Neuroprotective role of (Val8)GLP-1-Glu-PAL in an in vitro model of Parkinson’s disease
Lin Li, Ke Liu, Juan Zhao, Christian Holscher, Guang-lai Li Yue-ze Liu
1 Key Laboratory of Cellular Physiology, Shanxi Medical University, Taiyuan, Shanxi Province, China
2 Second Hospital, Shanxi Medical University, Taiyuan, Shanxi Province, China
3 Biomedical and Life Sciences, Lancaster University, Lancaster, UK
RESEARCH
Neuroprotective role of (Val8)GLP-1-Glu-PAL in an in vitro model of Parkinson’s disease
Lin Li1, Ke Liu1, Juan Zhao1, Christian Holscher2,3,#, Guang-lai Li2, Yue-ze Liu2,#
1 Key Laboratory of Cellular Physiology, Shanxi Medical University, Taiyuan, Shanxi Province, China
2 Second Hospital, Shanxi Medical University, Taiyuan, Shanxi Province, China
3 Biomedical and Life Sciences, Lancaster University, Lancaster, UK
Graphical Abstract
orcid: 0000-0001-6639-8216 (Yue-ze Liu)
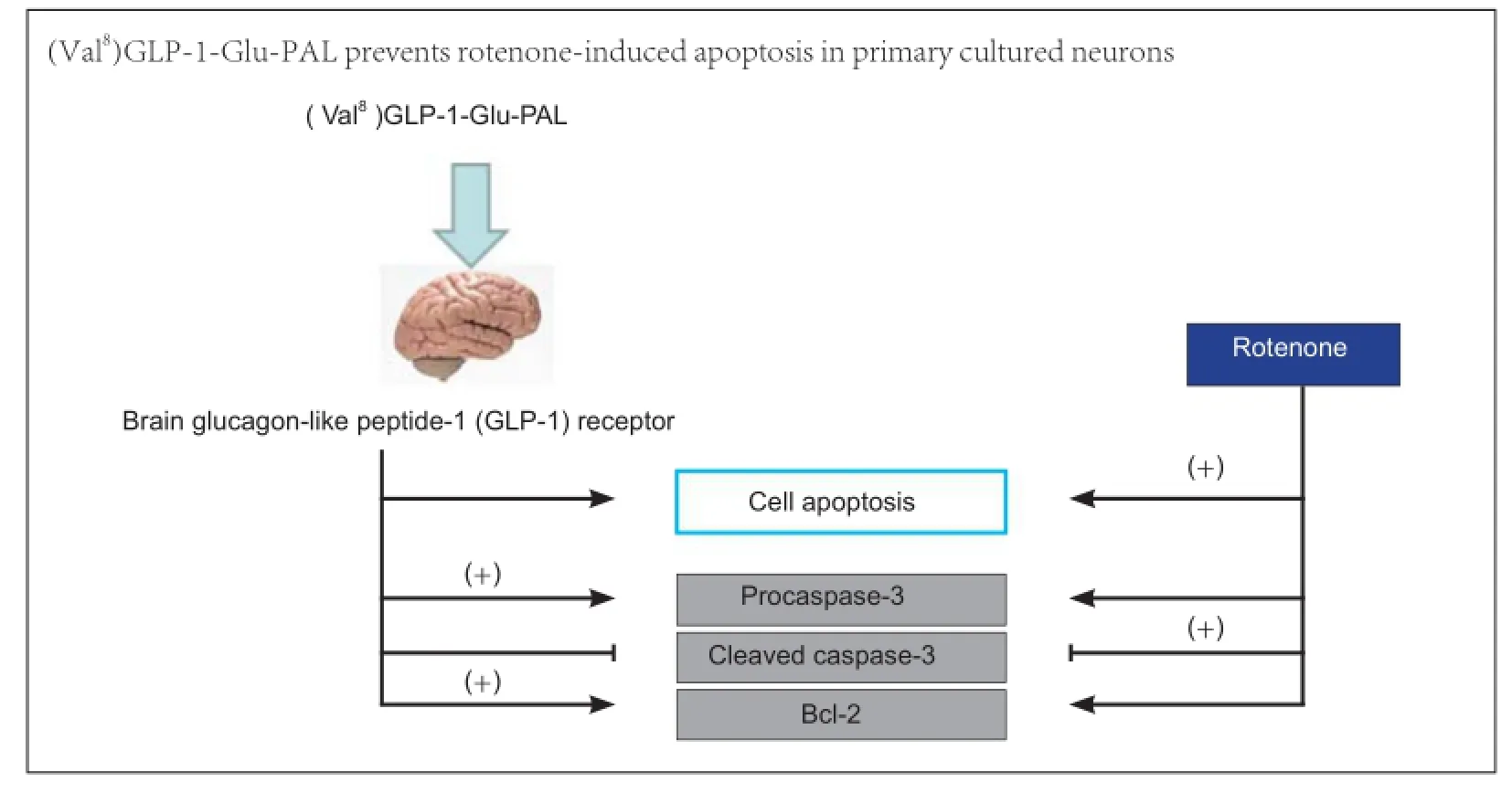
The growth factor glucagon-like peptide-1 (GLP-1) is neuroprotective in several animal models of neurodegeneration. Here, we analyzed the neuroprotective effects of a novel protease-resistant GLP-1 analogue, (Val8)GLP-1-Glu-PAL, which has advantages over older analogues, such as improvement of hippocampal neurogenesis, glucose homeostasis, and insulin secretion. We established an in vitro model of Parkinson’s disease using the mitochondrial stressor rotenone in primary cultured mouse neurons pretreated with (Val8)GLP-1-Glu-PAL. (Val8) GLP-1-Glu-PAL alone did not affect neuronal viability, but prevented the rotenone-induced reduction in cell viability in a dose-dependent manner. In addition, (Val8)GLP-1-Glu-PAL pretreatment prevented rotenone-induced proapoptotic changes manifesting as downregulation of procaspase-3 and Bcl-2 and upregulation of cleaved caspase-3. These results demonstrate that the novel agent (Val8)GLP-1-Glu-PAL shows promise as a drug treatment for Parkinson’s disease.
nerve regeneration; Parkinson’s disease; GLP-1; neurodegenerative disease; apoptosis; caspase-3; Bcl-2; cellular culture; rotenone; neural regeneration
Introduction
Parkinson’s disease is the second most common adult-onset neurodegenerative disorder. To date, no agent has been found that successfully prevents or reverses neurodegenerative processes in the brain (Bagetta et al., 2010). Mitochondrial toxins, such as the complex 1 inhibitor rotenone, are widely used as pesticides, and have been found to induce Parkinson’s disease in farmers who are exposed to the toxin (Chin-Chan et al., 2015). In rodents, administration of rotenone can lead to biochemical and histological changes similar to those observed in Parkinson’s disease (Fleming et al., 2004; Zhu et al., 2004).
