposite under visible-light irradiation;(d) wavelength dependence of the apparent quantum efficiencies for SPC-5 nanocomposite, the loading catalyst amount is 0.005 g.
In addition, the SPC-5 sample not only exhibits an excellent photocatalytic hydrogen evolution activity, but also has good photocatalytic stability. As shown in Fig.6c, after 9 h of Xenon lamp irradiation, the photocatalytic H2evolution activity of SPC-5 nanocomposite photocatalyst did not show significant attenuation and still remained at 119 μmoL·h-1. Fig. S1 and Fig.S2 (in Supporting Information) show the analysis of XRD, SEM,EDX and XPS spectra for SPC-5 sample after recycling photoreaction. It can be seen that the morphology and crystal structure of the SiC/Pt/CdS photocatalyst remained unchanged after three cycles of activity testing, indicating that the SiC/Pt/CdS nanocomposites are high-efficiency, inexpensive,easy to prepare and stable structure for H2evolution under visible light, which have outstanding commercial application prospects.
We further measured the apparent quantum efficiencies(AQEs) of the SPC-5 nanocomposite at different incident light wavelengths by using various band-pass filters. As can be seen clearly from Fig. 6d, the trends in the wavelength dependence of the AQEs which closely followed those of the absorbance measured by UV-Vis diffuse reflectance spectra (DRS). In addition, the AQEs decreases markedly with an increase in wavelength, the AQEs of SPC-5 are 5.5% and 4.2% at 365 nm and 425 nm, respectively.
3.5 Optical and photoelectric performance testing of the samples
In order to further understand the origin of prominent photoactivity for the SiC/Pt/CdS nanorods, the optical absorption properties were tested by UV-Vis DRS measurement.As can be seen from Fig. 7a, besides the SiC sample, the other samples exhibit significant light absorption in both the ultraviolet and visible-light regions. It is clear that the maximum absorption edge of pure CdS is 516 nm. Compared with pure CdS nanoparticles, the absorption edges of the SiC/Pt/CdS nanorods have a conspicuous red-shift. The maximum absorption wavelengths of SPC-3, SPC-5 and SPC-7 nanocomposites are 538 nm, 559 nm and 618 nm, respectively,suggesting that the constructed Z-scheme heterojunction nanorods broaden the light absorption range, thereby enhancing the photocatalytic hydrogen evolution activity. In Fig. 7b, the(αhν)2is plotted against(hν) to obtain a band gap of 2.4 eV for CdS. In contrast, pure SiC has the weakest absorption intensity,and the pure SiC shows a band gap of 2.85 eV12,24,27. The results of light absorption show that the nanocomposite with the optimum molar ratio of SiC and CdS not only shortens the migration distance of photoexcited carriers, but also reduces the"shielding effect" of the catalytic system on incident light, thus,it contributes to the absorption of incident light, and promotes the generation of more photoexcited electrons and holes in the light absorption center, and finally improves the photocatalytic hydrogen evolution activity.
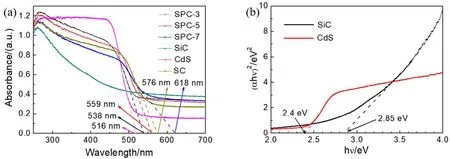
Fig. 7 (a) UV-Vis DRS spectra of all samples, (b) the plot of (αhν)2 versus photon energy (hν) with SiC and CdS.
The separation efficiency of photogenerated electrons and holes in SiC/Pt/CdS Z-scheme heterojunction nanorods was analyzed by photoluminescence spectra (PL). As shown in Fig.8a, SPC-3 and SPC-7 show much strong PL spectral peak intensity due to a relatively high recombination efficiency of photogenerated electron-hole pairs. Obviously, the SPC-5 sample has the lowest PL intensity among all the catalysts,indicating the fantastic charge separation ability over the Zscheme photocatalysis system. Because the SiC/Pt/CdS nanorods with an exceptionally large aspect ratio not only shorten the charge carrier transfer distance, but also provide directional transfer nanochannels for charges, as a result of making the SiC/Pt/CdS nanorods have the prolonged lifetime of photogenerated charges.
In addition to the prolonged lifetime, the charge transfer efficiency is also important for the increase of photocatalytic activity. The transient photocurrent responses of various samples are shown in Fig. 8b. Pure SiC has almost no photocurrent response under visible light illumination. Although the light absorption intensity of pure CdS in the wavelength range of below 500 nm is higher than that of binary and ternary heterojunction composites, the photocurrent intensity of pure CdS is significantly decreased. As expected, the SPC-5 show the highest photocurrent density response, in highly consistent with the PL spectra, which mean that the SPC-5 composite has the excellent capacity of charge transfer.
3.6 Photocatalytic mechanism
In order to clearly illustrate the migration path of photogenerated charges in the SiC/Pt/CdS nanorods during photocatalytic hydrogen production, we used the selective photo-deposition technology to simultaneously carry out the photo-reduction deposition of Au nanoparticles and photooxidation deposition of Mn3O4nanoparticles in the photoreaction28,29. It can be distinctly seen from Fig. 9a, b that numerous nanoparticles are successfully loaded on the surface of SiC/Pt/CdS nanorods after photodeposition testing. And their detailed structure information can be further given by HRTEM measurement. Fig. 9c shows the HRTEM image of photodeposited Au nanoparticles, wherein the lattice fringe of 0.221 nm corresponds to the (111) crystal plane of Au, and the lattice fringe of 0.250 nm corresponds to the (102) crystal plane of the hexagonal phase CdS, implying that Au ions can be completely photo-reduced by the electrons generated from the CdS.Similarly, as shown in Fig. 9d, a number of Mn3O4nanoparticles can be observed and which compactly attached on the SiC nanorods, this is due to the fact that Mn ions are photo-oxidated by holes produced from the positively charged surface of SiC nanorods. And the above selective photo-deposited process is further proved by the EDX spectrum and the XRD pattern. As shown in Figs. S3 and S4, after simultaneously photo-depositing Au and Mn3O4on the SPC-5 sample, the obvious elemental signals of Mn, O and Au can be detected. Therefore, from the af orementioned analysis, we can deduce that, in the SiC/Pt/CdS nanorods, the electrons on the conduction band of CdS are mainly participated in the reduction reaction during photocatalysis, and the holes on the valence band of SiC are more likely to undergo oxidation reaction.
