Advances in immunotherapy for treatment of lung cancer
Jean G.Bustamante Alvarez, María González-Cao, Niki Karachaliou, Mariacarmela Santarpia, Santiago Viteri,Cristina Teixidó, Rafael Rosell,,5,6
1Albert Einstein Medical Center, Philadelphia 19141, USA; 2Translational Cancer Research Unit, Instituto Oncológico Dr Rosell,Quirón Dexeus University Hospital, Barcelona 08028, Spain; 3Medical Oncology Unit, Human Pathology Department, University of Messina, Messina 98100, Italy; 4Pangaea Biotech S.L, Barcelona 08028, Spain; 5Cancer Biology & Precision Medicine Program,Catalan Institute of Oncology, Germans Trias i Pujol Health Sciences Institute and Hospital, Campus Can Ruti, Badalona, Barcelona 08916, Spain; 6Fundación Molecular Oncology Research, Barcelona 08028, Spain
Introduction
Efforts in the development of therapy for metastatic lung cancer have led to an evolving arsenal of different approaches including chemotherapy and molecular targeted therapies.However,metastatic lung cancer remains the leading cause of cancer death worldwide and 5-year survival for advanced disease is less than 5%.
Multiple studies have shown how post-transplant patients undergoing immunosuppressive treatment have increased incidence of different types of cancer, including lymphoproliferative disorders, head and neck and lung cancer,illustrating how immunosuppression is involved in cancer development1,2.By means of different immunosuppressive mechanisms, including “immune check points”, lung cancer manages to evade immunosurveillance.These immune checkpoints are receptors expressed on T cells that regulate the immune response.The first immune checkpoints described were cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and the programmed cell death protein 1 (PD-1) and its ligand (PD-L1)3.
CTLA-4 is expressed on the surface of T-cells and regulates the amplitude of T-cell activation, down-modulates T helper cell activity and enhances regulatory T (Tregs) cell immunosuppressive activity4.PD-1 receptor is an inhibitory molecule present in T activated cells, B cells, monocytes, and natural killer (NK) cells.This receptor binds to its ligand PD-L1(or B7-H1 or CD274) and PD-L2 (or B7 DC or CD273) which are expressed in tumor cells and antigen presenting cells.Up to 57% of non-small cell lung cancers (NSCLCs) express PD-L1 constitutively or as an acquired adaptive mechanism of immune resistance.Expression of tumor PD-L1 protein in NSCLC was associated with increased local lymphocytic infiltration and longer overall survival (OS)5.PD-1 acts within the tumor microenvironment peripherally6, inhibiting T-cell signaling,cytotoxic activity, proliferation, survival and effector function of T cells and also promoting differentiation of CD4+T cells into T regulatory cells and inducing T cell apoptosis6.
In view of these immunosuppressive mechanisms, clinical research in the last decade has encompassed targeting these immune check points using monoclonal antibodies like ipilimumab, an anti CTLA-4, and pembrolizumab and nivolumab against PD-17,8.These promising monoclonal antibodies were initially approved for metastatic melanoma and now several PD-1/PD-L1 inhibitors have recently been approved by the FDA also for lung cancer.
Antigen presenting cells present peptides to tumor activated specific T lymphocytes through the major histocompatibility complex class I9,10.Cancer cells also augment secretion of gamma interferon (INF-γ) and tumor necrosis factor α (TNF-α)by CD4+T helper lymphocytes (TH)10.High intratumoral T cell density is required to eliminate cancer cells.Prognosis can be affected by the quantity, localization, and phenotype of infiltrating T cells11; high infiltration by cytotoxic T cells confers good prognosis12.It is also important to consider that some other inhibitory cells infiltrate tumors, like regulatory T CD4+cells FOXP3+(Tregs), cancer associated fibroblasts, myeloid derived suppressor cells and tumor associated macrophages13.These cells generate an immunosuppressive microenvironment by several mechanisms, such as TGF-β and interleukin-10 secretion,secretion of platelet derived growth factor (PDGF) and vascular endothelial growth factor (VEGF).Tumor cells, on the other hand, produce down-regulation of the major histocompatibility complex class I (MHC-I) and antigen expression and increase PD-L1 expression in tissue.As a result, solid tumors attain an immunological response insufficient to eliminate cancer cells,which is the reason why enhancing the function and quantity of CTLs may be of clinical benefit14.
Inhibitory receptors like CTLA-4, PD-1, TIM3 and LAG3,killer cell immunoglobulin-like receptor (KIR) and VISTA are co-expressed by T cells, secondary to chronic antigen stimulation15-29.This hyporesponsiveness makes cancer behave like a self-protein, inducing immune tolerance by transforming T-cell into an “exhaustion phenotype”30-32.