The growth factor glucagon-like peptide-1 (GLP-1) is a member of the incretin hormone family (Perry and Greig, 2002; H?lscher, 2014a), and the GLP-1 receptor is expressed in neurons in the central nervous system (Hamilton and H?lscher, 2009; Lee et al., 2011; Darsalia et al., 2012). Agonists at the GLP-1 receptor were originally developed as a treatment for type 2 diabetes, and several remain on the market (Camp-bell and Drucker, 2013). GLP-1 and long-acting, protease-resistant GLP-1 receptor agonists have shown a range of neuroprotective effects in cell culture (Perry and Greig, 2002; Sharma et al., 2014) and in animal models of Parkinson’s (Bertilsson et al., 2008; Li et al., 2009) and Alzheimer’s diseases (McClean et al., 2011). In cellular and functional studies in rodents, the GLP-1 analogue exendin-4 protects against Parkinson’s disease-like pathologic changes such as 6-hydroxydopamine-induced dopaminergic neuronal loss (Bertilsson et al., 2008). This was confirmed in another model of Parkinson’s disease in which 6-hydroxydopamine and lipopolysaccharide were used to lesion the substantia nigra (Harkavyi et al., 2008). Exendin-4 also protects dopaminergic neurons and rescues motor function in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease (Li et al., 2009). Based on these encouraging preclinical studies, a pilot clinical trial of exendin-4 was conducted in patients with Parkinson’s disease (Aviles-Olmos et al., 2013). The study examined the effects of exendin-4 in a randomized single-blind trial in 45 patients. The drug was given for 12 months followed by a 2-month wash-out period. Clinically relevant improvements in motor and cognitive measures were observed. At 12 months, patients who had received exendin-4 showed a mean improvement of 2.7 points on the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), compared with a mean decline of 2.2 points in control patients taking conventional Parkinson’s disease medication. Most interestingly, patients taking exendin-4 showed a clear improvement in the Mattis DRS-2 cognitive score, suggesting that the drug has beneficial effects on cognition and memory (Aviles-Olmos et al., 2013). A follow-up study showed that the protection of motor skills and cognitive scores remained at 12 months after cessation of exendin-4 treatment (Aviles-Olmos et al., 2014). These results support the hypothesis that GLP-1 receptor agonists may also be effective in the treatment of Alzheimer’s disease (H?lscher, 2014b).
