Problems and Suggestions for Domestic Non-special Use Cosmetics Record Filing in China
Chunli Hu, Li Wang
Liaoning Center for Drug and Device Evaluation and Monitoring, China
Yanan Li
Dandong City Drug Inspection Test Center, China
With the improvement of Chinese people’s living standards, the demand for cosmetics is increasing day by day. But the regulation of cosmetics is lagging behind,especially the confused label management indicates that illegal problems happen frequently.[1]Cosmetic products in China are divided into two classes: special use cosmetics and non-special use cosmetics. Just as health and heauty trends continue to change, so do industry regulations. In order to regulate the market order and protect people’s health, China Food and Drug Administration (hereinafter referred to as CFDA) has carried out the work of domestic non-special use cosmetics record filing management in July 2014 (this category includes all the products excluded from special use cosmetics which are classified into 9 types: hair growth products, hair dyes, hair perming products, depilating products, breast beauty products, slimming products,deodorants, freckle-removing products, sunscreens), to standardize the ingredients, labels and specifications of nonspecial use cosmetics for public supervision and inquiry.Some problems encountered in the process of domestic nonspecial use cosmetics record filing management has been collected, and suggestions like combining with the current regulations and technical requirements also has been put forward, which will efficiently help the cosmetics industry develop more healthily.
Background of domestic non-special use cosmetics record filing
In 2011, CFDA started the work of domestic nonspecial use cosmetics record filing. “Since 1 October 2011,the domestic non-special use cosmetics, which was first placed on the market, should be filed in accordance with the relevant requirement of Administrative Measures for Record keeping of Domestic Non-special Use Cosmetics Record Filing.”[2]However, the implementation of the situation was not operated well in a period of time and lack of enthusiasm.[1]By the end of 2013, CFDA adjusted the management model of cosmetics registration, published Notice on Adjusting Related Matters on Cosmetics Registration and Notification Management (Notice NO.10,2013), which lays down the domestic non-special use cosmetics enterprises to file their non-special use products with provincal FDA through the online Record Filing System before placing their products on the market. The Product record information confirmed by the provincial FDA should unified announced on the government website for public inquiry. Provincial FDA will no longer issue record-keeping certificates for approved domestic non-special use cosmetics. At the same time, whitening cosmetics will be classified as special use cosmetics.[3]
From unable to query the information to all the record information unified announced on the website, it was a major highlight and breakthrough. It also strengthen the enterprises safety consciousness. As of May 2016, there were more than 400,000 records on the platform (the domestic non-special use cosmetics record information management system), many of which were new records.The rate of domestic non-special use cosmetics record has been significantly improved.
Regulation and technical requirements of Domestic Non-special Use Cosmetics Record Filing
Cosmetic labeling means any tag, brand, mark,pictorial or other descriptive matter, and other written,printed or graphic matter on or accompanying a cosmetic product. Proper labeling is an improtant aspect of marketing a cometics product. Labeling is used to help imform consumers of a product’s intended use and any related warning, its ingredients and net quantity of contents, and its place of manufacture or distribution.
Before the supervision system reformed in 2013, the supervision of cosmetic labels was adopt an sectioned management system. Administration of Quality Supervision was responsible for the examination and supervision of cosmetic labels; Drug Regulation Administration Department was responsible for the supervision of the Cosmetics circulation; Administration for Industry & Commerce was responsible for advertising,marketing, consumer protection, etc. After the CFDA was founded, the duties of administration and supervision were transferred to CFDA.[4,5]Therefore, the existing laws and regulations for Domestic Non-special Use Cosmetics Record Filing include multiple documents and issued by the Quality Supervision Bureau, Food and Drug Administration, etc. The existing laws and regulations related to domestic non-special use cosmetics labels are shown in Table 1.