PD-1 receptor is expressed by activated T cells, NK cells,monocytes and B cells and binds PD-L1 (B7-H1 or CD274)and PD-L2 (B7-DC or CD273).PD-L1 is present in malignant cells as well as antigen presenter cells, myeloid cells, epithelial and lymphoid cells, and represents a constitutive or acquired mechanism of immune resistance33.PD-1/PD-L1 inhibit cytotoxic T lymphocyte proliferation, survival and effector activity, induce apoptosis of infiltrative T cells and increase the amount of regulatory T cells in the tumor microenvironment34.
Clinical development of anti PD-1 and PDL1 inhibitors in NSCLC
Currently, there are three PD-1/PD-L1 inhibitors available for lung cancer: nivolumab for squamous NSCLC (approved by FDA March 2015), pembrolizumab for the treatment of NSCLC and MPDL3280A from Roche (both granted breakthrough therapy designation) for NSCLC that has progressed on prior treatment (platinum based chemotherapy or targeted therapy for EGFR or ALK positive patients).
NSCLC was found to express PD-L1 in 27% to 57% of cases either in the cellular membrane or the cytoplasm35,36.PD-L1 and other molecules that act as immune checkpoints are upregulated in response to substances secreted by lung tumor cells,such as the enzyme IDO which also down-regulates MHC-137.Other immunosuppressive factors like IL-10 and TGF-β are secreted by tumor cells, editing the tumor microenvironment to attenuate immune response38.There are several clinical trials ongoing in lung cancer with different immune checkpoints blockers (Tables 1,2).
Anti PD-1 drugs