Type 2 diabetes is a risk factor for Alzheimer’s and Parkinson’s diseases. Insulin signaling is impaired in the brains of patients with these diseases, and recent studies have shown that the pharmacologic agents used to treat diabetes also improve symptoms in Alzheimer’s and Parkinson’s diseases. In particular, three licensed GLP-1 mimetics are very effective in crossing the blood-brain barrier, and show good effects in animal models of Alzheimer’s and Parkinson’s diseases (H?lscher, 2014c). GLP-1 itself has a relatively short circulating half-life, making it impractical as a therapeutic agent (Kieffer et al., 1995). Therefore, the focus of ongoing research is the development of new GLP-1 analogues with increased enzymatic stability and improved biological efficacy (Vilsb?ll and Knop, 2008). (Val8)GLP-1 is a human GLP-1 analogue developed by substitution of Ala with a Val residue at N-terminal position 8. This renders the peptide resistant to degradation by dipeptidyl peptidase-4 by masking its cleavage site (Green et al., 2006), but it is still subject to rapid renal clearance. Renal filtration can be minimized by the incorporation of a fatty acid moiety into a peptide chain, thereby facilitating binding to serum proteins such as albumin and thus prolonging the duration of action (Kurtzhals et al., 1995). Liraglutide, a GLP-1 analogue, is characterized by a C16 fatty acid moiety conjugated to Lys26 (Madsen et al., 2007). Based on the structural properties of liraglutide, Lennox et al. (2013) developed a novel GLP-1 peptide, (Val8)GLP-1-Glu-PAL, which contains a C16 fatty acid moiety (designated PAL) conjugated to Lys26 via a glutamic acid linker (Glu) in addition to an amino acid substitution at position 8. This Glu-PAL moiety is the same linker-fatty acid conjugate found in GLP-1. They examined the enzymatic stability and in vitro insulinotropic activity together with the acute and persistent in vivo actions of (Val8) GLP-1-Glu-PAL on glucose tolerance and insulin response. Furthermore, the longer-term effects of (Val8)GLP-1-Glu-PAL were assessed by measuring bodyweight, food intake, glycemic and insulinotropic responses, insulin sensitivity, and hippocampal neurogenesis. Their study demonstrated that (Val8)GLP-1-Glu-PAL is a long-acting GLP-1 peptide that significantly improves hippocampal neurogenesis, glucose homeostasis, and insulin secretion in high-fat-fed mice (Lennox et al., 2013). In the present study, we investigated whether (Val8)GLP-1-Glu-PAL prevents rotenone-induced apoptosis in primary cultured neurons.
Materials and Methods
Primary culture of mouse neurons
Swiss mouse pups (1-3 days old) were obtained from the Animal Center at Shanxi Medical University, China (license No. SCXK (Jin) 2009-0001). All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications, No. 80-23, revised 1978) and approved by the Animal Ethics Committee in Shanxi Medical University, China. All procedures involving fresh tissue were performed on ice. Neonatal mice were sacrificed by decapitation. Cortical and hippocampal tissues (Paxinos and Watson, 2005) were isolated and washed in Ca2+- and Mg2+-free Hanks balanced salt solution. The tissue was cut into small pieces and incubated with 0.25% trypsin for 10 minutes at 37°C. The trypsin was inactivated using fetal bovine serum to prevent excessive dissociation. The dissolved tissues were centrifuged (1,000 r/min for 5 minutes) and cells were taken. The cells were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin (Trans, Beijing, China) in a humidified atmosphere at 37 ° C with 5% CO2for 6 hours. Neurobasal medium (Gibco, Carlsbad, CA, USA) containing 2% B27 supplement, 1% L-glutamine and 1% penicillin and streptomycin was used. Neurons were cultured and grew for 7 days until they reached 70-80% confluency.