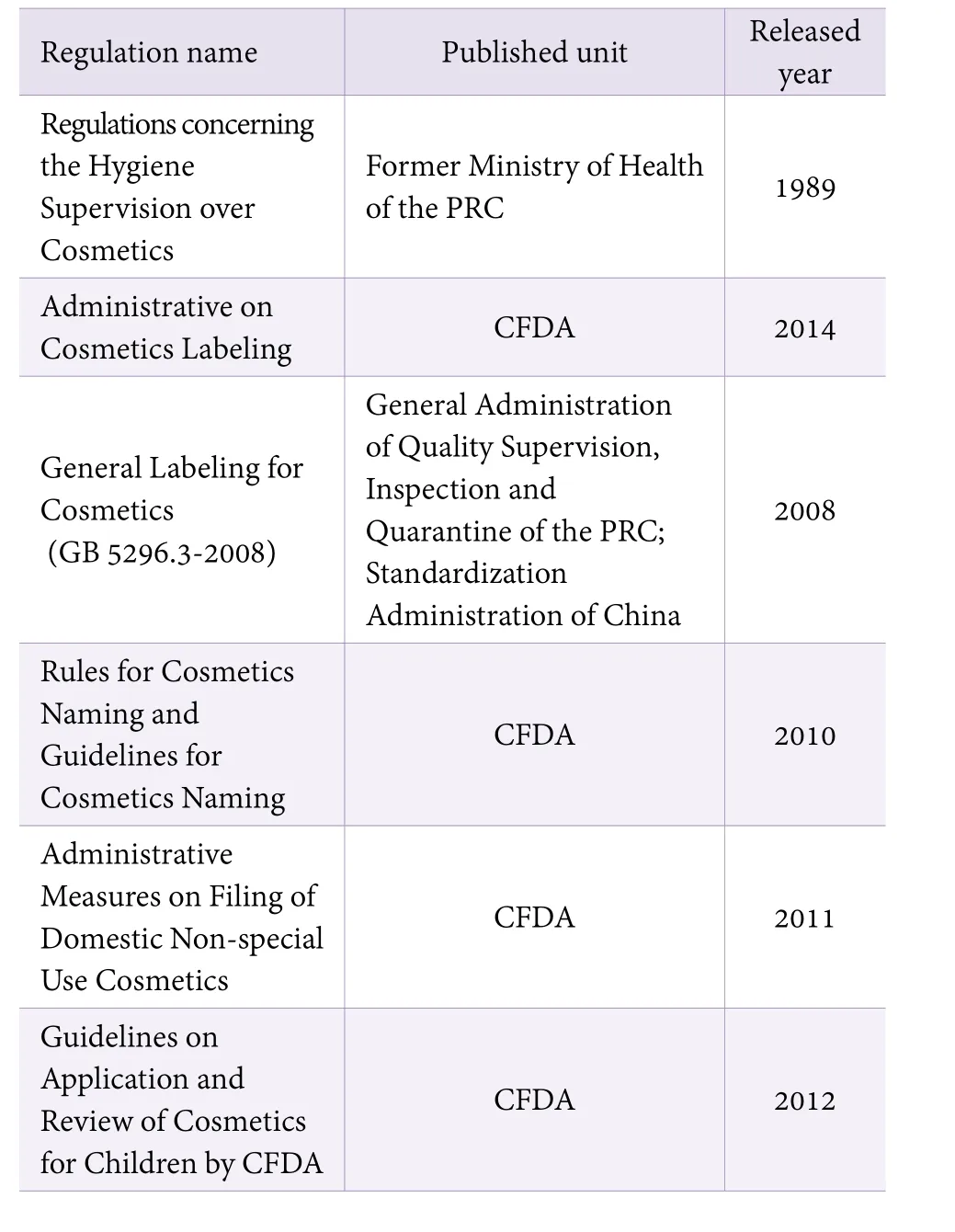
Table 1. Existing regulations that the domestic non-special Use Cosmetics Record Filing involved
It should be noted that the above regulations are made only for the current audit process, the actual record should include but not only limited to the above regulations.
In addition to technical information of product process, toxicological test and risk assessment for future examination, the manufacturer is required to submit product formula and packaging label information online,which will reflect the critical safety information. Product formula is the key point of technical risk assessment.Therefore, the applicant should clarify the product formula in details including all the raw materials and the standard Chinese name, raw material serial number,restricted substance content, use purpose and so on. If the formula contains colorants, it should provide CI number;if the formula contains raw materials for hydrocarbon(except for single component) from petroleum and coal tar, it should provide CAS number. All the raw materials should be in the catalogue of raw materials, especially pay attention to the banned substances, restrictive substances,colorants and preservatives allowed to use. The existing technical requirements related to domestic non-special use cosmetics are shown in Table 2.

Table 2. Technical requirements that the domestic nonspecial use cosmetics executed
Common problems and suggestions
Laws and regulations issued early and are in urgent need of upgrading.Regulations Concerning the Hygiene Supervision Over Cosmeticshas significant impact on the cosmetics industry in China and has provided the foundation for regulating both cosmetics manufacturing and cosmetics importing into China. The law was released in 1989, but after more than twenty years in practice, discussions around the need for amendents have recenty emerged.[6]Administrative of Provisions on Cosmetic Labeling released by Administrative Quality Supervision Inspection and Quarantine, has been unable to adapt to the current stage. Therefore, CFDA should improve cosmetics supervision legal basis to enhance the regulation operation as soon as possible,[1]make more detailed rules and regulations to standardize the forms and contents of label declaration.