Nivolumab is a fully humanized anti-PD-1 monoclonal antibody from Bristol Meyer Squibb (BMS).A phase I trial in 2012 in previously treated NSCLC patients treated with intravenous nivolumab every 2 weeks for 12 cycles at different doses (0.1 to 10.0 mg per kilogram of body weight) demonstrated an overall response rate (ORR) of 18% by RECIST 1.1 criteria.These responses were seen in non-squamous as well as squamous NSCLC (ORR of 12% and 33%, respectively)7.Long term follow-up of the phase I trial with nivolumab in 129 previously treated NSCLC patients showed that in 50% of patients who responded, response was already evident by week 8 after starting treatment.In this trial, the ORR was 14% up to 25% and OS was 18% at 3 years.The median duration of response was 17 months.Pneumonitis was observed in 7% of patients, and grade 3-4 toxicities in 14%.The most common side effects were fatigue, diarrhea, and anorexia63.
The phase III CheckMate 017 trial reported an OS advantagefor nivolumab in pretreated squamous NSCLC.It included 272 squamous NSCLC patients regardless of PD-L1 status.Patients were randomized to nivolumab (3 mg/kg i.v.in 60 min every 2 weeks) versus docetaxel.The OS endpoint was met early and the study was therefore stopped in January 2015.Median OS was 9.2 months in the nivolumab arm (95% CI: 7.3-13.3) and 6.2 months in the docetaxel arm (95% CI: 5.1-7.3).At 1-year,the OS was 42% for the nivolumab group versus 24% in the docetaxel group.The hazard ratio (HR) was 0.59 (95% CI: 0.44-0.79; P=0.00025).Nivolumab also improved median progression free survival (PFS) 3.5 vs.2.8 months with docetaxel (HR =0.62;95% CI: 0.47-0.81; P=0.0004) (Table 1)47.
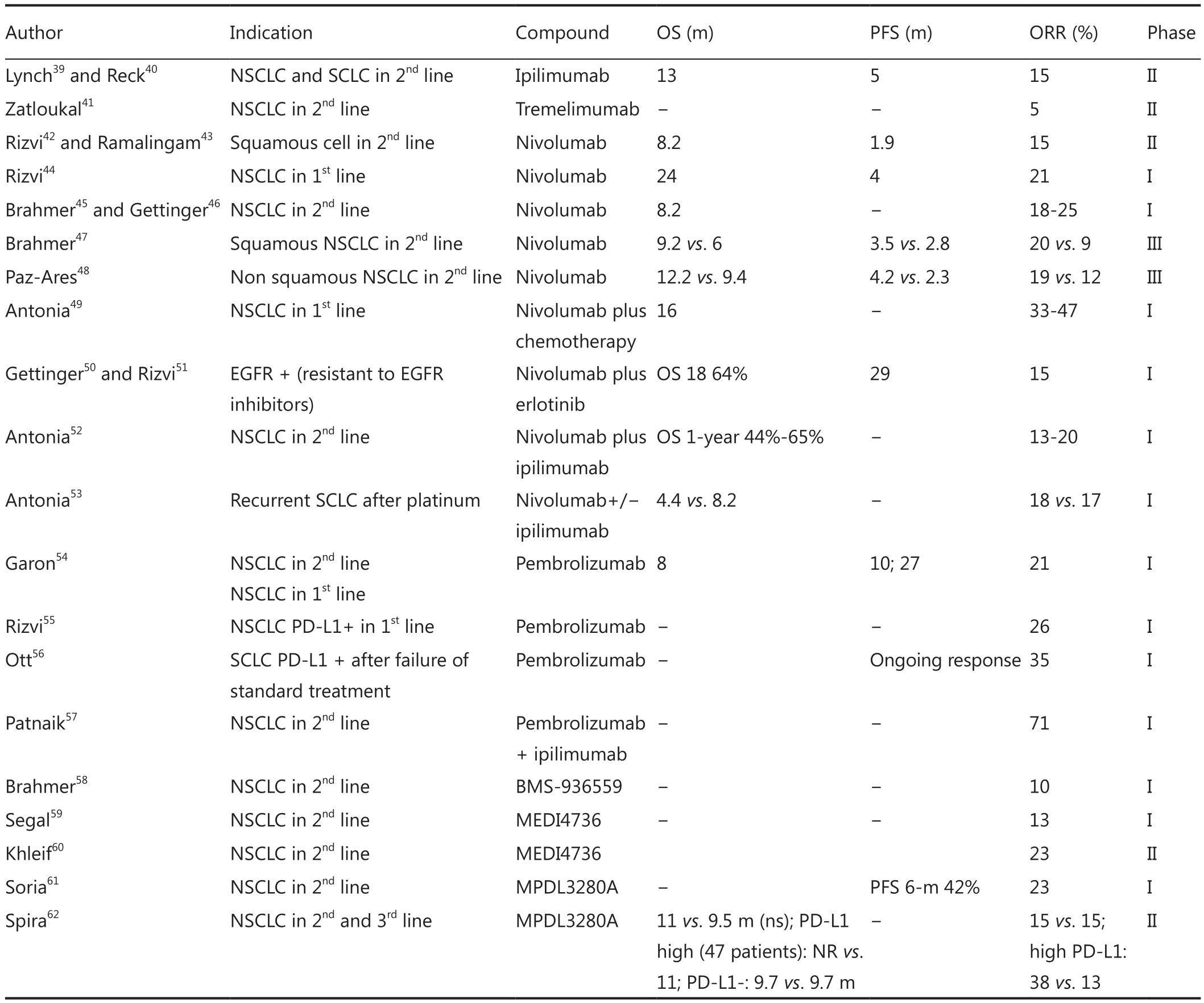
Table 1 Results of trials with PD-1 and PD-L1 inhibitors