Cell counting kit-8 (CCK-8) assay

Figure 1 Viability of neurons exposed to rotenone at different concentrations.
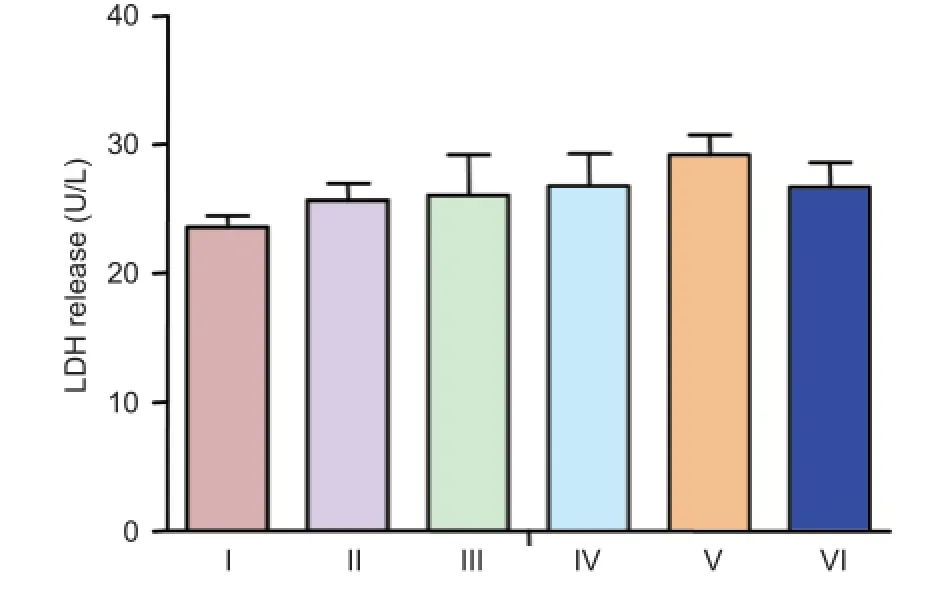
Figure 2 LDH release in cultured neurons treated with different concentrations of (Val8)GLP-1-Glu-PAL.

Figure 3 Neuroprotective effects of (Val8)GLP-1-Glu-PAL on neuron survival after rotenone exposure.
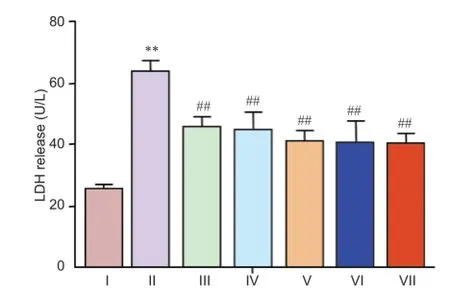
Figure 4 Rotenone-induced elevation of LDH release was inhibited by (Val8)GLP-1-Glu-PAL.
We used the CCK-8 assay (Dojindo Laboratories, Tokyo, Japan) to identify the lowest concentration of rotenone that would inhibit cellular survival. Primary neurons were seeded at a concentration of 1 × 106cells per well in 96-well cell culture plates at 37°C for 7 days. Rotenone (Solarbio, Beijing, China) was prepared in 1 mM dimethyl sulfoxide and diluted in the culture medium at concentrations of 0.5, 1, 3, and 5 nM. Cell survival was assessed at 48 hours by adding CCK-8 solution (10 μL per well) directly to the cell suspension and incubating for 2 hours at 37°C. Absorbance values of all wells were measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA). The results are expressed as a percentage of values in control cells not exposed to rotenone.
Lactate dehydrogenase (LDH) assay
The neuroprotective effects of (Val8)GLP-1-Glu-PAL against rotenone were explored at a range of concentrations using the LDH cell viability assay (de Carvalho et al., 2014). Cultured neurons were incubated for 7 days, then pretreated with 0, 10, 50, 100, 200 and 500 nM (Val8)GLP-1-Glu-PAL for 2 hours, before being exposed to rotenone (0.5 nM) for 48 hours. Activity of LDH released into the medium after freeze-thaw lysing of the cells was evaluated using an LDH assay kit (Jiancheng Company, Nanjing, Jiangsu Province, China) according to the manufacturer’s instructions, and quantified by measuring the optical density at 440 nm with a 722 visible spectrophotometer (Shanghai Optical Instrument Factory, Shanghai, China). Thereleased LDH activity was expressed as U/L.