In addition, due to the history of sectioned management,the current version regulations are issued by previous different administrative units and are lack of authority and continuity. For example, “cosmetics” was not entirely consistently defined in Regulations cocerning the Hygiene Supervision over Cosmetics, GB5296.3 and Administrative Provisions on Cosmetic Labels.Regulations cocerning the Hygiene Supervision over Cosmetics defines cosmeticsas “daily products of chemical industry”, but GB 5296.3 and Administrative Provisions on Cosmetics labelingdefine cosmetics as“generalized products”; the part of the human body cosmetics applied on has been limited to “the human lips or teeth, etc.” in Administrative Provisions on Cosmetics Labeling; but in Regulations cocerning the Hygiene Supervision over Cosmetics and GB 5296.3, it has been limited to “l(fā)ips”, and so forth.
CFDA has released draft Regulations concerning Supervision and Administration over Cosmetics for public consultation. We hope that the official regulation can be released early to ensure the seriousness and authority of supervision and evaluation. If the rivision is approved, it will be the first time the regulation has been changed since it was founed. The CFDA said the purpose of the revision was to bring the rules more in line with those of the EU and US.
Certain varieties do not belong to non-special use cosmetics.Cosmetics munufacturers of non-special use cosmetics shall notify the provincial FDA through an online platform. Whitening products were classified as the special use cosmetics for the first time. Many applicants do not understand the requirements of CFDA thoroughly and are not sensitive to the word “white”. For example,although the name of some products does not contain“white”, the label is still marked words close to “white”.Some labels are not suitable, such as shampoo with fertility function, declaring “improve blood circulation”,“dilate the capillaries” or some medical claims and function words. These are all beyond the requirement of non-special use cosmetics.
The purpose of cosmetics record is to standardize the management, to avoid concept confusion and misleading customers. Administrative Measures on Cosmetic labeling can be used as a reference released by CFDA. Meanwhile,enterprises should enhance the sense of responsibility and identify carefully, and avoid using the ambiguous words and phrases.

Record information is incomplete.First of all, the label information is incomplete, such as not all stereo and planar graphs of labels are provided, the information of manufacturer is not complete. Secondly, the product formulation is not complete, such as restricted substances or limited preservatives are not listed in the content, the specific name of raw materials, extract parts are not clear,etc.
Labels and ingredients are the key control points of cosmetic technical evaluation. The applicant should provide corresponding product information according to the actual situation, and fill in the standard Chinese name, INCI name, content of restricted substance and the use purpose in the recipe list. Particularly, as a risk control point of the product quality, the type of restricted substance should be included in Hygienic Standard for Cosmetics and the amount should meet the requirements of the standard.
Cosmetics labels do not meet the requirements.At present, the cosmetic packages on the market are more and more exquisite, but the labels are lack of standardization increasingly.[7,8]To meet the needs for import cosmetics of customers, some domestic cosmetic labels printed information in English, Japanese, korean and other foreign languages. If the label contains any representation in a foreign language, all label information required under the current regulations must also appear in that language.[9]It should be further stipulated labels.Naming, ingredients and shelf life must appear on the panel of the package in the proper position of the product package in standard Chinese characters. The language used in labeling must be standard Chinese.Foreign language can be used at the same time but must be a correct translation of the Chinese and the font size must not be bigger than the corresponding Chinese characters.
In order to highlight the effectiveness or raise the price of cosmetics, enterprises often claim that cosmetics contain certain ingredients in order to attract consumers. For example, a “red ginseng mask” is labeled as containing the ingredient of “ginseng extract” in the content. According to Chinese Pharmacopoeia 2015, red ginseng is a product made by the primary processing of ginseng, and the active ingredient and functional indications of them are not completely the same. Ginseng extract and red ginseng extract are both listed in the IECIC 2015, so the product named red ginseng belongs to stealing concept.Such problems are seen frequently, such as ingredients containing “chondrus crispus extract”, “panicum miliaceum seed extract” and “fragrance”, which were named “seaweed”, “millet”, “milk” the common name respectively. CFDA should define such problems. All cosmetics must be labeled with their ingredients. If a product named after raw material, the raw material should be listed in product ingredients list.
Pictures of product label and description should be relatively corresponding. Integrated packaging of the overall sales should be declared in accordance with the package, and all the ingredients of products should be declared in the package. But if you declare one single product and provide a combination packaging label picture, it can’t be investigated that whether the ingredients of other products in combination packaging meet the requirements. It is proposed to be clear about how to declare such products in the new Administrative Measures on Cosmetic Labeling, fully consider the diversity and complexity of cosmetics.