CheckMate 063 assessed safety profile of nivolumab in squamous NSCLC.This was a phase II open label, multinational and multicenter single arm trial in 117 patients.Nivolumab was given to squamous NSCLC patients who had progressed to platinum based therapy and second line systemic chemotherapy.As with CheckMate 017, this trial included patients regardless of PD-L1 status.The most common adverse reactions were fatigue (50%), dyspnea (38%), musculoskeletal pain (36%),decreased appetite (35%), cough (32%), nausea (29%), and constipation (24%).There were two treatment-associated deaths caused by pneumonia and ischemic stroke that occurred in patients with multiple comorbidities and progressive disease.In 27% of patients, nivolumab was stopped due to severe adverse reactions.Seventeen out of 117 patients (ORR: 15%; 95% CI:9-22) had partial responses with durability ranging from 1.9 to 11.5 months; 59% had responses of 6 months or longer42.
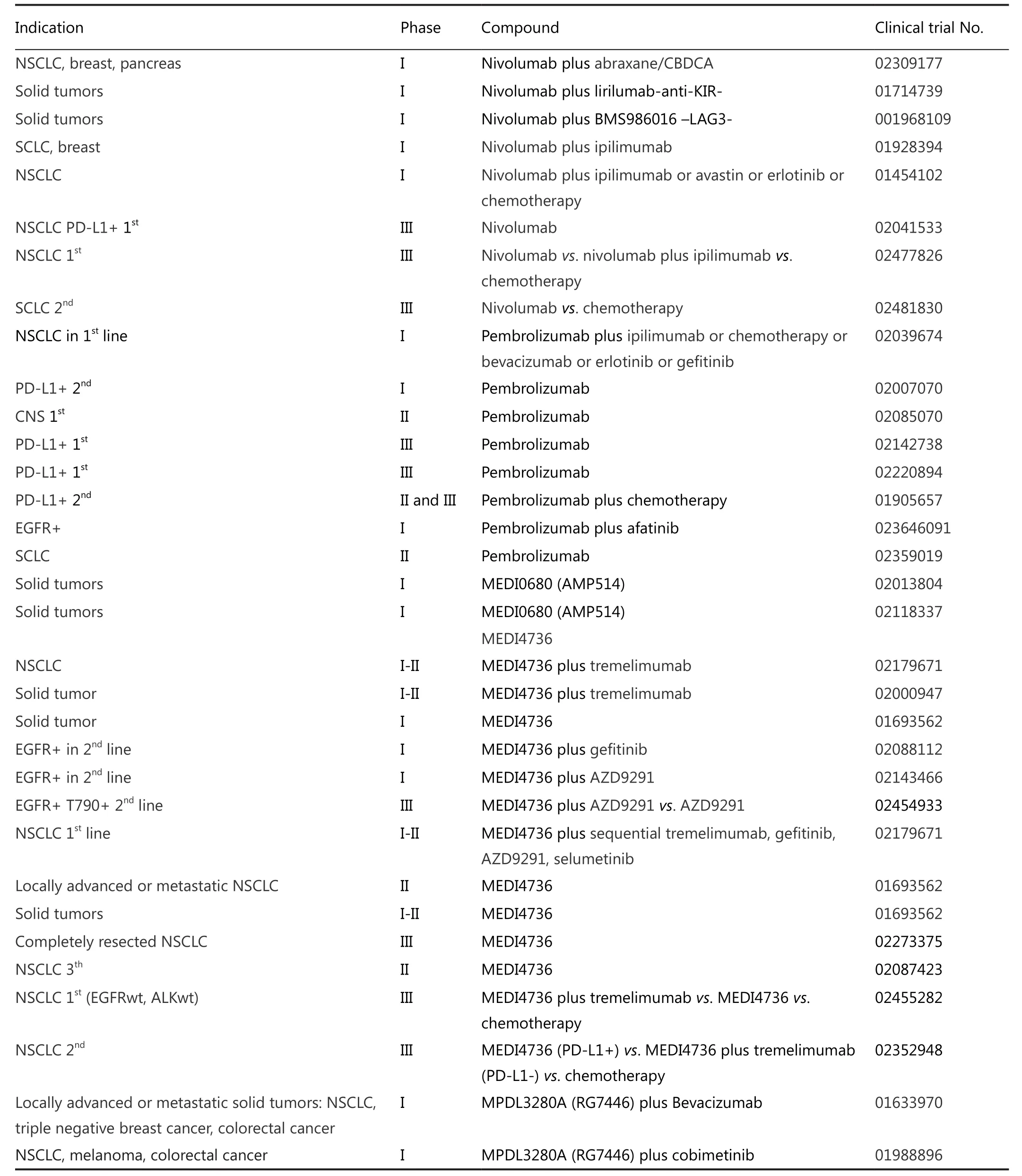
Table 2 Current trials on anti PD-1 and anti PD-L1 inhibitors
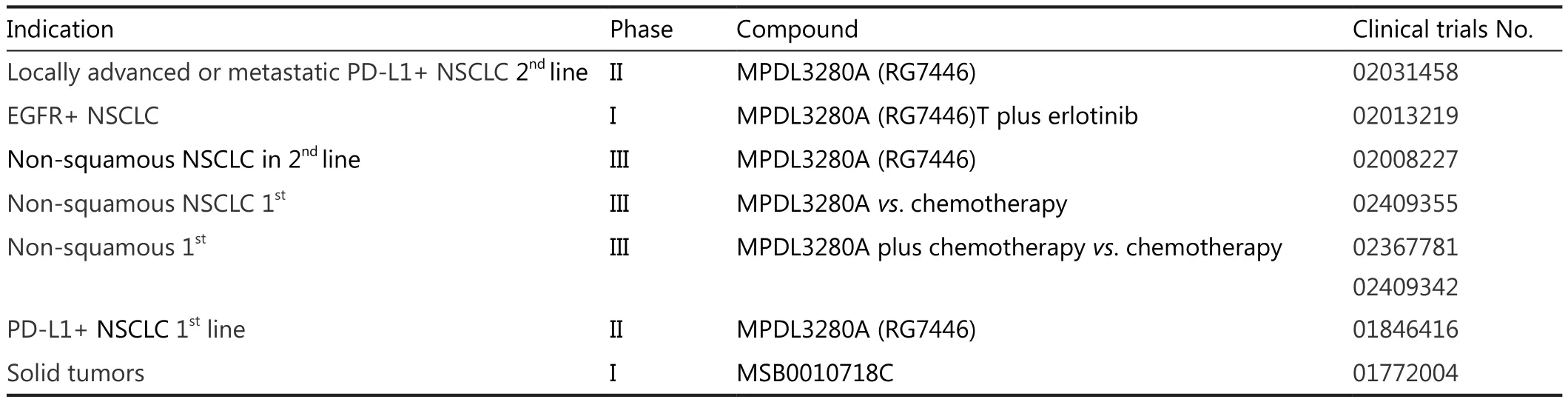
Table 2 Current trials on anti PD-1 and anti PD-L1 inhibitors
A phase III trial (CheckMate 057) randomized 582 patients with advanced non-squamous NSCLC after failing platinum doublet chemotherapy to nivolumab at 3 mg/kg i.v.every 2 weeks (n=292) or docetaxel (n=290).The study was stopped early after the primary endpoint of improved OS was reached.Median OS was 12.2 months with nivolumab vs.9.4 months with docetaxel (HR =0.73; 95% CI: 0.59-0.89; P=0.00155),with a 1-year OS of 50.5% vs.39.0% for docetaxel.ORR was 19%vs.12% (P=0.0246).Fifty-two percent of the PD-1 inhibitor responses are still ongoing compared with 14% of the docetaxel responses48(Table 1).