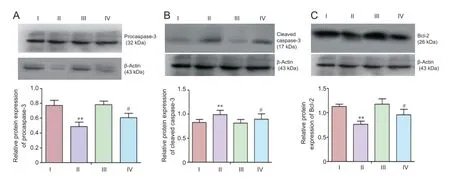
Figure 5 (Val8)GLP-1-Glu-PAL inhibited apoptosis signaling induced by rotenone.
Western blot analysis
Western blot analysis was used to determine expression of the apoptotic signaling proteins procaspase-3, cleaved caspase-3 and Bcl-2. Cultured neurons were incubated for 7 days, then treated with (Val8)GLP-1-Glu-PAL (100 nM) for 2 hours before exposure to rotenone (0.5 nM) for 48 hours. Cells were plated at a concentration of 1 × 106per dish in cell culture dishes of 100 mm × 20 mm, and grown for 7 days until they reached 70-80% confluence. Cells were collected in ice-cold RIPA lysis buffer (Beyotime, Shanghai, China). Samples were centrifuged (12,000 r/min for 5 minutes at 4°C) before the supernatant was taken. Total protein concentration of the cells was determined using the BCA Protein Assay Kit (Beyotime), and an equal concentration of loading buffer was added to the samples. Proteins (30 μg) were run in a 15% Tris-Tricine gel and electrophoretically transferred onto polyvinylidene fluoride membranes. After blocking with 5% bovine serum albumin in PBS containing 0.05% Tween-20 (PBST) at room temperature for 2 hours, the membranes were incubated overnight at 4 ° C with rabbit anti-procaspase 3 polyclonal antibody (1:500; Abcam, Cambridge, UK), rabbit anti-cleaved caspase 3 polyclonal antibody (1:1,000; Cell Signaling Technology, Boston, MA, USA), rabbit anti-Bcl 2 polyclonal antibody (1:500; Abcam), or rabbit anti-mouse β-actin monoclonal antibody (1:500; Abcam). After washing with PBST, the membranes were incubated with goat anti-rabbit IgG (1:5,000, Abcam) for 2 hours at room temperature. The bands were detected using an enhanced chemiluminescence system (Transgen, Beijing, China). The blots were scanned and the relative protein content quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Protein content was expressed as the target protein/β-actin optical density ratio.
Statistical analysis
Data was expressed as the mean ± SEM and were compared between groups by one-way analysis of variance (SPSS 16.0 software; SPSS, Chicago, IL, USA). Dunnett’s t-test was used for pairwise comparison between groups. Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) was used to present the data. P < 0.05 was considered statistically significant.
Results
Neurotoxicity of rotenone in primary cultured neurons
Evident morphological changes such as cell loss, shrinkage, enhanced refraction, string-of-beads appearance, and even floating and cell death were seen in the cultured hippocampal and cortical neurons exposed to rotenone (5 nM) for 48 hours, compared with control. Neuronal density was markedly reduced (Figure 1).
The CCK-8 assay showed a dose-dependent decrease in the number of viable cultured neurons after rotenone exposure, with minimal cellular loss observed after low concentrations and a steady decline in number at higher concentrations. Of the concentrations tested, the lowest to reduce cell numbers was 0.5 nM (Figure 1).
LDH release in cultured neurons treated with different concentrations of (Val8)GLP-1-Glu-PAL
Cellular viability was determined by measuring LDH release into the culture medium from dead or dying cells. (Val8)GLP-1-Glu-PAL alone did not affect total LDH release at any concentration tested (P > 0.05, vs. control; Figure 2).
Protective effect of (Val8)GLP-1-Glu-PAL on cell survival after rotenone treatment
A 20% decrease in CCK-8 value was shown in cultured neurons exposed to 0.5 nM rotenone compared with control (P <0.05), which was prevented by pretreatment with (Val8)GLP-1-Glu-PAL. No significant difference was observed between rotenone-damaged neurons after pretreatment with 10 or 50 nM (Val8)GLP-1-Glu-PA (P > 0.05); however, significantly higher CCK-8 values were observed after pretreatment with higher concentrations of (Val8)GLP-1-Glu-PAL (100, 200 and 500 mN; P < 0.05). The lowest concentration of (Val8)GLP-1-Glu-PAL to prevent rotenone damage was 100 nM (Figure 3).