Evaluation standards are inconsistent in different provinces.A major highlight of cosmetics record filing is the whole network shares, and both of the two entrusting parties companies are required to file. If the two entrusting parties are in different provinces, it is required to file in two provinces respectively, which can cause a problem that if the two provinces have different requirements, two opposite conclusions will be gaven and influence the authority of technical evaluation. For example, can designed sample manuscript uploaded as product pictures? Dose the ingredients in the label need to be strictly as same as ingredient list? Does the restricted substance monomer need to be marked?
Is the design sample manuscript feasible? The author believes that if the product has been filed and in the market, it should upload actual photos; if the new products are not sold in the market and the record number isn’t confirmed, it can upload sample design manuscript; if the recorded products need to modify the labels and hasn’t be printed, it can upload sample design manuscript, too.

Before theIECIC 2015 published, ingredients are not be required to registered. Therefore, there are many differences between labels and ingredient list. It is suggested that based on the history of cosmetics approval,allowing it to be recorded, and modifying the ingredients according to the latest regulatory requirements.Enterprises can upload the sample designs or devise labels and provide instructions, and ultimately achieve the strategies of filing.
Should the amount of restricted substance or monomer residue be marked? For example, it isn’t stipulated the cosmetics maximum concentration of “polyacrylamide”in the restricted substance table in Hygienic Standard for Cosmetics, but the maximum residue amount of acrylamide monomer is stipulated. Restricted substance is the risk ingredient of cosmetics, and the best way to ensure the product safety is to mark the amount of restricted ingredients and amount of monomer residues simultaneously. Under the current background of cosmetics record filing, the monomer residue met the requirement of specification can be approved.
It is suggested that CFDA should hold work exchange meetings to collect the problems encountered by provincial administration and unify standards, ensuring the consistency of provincial evaluation standard, and reducing the burden of enterprises.

Conclusion
Domestic Non-special Use Cosmetics Record Filing is a meaningful attempt for CFDA decentralization. CFDA may transfer power to provincial FDA. Therefore, it should summary the questions existing in the technical reviewing process of domestic non-special use cosmetics timely. The qustions are fed back to CFDA and transfer to provincial FDA. It will guide the enterprises to complete the declaration, and ensure the declaration quality finally. CFDA should also promulgate relevant laws and regulations, implementation details and guidelines as soon as possible, providing accurate basis for enterprises to declare and references for technical evaluation, leading customers to make the right choice. Enterprises should strengthen the sense of responsibility and risk awareness continuously, ensuring the product safety and label legitimacy. Finally, it should enhance exchanges between government and enterprises to promote the healthy development of the cosmetics industry.[10]
[1] Ziyu Song. Analysis on Problems and Countermeasures in Supervision of Cosmetic Industry. Capital Medicine 2014(11),53.
[2] CFDA. Notice on Printing and Issuing Measures for the Administration of Domestic Non-special Use Cosmetics Record Filing 2011.
[3] CFDA. Notice on the Adjustment of the Relevant Matters Concerning the Registration and Management of Cosmetics 2013.
[4] Shuyi Lv; Xiaodong Zhu; Ying Zhou; et al. A Comparative Study on the Regulation and Standards of 11 Countries(Regions)Cosmetics Labels. Health Care Products and Cosmetics 2014,2(126), 27-43.
[5] Qichen You. Status and Development Trend of Chinese Cosmetic industry. Detergent & Cosmetics 2007, 30(1), 1-3.
[6] Jincheng Yao; Linggui Zeng; Xinwen Lin; et al. Analysis of Status Quo and Related Strategies of Cosmetics Safety Control System in China. China Pharmacy 2014, 25(9), 775-777.
[7] Hui Wang; Jiong Zhu. Analysis of Quality Conditions in the Acne Removing Cosmetics Market and Study on Its Supervision. Chinese Pharmaceutical Affairs 2013, 27(10),1021-1024.
[8] Zihan Liu. Don’t be Fooled by the Concept of Drug Cosmetics’.China Pharmaceutical News 2010.
[9] General Administration of Quality Supervision, Inspection and Quarantine of the PRC. Provisions on the Administration of Cosmetics Labels 2007.
[10] Yu. Cosmetic Safety and Risk Control Measures. Fujian Textile 2014(10), 25-26.
 China Detergent & Cosmetics2016年2期
China Detergent & Cosmetics2016年2期
- China Detergent & Cosmetics的其它文章
- Synthesis of Monoglycerides with Cinnamomum Burmannii Seeds Oil and Its Application in Moisturizing Cream
- New Application of a Series of Preservatives Derived from Amino Acid for Cosmetic Products
- Cosmetic Safety Evaluation Based on In Vitro Three-dimensional Reconstructed Human Epidermis(3D-RHE) Models
- Patent Protection of Daily Chemical Industry in China
- New Trends of Comsmetic Regulations in China
- Development of Household Care Chemical Industry in Pakistan