Results from a phase III trial CheckMate 026 comparing nivolumab versus chemotherapy in the first line setting for PDL1 positive NSCLC patients are pending (NCT02041533).
There is also an ongoing phase I trial with multiple arms using combinations of nivolumab with chemotherapy, bevacizumab,ipilimumab or erlotinib (CheckMate 012, NCT01454102).
Now a phase III trial is planned to test the combination of nivolumab plus ipilimumab vs.chemotherapy in the first line setting (CheckMate 227, NCT02477826).
In small cell lung cancer (SCLC) a phase III trial will test nivolumab vs.topotecan in the second line setting (CheckMate 331, NCT02481830).
Preliminary results of platinum doublets combined with nivolumab have showed similar response rates when compared to chemotherapy alone in the first line setting (ORR: 33%-47%)and OS rate was 86 % at 18 months in the nivolumab combined with carboplatin and taxol arm49.
Pembrolizumab (MK-3475) is an anti PD-1 monoclonal antibody from Merck Sharp & Dohme laboratory.Data from a phase Ib trial in previously treated patients showed an ORR of 21% by RECIST1.1 (26% in treatment na?ve and 20% in previously treated patients).In treatment na?ve patients, median PFS was 27 weeks, and median overall survival was not reached(OS at 6 months was 86%).In previously treated patients,median PFS was 10 weeks and OS 8.2 months.In the pooled population, median PFS was 13 weeks and 6-month PFS rate was 30%; median OS was 8.2 months with a 6-month OS rate of 64%.The drug was well tolerated with grade 2-4 adverse events in 10% of cases, most commonly pneumonitis54,64.In view of these results, pembrolizumab (formerly known as lambrolizumab) was designated as breakthrough therapy for lung cancer treatment by the FDA.
Currently pembrolizumab is being compared with docetaxel in a phase II/III trial in advanced PD-L1 positive NSCLC(NCT01905657).Pembrolizumab is administered every 3 weeks at two different doses (2 mg/kg i.v.every 3 weeks and 10 mg/kg i.v.every 3 weeks) vs.docetaxel (75 mg/m i.v.every 3 weeks).
A phase I trial in PD-L1 positive NSCLC with pembrolizumab(NCT02039674) is currently ongoing, as is a phase I/II trial with ipilimumab or chemotherapy in combination with pembrolizumab (NCT02039674) and a phase II trial of adjuvant pembrolizumab after chemo-radiotherapy for stage III NSCLC patients (NCT02343952) (Table 2).
In addition to the above mentioned trials, two phase III trials of pembrolizumab at a fixed dose of 200 mg i.v.every 3 weeks for up to 35 treatments is also being compared in the first line setting vs.platinum based chemotherapy in PD-L1 positive and PD-L1 strong positive NSCLC patients (Keynote 042, NCT02220894 and Keynote 024, NCT02142738) (Table 2).
Data in SCLC patients were presented in May 2015 as preliminary results of an ongoing multi-cohort, phase Ib study of pembrolizumab in patients with PD-L1+ advanced solid tumors(Keynote 028).The SCLC cohort had an ORR of 35% with durable responses56(Table 1).
An ongoing phase II trial is testing pembrolizumab in patients with extensive stage small cell lung cancer after completion of combination chemotherapy (NCT02359019) (Table 2).
Anti PD-L1 drugs
BMS-936559 is a fully humanized IgG4 monoclonal antibody against PD-L1.This anti PD-L1 was used in 207 patients with advanced stage solid tumors, 75 of whom had NSCLC.The results of the study showed an ORR close to 10% and stable disease for up to 6 months in 18% patients58.However, further development of this drug has been halted by Brystol Meyer Squibb (Table 1).
MEDI4736 (Astra Zeneca) is a monoclonal antibody designed with a mutated FC domain in order to prevent antibody-dependent cell mediated cytotoxicity (ADCC).Preliminary results of a phase I trial in patients with different solid tumor types including NSCLC reported clinical benefit and durable disease control with no dose limiting toxicities or grade 3-4 toxicities60.Objective response was seen in 23% of patients with pretreated NSCLC (12 out of 53 evaluable patients) in the phase II trial60.Results reported at the 2014 ASCO annual meeting showed the drug was well tolerated at all tested doses.Doses were escalated from 0.1 to 10 mg/kg every 2 weeks,with extension to 15 mg/kg every 3 weeks65.Pneumonitis,hyperglycemia and colitis were not reported.