Neuroprotective effect of (Val8)GLP-1-Glu-PAL against rotenone toxicity
Release of LDH in cultured neurons exposed to 0.5 nM rotenone was significantly greater than in control cells (P < 0.05). This increase was prevented by pretreatment with different concentrations of (Val8)GLP-1-Glu-PAL (P < 0.01, vs. rotenone; Figure 4).
(Val8)GLP-1-Glu-PAL inhibited apoptosis signaling induced by rotenone
Western blot analysis showed that 48 hours after exposure to rotenone (0.5 nM), neurons expressed a lower level of procaspase-3, and higher level of cleaved caspase-3, than control neurons (P < 0.01). These effects were significantly reduced by pretreatment with 100 nM (Val8)GLP-1-Glu-PAL (P < 0.01 or P < 0.05). A rotenone-induced decrease in Bcl-2 expression was also prevented by pretreatment with 100 nM (Val8)GLP-1-Glu-PAL (P < 0.05; Figure 5).
Discussion
The mechanisms underlying the initiation of neurodegenerative processes in Parkinson’s disease are incompletely understood. Chemicals known to induce disease-like symptoms and depletion of dopaminergic metabolism and transmission in neurons, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (Herrero et al., 1995), 6-hydroxydopamine (Jolicoeur et al., 1991), and rotenone, are used to study the disease and test potential treatments. The mode of action appears to be that these chemicals are preferentially taken up or metabolized by dopaminergic neurons, increasing oxidative stress and blocking mitochondrial activity. We therefore used the rotenone-induced cellular model of Parkinson’s disease to investigate the neuroprotective properties of (Val8)GLP-1-Glu-PAL in vitro.
Exendin-4 was the first GLP-1 receptor agonist developed, and was originally used to treat type 2 diabetes. It has a biological half-life of about 4 hours, and has to be injected subcutaneously twice daily. Several GLP-1 receptor agonists have since been developed, with longer half-lives and superior effects than exendin-4 (Campbell and Drucker, 2013). For example, liraglutide (GLP-1-Glu-PAL) only needs to be injected once daily (Vilsb?ll et al., 2008). (Val8)GLP-1 is a protease-resistant GLP-1 analogue that is also neuroprotective (Wang et al., 2010; Gengler et al., 2012; Li et al., 2012). The combination of these two analogues creates a powerful new GLP-1 analogue, (Val8)GLP-1-Glu-PAL (Lennox et al., 2013).
In the present study, we have shown that (Val8)GLP-1-Glu-PAL protects primary cultured neurons from the toxic effects of rotenone. It preserved neuronal cell viability, suggesting that it may prevent dopaminergic degenerative processes induced by rotenone or similar oxidants. Importantly, (Val8) GLP-1-Glu-PAL antagonized rotenone-activated apoptotic signaling pathways. Caspase-3 is a key protease activated by mitochondrial damage (Kashyap et al., 2010). Rotenone-induced oxidative mitochondrial stress activated this apoptotic pathway, upregulating total procaspase and cleaved activated caspase-3 expression. This was inhibited by (Val8)GLP-1-Glu-PAL, suggesting that (Val8)GLP-1-Glu-PAL prevents apoptosis and neuronal death. The effect is most likely due to the activation of growth factor signaling via the GLP-1 receptor, which inhibits apoptotic signaling (Li et al., 2010; Sharma et al., 2014). In addition, the GLP-1 receptor induces upregulation of the anti-apoptotic protein Bcl-2 (Li et al., 2010; Sharma et al., 2014). Bcl-2 acts to preserve mitochondrial integrity by preventing the loss of mitochondrial membrane potential and/or release of pro-apoptotic proteins such as cytochrome C into the cytosol (Harada and Grant, 2003). In the present study, (Val8)GLP-1-Glu-PAL was also able to restore normal levels of Bcl-2 that had been reduced by rotenone.