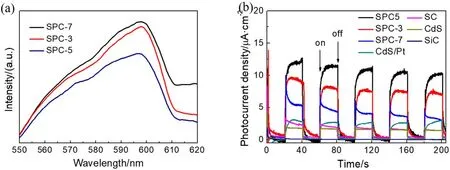
Fig. 8 (a) Photoluminescence (PL) spectra of SPC-3, SPC-5 and SPC-7, (b) transient photocurrent responses versus time.

Fig. 9 (a, b) TEM and (c, d) HRTEM images of SPC-5 nanocomposite with simultaneously photodeposited Mn3O4 and Au nanoparticles.
Based on the above characterization and experimental results,the tentative mechanism for photocatalytic hydrogen production over the ternary SiC/Pt/CdS nanorod photocatalyst was proposed, and the possible schematic diagram is shown in Scheme 2. From UV-Vis DRS spectroscopy, we have a clear cognition of the energy band structure for SiC and CdS, and their conduction band potential (ECB) and valence band potential(EVB) can be estimated according to the empirical equation under the standard hydrogen electrode30-32. Therefore, it can be seen from Scheme 2 that the CB potential (-0.51 eV) of CdS is more negative than the CB potential (-0.455 eV) of SiC, while the VB potential (+2.395 eV) of SiC is more positive than the VB potential (+1.89 eV) of CdS. In addition, since the Pt nanoparticles are situated at the interface between SiC nanorods and CdS nanoparticles, a tightly coupled Z-scheme heterojunction structure can be formed, which may provide a driving force for the charges transfer and greatly shorten their migration path.
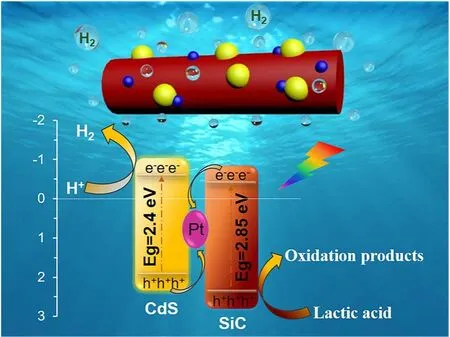
Scheme 2 The mechanism of electron/holes transfer and separation of the SPC-5 composite under visible light irradiation (λ > 420 nm).
As shown in Scheme 2, both SiC and CdS are excited firstly to generate numerous electrons and holes under visible light illumination, and then the electrons are rapidly migrated from the VB to the CB of SiC and CdS, respectively, while the corresponding holes are left on the VB of the two catalysts. Due to the formation of Z-scheme heterostrutured system, the photogenerated electrons on the CB of SiC can inject into the metal Pt through the schottky barrier along the interface of SiC and CdS. In the meanwhile, the photogenerated holes on the VB of CdS would be transferred to metal Pt to recombine with the electrons from SiC nanorod. Benefiting to the Z-scheme mechanism of charge transfer, much more electrons on the CB of CdS can effectively participate in the hydrogen production reaction rather than recombination. At the same time, the holes retained on the VB of SiC are quenched by the sacrificial lactic acid in the solution. As a result, such a Z-scheme charge transport path significantly promotes the separation and migration of photogenerated electrons and holes, thereby greatly improving the photocatalytic water splitting performance33-38.
4 Conclusions
In this work, a novel SiC/Pt/CdS Z-scheme heterojunction nanorod photocatalyst was prepared by a simple chemical reduction-assisted hydrothermal method. The construction of tightly connected interfacial Z-scheme heterojunction not only can supply directional migration nanochannel to promote the spatial separation of photogenerated electron-hole pairs, but also improve the light trapping efficiency. When the molar ratio of SiC to CdS is 5 : 1, the prepared SiC/Pt/CdS Z-scheme heterojunction photocatalyst exhibits excellent visible light photocatalytic activity which as high as 367.0 μmol after visible light irradiation for 3 h. According to the comprehensive analysis of photoluminescence spectroscopy and photoelectrochemical test, the possible Z-scheme mechanism of charge transfer in SiC/Pt/CdS heterojunction was proposed. This strategy would provide new impetus to design SiC-based high-performance nanorod photocatalyst in hydrogen evolution from water splitting.
Acknowledgement:We thank the technical help from International Center for Dielectric Research (ICDR), Xi’an Jiaotong University, Xi’an, China. And also thank Ms. Dai and Mr. Ma for their help in using SEM and EDX and TEM,respectively.Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
- 物理化學(xué)學(xué)報的其它文章
- 魅力光催化劑
- Rod-Shaped Metal Organic Framework Structured PCN-222(Cu)/TiO2 Composites for Efficient Photocatalytic CO2 Reduction
- Cu2+ Modified g-C3N4 Photocatalysts for Visible Light Photocatalytic Properties
- Controlling Self-Assembly of 3D In2O3 Nanostructures for Boosting Photocatalytic Hydrogen Production
- 硫化鎘反蛋白石光子晶體制備及光解水制氫
- 光催化甲烷轉(zhuǎn)化研究進(jìn)展