Preliminary results from an ongoing study with 346 patients with solid tumors, of whom 143 had NSCLC, used MEDI4736 at dosages of 10 mg/kg every 2 weeks for 1 year.Only 6% of patients had grade 3-4 drug related serious adverse events.The median treatment duration was 8 weeks, and activity was seen as early as 6 weeks.After finishing active therapy, ORR in NSCLC was 13%59.
Adjuvant MEDI4736 after chemo-radiotherapy for unresectable stage III NSCLC is currently being tested in a phase III trial (10 mg/kg i.v.every 2 weeks) (NCT02125461).Safety of doses of 0.1-10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks is also been evaluated in a phase I trial in advanced solid tumors including NSCLC (NCT01693562).The anti CTLA-4 IgG2 monoclonal antibody tremelimumab is also being tested in combination with MEDI4736 in a phase Ib trial in NSCLC.(NCT02000947), and a phase III trial in the first line setting will compare the combination with tremelimumab vs.MEDI4736 as single agent vs.chemotherapy in advanced NSCLC according to PD-L1 status (Table 2).
For EGFR positive patients with T790 mutation, a phase III trial is comparing AZD9291 as single agent vs.combination with MEDI4736 (NCT02143466).Other ongoing phase I trials are combining small molecules with MEDI4736 (NCT02179671,NCT02143466).There is currently a phase II trial recruiting PD-L1 positive patients who have received at least two prior systemic treatment regimens including one platinum-based chemotherapy regimen to receive MEDI4736 (NCT02087423)(Table 2).
Atezolizumab MPDL3280A from Hoffman la Roche is also an anti-PD-L1 monoclonal antibody, IgG type with Fc domain engineered modification to avoid ADCC.Preliminary phase I results in previously treated advanced NSCLC patients reported a response rate of 23% in squamous and non-squamous NSCLC,24-week PFS was 48% and median time to response was 12 weeks61,66.Results from the phase II POPLAR study presented at the 2015 ASCO annual meeting showed that atezolizumab improved median OS compared with docetaxel in previously treated patients with PD-L1 strong positive NSCLC.For the 47 patients with high PD-L1 expression from 277 included in the study, median OS was not reached in those treated with atezolizumab vs.11 months in patients treated with docetaxel (HR =0.46; 95% CI: 0.19-1.09).In high expression PD-L1 patients, ORR was 38% with atezolizumab vs.13% with chemotherapy62(Table 1).
The FIR trial is completed and results are pending.This is a phase II study using this drug in the first setting of PD-L1 positive NSCLC patients (NCT01846416).Other ongoing trials are a phase I study assessing the combination of erlotinib and MPDL3280A in EGFR mutated adenocarcinoma(NCT02013219), a phase III trial where MPDL3280A is being tested at a dose of 1,200 mg i.v.every 3 weeks vs.docetaxel at dose of 75 mg/m2i.v.every 3 weeks after chemotherapy failure(NCT02008227), a phase III trial in the first line setting in nonsquamous NSCLC (NCT02409355) and three phase III trials testing the combination of MPDL3280A in the first line setting of non-squamous NSCLC (NCT022367781, NCT02409342,NCT02367794).
Anti CTLA-4 antibodies
Ipilimumab (anti CTL-4 antibody) is a fully humanized Ig G1 monoclonal antibody approved for metastatic melanoma.It was tested in a phase II trial in 334 lung cancer patients (204 NSCLC and 104 SCLC patients) in the first line setting.The trial featured two ipilimumab arms in combination with chemotherapy(paclitaxel plus carboplatin), one arm in a phased schedule and the other one in concomitant schedule vs.a third arm of chemotherapy alone.Median PFS was longer with the phased combination (5.1 vs.42 months; HR =0.69; 95% CI: 0.48-1.00;P=0.02).Subgroup analysis showed higher activity in squamous cell lung cancer.Toxicity was moderate with grade 3-4 toxic effects in 15% of patients39.A phase III trial with ipilimumab in combination with chemotherapy in a phased schedule in squamous NSCLC was completed and results are pending(NCT01285609).Ipilimumab is also being tested in SCLC(NCT01331525, NCT01450761, and NCT02046733) and in combination with ALK and EGFR inhibitors (NCT01998126).
Tremelimumab is a monoclonal antibody similar to ipilimumab and is developed simultaneously.Although tremelimumab is similar to ipilimumab, the pivotal trail in advanced melanoma was negative, and this was the reason why clinical development of the drug was stopped during years.Tremelimumab was also tested in NSCLC in a phase II study including pre-treated patients.Patients were randomized to tremelimumab or best supportive care after four cycles of a platinum combination.The response rate was poor,just 5%, and there were no differences in PFS41.