In conclusion, (Val8)GLP-1-Glu-PAL shows good effects in this neuronal culture assay. Preclinical tests in rodent models of Parkinson’s disease will determine its effects on motor activity, dopamine transmission and neuronal survival. Direct comparisons with exendin-4, liraglutide and other drugs for Parkinson’s disease will have to be conducted before a conclusion on the efficacy of this GLP-1 analogue can be drawn. The primary neuronal cell culture results presented here are the first encouraging demonstrations of neuroprotection that pave the way to further studies of this novel analogue.
Author contributions: LL and YZL wrote the paper and were responsible for the study protocol, design, and grant support. CH directed the study and was responsible for chemical supply. GLL performed western blot analysis. KL and JZ performed all remaining experimental procedures. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T (2013) Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest 123:2730-2736.
Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T (2014) Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis 4:337-344.
Bagetta V, Ghiglieri V, Sgobio C, Calabresi P, Picconi B (2010) Synaptic dysfunction in Parkinson’s disease. Biochem Soc Trans 38:493-497.
Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, R?nnholm H, Wikstr?m L (2008) Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res 86:326-338.
Campbell JE, Drucker DJ (2013) Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 17:819-837.
Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9:124.
Darsalia V, Mansouri S, Orts?ter H, Olverling A, Nozadze N, Kappe C, Iverfeldt K, Tracy Linda M, Grankvist N, Sj?holm ?, Patrone C (2012) Glucagon-like peptide-1 receptor activation reduces ischaemic brain damage following stroke in type 2 diabetic rats. Clin Sci (Lond) 122:473-483.
de Carvalho ND, Garcia RC, Kleber Ferreira A, Rodrigo Batista D, Carlos Cassola A, Maria D, Lebrun I, Mendes Carneiro S, Castro Afeche S, Marcourakis T, Sandoval MRL (2014) Neurotoxicity of coral snake phospholipases A2 in cultured rat hippocampal neurons. Brain Res 1552:1-16.
Fleming SM, Zhu C, Fernagut PO, Mehta A, DiCarlo CD, Seaman RL, Chesselet MF (2004) Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol 187:418-429.
Gengler S, McClean PL, McCurtin R, Gault VA, H?lscher C (2012) Val(8)GLP-1 rescues synaptic plasticity and reduces dense core plaques in APP/PS1 mice. Neurobiol Aging 33:265-276.
Green BD, Lavery KS, Irwin N, O’Harte FPM, Harriott P, Greer B, Bailey CJ, Flatt PR (2006) Novel glucagon-like peptide-1 (GLP-1) analog (Val8)GLP-1 results in significant improvements of glucose tolerance and pancreatic beta-cell function after 3-week daily administration in obese diabetic (ob/ob) mice. J Pharmacol Exp Ther 318:914-921.
H?lscher C (2014a) The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer’s disease. Alzheimers Dement 10:S47-54.
H?lscher C (2014b) Insulin, incretins and other growth factors as potential novel treatments for Alzheimer’s and Parkinson’s diseases. Biochem Soc Trans 42:593-599.
H?lscher C (2014c) Drugs developed for treatment of diabetes show protective effects in Alzheimer’s and Parkinson’s diseases. Sheng Li Xue Bao 66:497-510.
Hamilton A, H?lscher C (2009) Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport 20:1161-1166.
Harada H, Grant S (2003) Apoptosis regulators. Rev Clin Exp Hematol 7:117-138.
Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS (2008) Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation 5:19.
Herrero MT, Augood SJ, Hirsch EC, Javoy-Agid F, Luquin MR, Agid Y, Obeso JA, Emson PC (1995) Effects of l-DOPA on preproenkephalin and preprotachykinin gene expression in the MPTP-treated monkey striatum. Neuroscience 68:1189-1198.
Jolicoeur FB, Rivest R, Drumheller A (1991) Hypokinesia, rigidity, and tremor induced by hypothalamic 6-OHDA lesions in the rat. Brain Res Bull 26:317-320.
Kashyap MP, Singh AK, Siddiqui MA, Kumar V, Tripathi VK, Khanna VK, Yadav S, Jain SK, Pant AB (2010) Caspase cascade regulated mitochondria mediated apoptosis in monocrotophos exposed PC12 cells. Chem Res Toxicol 23:1663-1672.
Kieffer TJ, McIntosh CH, Pederson RA (1995) Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136:3585-3596.
Kurtzhals P, Havelund S, Jonassen I, Kiehr B, Larsen UD, Ribel U, Markussen J (1995) Albumin binding of insulins acylated with fatty acids: characterization of the ligand-protein interaction and correlation between binding affinity and timing of the insulin effect in vivo. Biochem J 312:725-731.
Lee CH, Yan B, Yoo KY, Choi JH, Kwon SH, Her S, Sohn Y, Hwang IK, Cho JH, Kim YM, Won MH (2011) Ischemia-induced changes in glucagon-like peptide-1 receptor and neuroprotective effect of its agonist, exendin-4, in experimental transient cerebral ischemia. J Neurosci Res 89:1103-1113.
Lennox R, Porter DW, Flatt PR, Gault VA (2013) (Val(8))GLP-1-Glu-PAL: a GLP-1 agonist that improves hippocampal neurogenesis, glucose homeostasis, and β-cell function in high-fat-fed mice. ChemMed-Chem 8:595-602.
Li L, Zhang ZF, Holscher C, Gao C, Jiang YH, Liu YZ (2012) (Val8) glucagon-like peptide-1 prevents tau hyperphosphorylation, impairment of spatial learning and ultra-structural cellular damage induced by streptozotocin in rat brains. Eur J Pharmacol 674:280-286.
Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH (2010) Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem 113:1621-1631.
Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH (2009) GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A 106:1285-1290.
Madsen K, Knudsen LB, Agersoe H, Nielsen PF, Th?gersen H, Wilken M, Johansen NL (2007) Structure-activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: importance of fatty acid length, polarity, and bulkiness. J Med Chem 50:6126-6132.
McClean PL, Parthsarathy V, Faivre E, H?lscher C (2011) The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci 31:6587-6594.
Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates. London: Academic Press.
Perry T, Greig NH (2002) The glucagon-like peptides: a new genre in therapeutic targets for intervention in Alzheimer’s disease. J Alzheimers Dis 4:487-496.
Sharma MK, Jalewa J, H?lscher C (2014) Neuroprotective and anti-apoptotic effects of liraglutide on SH-SY5Y cells exposed to methylglyoxal stress. J Neurochem 128:459-471.
Vilsb?ll T, Knop FK (2008) Long-acting GLP-1 analogs for the treatment of type 2 diabetes mellitus. Biodrugs 22:251-257.
Vilsb?ll T, Brock B, Perrild H, Levin K, Lervang HH, K?lendorf K, Krarup T, Schmitz O, Zdravkovic M, Le-Thi T, Madsbad S (2008) Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med 25:152-156.
Wang XH, Li L, H?lscher C, Pan YF, Chen XR, Qi JS (2010) Val8-glucagon-like peptide-1 protects against Aβ1-40-induced impairment of hippocampal late-phase long-term potentiation and spatial learning in rats. Neuroscience 170:1239-1248.
Zhu C, Vourc’h P, Fernagut PO, Fleming SM, Lacan S, Dicarlo CD, Seaman RL, Chesselet MF (2004) Variable effects of chronic subcutaneous administration of rotenone on striatal histology. J Comp Neurol 478:418-426.
Copyedited by Slone-Murphy J, Stow A, Yu J, Wang L, Li CH, Song LP, Zjao M
10.4103/1673-5374.177742 http://www.nrronline.org/
How to cite this article: Li L, Liu K, Zhao J, Holscher C, Li GL, Liu YZ (2016) Neuroprotective role of (Val8)GLP-1-Glu-PAL in an in vitro model of Parkinson’s disease. Neural Regen Res 11(2):326-331.
Funding: This study was supported by a grant from the Shanxi Science and Technology Department of China, No. 2011081060; a grant from Shanxi Scholarship Council of China, No. 2011-44; and a grant from the Cure Parkinson’s Trust UK to CH.
Accepted: 2015-12-17
*Correspondence to: Christian Holscher or Yue-ze Liu, Ph.D., yuezeliu@163.com.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Impaired consciousness caused by injury of the lower ascending reticular activating system: evaluation by diffusion tensor tractography
- Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway
- Influence of immobilization and sensory re-education on the sensory recovery after reconstruction of digital nerves with direct suture or muscle-in-vein conduits
- Effects of microtubule-associated protein tau expression on neural stem cell migration after spinal cord injury
- Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons
- Neural differentiation and synaptogenesis in retinal development