This drug is now being tested in combination with the target drug gefitinib (NCT02040064), with the anti PD-L1 MEDI4736 (NCT02000947, NCT02179671) and with the OX-40 agonist MEDI6469 (NCT02205333) (Table 2).
PD-L1 immunohistochemical expression and response to therapy
Immunohistochemistry (IHC) has been used to measure PDL1 expression in cancer cells, as well as in tumor infiltrating lymphocytes67.Interpretation of outcomes is complicated due to the range of techniques and because there are different antibodies used by every pharmaceutical company.Also, timing of the biopsy is a variable that can affect results since expression of the PD-L1 changes during tumor evolution68,69.The cut-off at which PDL1 positivity is considered positive, is an important factor for the interpretation of results.For example, 1% of cut-off has been used in studies with pembrolizumab and depending on this percentage,negativity, light positivity or intense positivity has been defined.Taking into account these different cut-off values, there is a reported 30% of intense positivity of PD-L1 for NSCLC.In the reported studies with nivolumab, a 5% of membrane staining of tumor cells was considered as positive.About 33%-48% of tumor samples were PD-L1 positive in the nivolumab studies.In the studies of MPDL3280A, PD-L1 positivity criteria included 5% of IHC staining on tumor infiltrating lymphocytes and tumor cells.According to these criteria, 25% of NSCLC samples were positive for PD-L1 expression66.
Further studies are required to demonstrate if PD-L1 expression by IHC correlates with a higher response rate when tumor cells are positive for staining.Median response rate of 38% in PD-L1 positive patients (ranging from 23% to 83%) vs.7%(ranging from 0%-15%) in PD-L1 negative patients have been
A pembrolizumab trial, Keynote 001, has shown improved ORR in patients with positivity for PD-L1 expression.A total of 495 patients were assigned to receive pembrolizumab(at a dose of either 2 or 10 mg per kilogram of body weight every 3 weeks or 10 mg per kilogram every 2 weeks) to either a training group (182 patients) or a validation group (313 patients).Results of PD-L1 expression were reported as the percentage of neoplastic cells with PD-L1 membrane staining;objective responses among all patients was 19.4%, and the median duration of response was 12.5 months.The median PFS was 3.7 months, and median OS was 12.0 months.Cutoff for the training group was PD-L1 expression in at least 50% of tumor cells.A response rate of 45.2% was seen among patients with a proportion score of at least 50% in the validation group, and, among patients with a proportion score of at least 50%; median PFS was 6.3 months while median OS was not reached68,72.Other studies as the study from Garon et al.72reported higher response rate and longer survival for PD-L1 positive cases.
In other types of tumors like melanoma, the predictive value of PD-L1 can be different, as the response rate in PDL1 negative cases is higher than in PD-L1 negative lung cancer cases73.In melanoma, PD-L1 expression, as well as the presence of infiltrating CD8 lymphocytes, has been described as a better predictive factor for response to anti PD-1 drugs than considering only the PD-L1 expression74.
Synergistic combinations and innovative approaches
Synergy can be achieved by targeting different immune checkpoints with combinations, as combining antibodies anti checkpoints with target drugs, antiangiogenic drugs or chemotherapy.
After promising activity in melanoma was reported, trials in lung cancer demonstrated that the combination of a PD-1 inhibitor and anti CTLA-4 antibody was active, with an ORR of 13%-20%52.Several ongoing trials are testing different combinations in lung cancer, including several phase III trials (Table 2).
In a phase I/II clinical trial (CheckMate 032), nivolumab was studied with or without ipilimumab for treatment of recurrent SCLC.This open-label study randomized patients to nivolumab 3 mg/kg i.v.every 2 weeks or nivolumab plus ipilimumab (1+1,1+3 or 3+1 mg/kg) i.v.every 3 weeks for four cycles, followed by nivolumab 3 mg/kg every 2 weeks.ORR was 18% with nivolumab and 17% with nivolumab/ipilimumab.Median OS was 4.4 months with nivolumab monotherapy and 8.2 months with combination therapy53(Table 1).
Interim results from a phase I study evaluating pembrolizumab plus ipilimumab in patients with recurrent NSCLC reported an ORR of 71%, showing a decrease in target lesion burden57(Table 1).
Another possible combination is MAPK pathway inhibitors.In the past two decades, targeted therapies in NSCLC, mainly EGFR inhibitors such as erlotinib, gefitinib, afatinib and ALK and ROS-1 inhibitors like crizotinib, have developed rapidly and have shown high response rates75.The possible combinations with immunotherapy could presumably allow long lasting effects by means of enhanced tumor antigen presentation by dendritic cells after apoptosis, necrosis and immune checkpoint blockade,allowing infiltrating cytotoxic T cells to attack tumor cells76.
Akbay et al.77showed that in murine lung tumor cells there is upregulation of tumor PD-L1 via mutant EGFR signaling,and that in preclinical models there was a survival benefit when therapeutic blockade of the PD-L1 pathway was attempted.
A study analyzing biopsies from 123 NSCLC patients reported 56 EGFR mutant and 29 KRAS mutant tumors,with a significant correlation of EGFR mutation with PDL1 expression, and KRAS mutation with PD-1 expression.Moreover, EGFR positive patients treated with EGFR inhibitors had better survival when they had PD-L1 molecule expression.In a phase I trial, erlotinib plus nivolumab showed a median PFS of 29 months, an OS rate of 64% at 18 months and ORR of 15%78.
Trials testing the combination of erlotinib, gefitinib, afatinib or novel anti EGFR drugs plus ipilimumab, nivolumab,MPDL3280A and tremelimumab are currently ongoing.Also a phase I trial is ongoing testing the combination for ALK positive of ceritinib and nivolumab tumors (NCT02393625) (Table 2).
Several trials are currently assessing different aspects of the combination of antiangiogenic and immunotherapy, including a phase I trial evaluating the safety and tolerability of nivolumab as maintenance therapy in combination with bevacizumab in NSCLC (NCT01454102) (Table 2).
With regard to combinations with chemotherapy, ipilimumab was studied in a phase III trial with dacarbazine for advanced melanoma.OS was significantly longer in the arm receiving ipilimumab plus dacarbazine than in the group receiving dacarbazine plus placebo (11.2 vs.9.1 months; HR for death=0.72; P<0.001)79.Ipilimumab is currently being studied in combination with lenalidomide for advanced or metastatic cancers with no available standard therapy (NCT01750983).As we have previously mentioned, ipilimumab was studied in a phase II trial in chemotherapy na?ve patients with NSCLC.Patients were randomly assigned to receive paclitaxel and carboplatin with either placebo or concurrent ipilimumab or phased ipilimumab.The study met its primary end point of improvement in terms of immune-related progression-free survival (irPFS) for phased ipilimumab vs.the control (HR =0.72; P=0.05), but not for the concurrent arm (HR =0.81; P=0.13)39.Nivolumab has also been studied in a phase I trial in combination with carboplatin and nab-paclitaxel in stage IIIB and IV NSCLC (NCT02309177).There is also a phase I multi-arm study of nivolumab in combination with gemcitabine/cisplatin, pemetrexed/cisplatin,carboplatin/paclitaxel, bevacizumab maintenance, erlotinib,ipilimumab or as monotherapy in subjects with stage IIIB/IV NSCLC (NCT02309177) (Table 2).
Radiation therapy has been described to induce tumor regression in non-irradiated sites, and this rare phenomenon is called the “abscopal effect”.There was a case of a melanoma patient treated with ipilimumab and radiotherapy; the patient had a systemic response to localized radiotherapy after progressing on ipilimumab.Non-irradiated areas also regressed and disease remained stable and minimal months later80.Radiotherapy has been shown to enhance antigen presentation by myeloid cells within the tumor microenvironment, therefore increasing T-cell killing of the malignant cells, a mechanism which is thought to mediate the abscopal effect.Combinations of radiotherapy with immune checkpoint inhibitors are currently being studied.For example, there is an ongoing phase II trial of ipilimumab with stereotactic body irradiation in advanced lung cancer (NCT02239900), pembrolizumab with hypofractionated stereotactic radiation therapy (NCT02444741), and pembrolizumab with concurrent chemo-radiation for SCLC(NCT02402920).
Toxicity
When combining ipilimumab with PD-1 inhibitors like nivolumab in melanoma, drug-related adverse events of grade 3 or 4 were reported in 53% of patients compared with 18% of patients who received ipilimumab monotherapy72,81,82.Grade 3 or 4 adverse events, regardless of attribution, were observed in 72%of patients, and grade 3 or 4 treatment-related events were noted in 53%, with the most common events being elevated levels of lipase (in 13% of patients), aspartate aminotransferase (in 13%),and alanine aminotransferase (in 11%).A total of 6 in 28 patients(21%) had grade 3 or 4 treatment-related events that were doselimiting.
Even though ipilimumab is generally well tolerated,severe immune mediated side effects have been observed including enterocolitis, hepatitis, dermatitis, neuropathies, and endocrinopathies.These reactions can manifest during treatment or even months after discontinuation of ipilimumab.In the phase III study of ipilimumab, enterocolitis was the most common severe toxicity, seen in 34 of 511 patients.One percent of patients had bowel perforation and 0.8% died83,84.Treatment of severe reactions mainly consists of discontinuation of ipilimumab and initiation of systemic corticosteroids at a dosage of 1 to 2 mg/kg per day of prednisone or equivalent with taper over a period of 1 month once the toxicities have considerably improved85.
Nivolumab is a better tolerated drug.The most common adverse reactions (≥20%) reported in a phase III clinical trial were fatigue (50%), dyspnea (38%), musculoskeletal pain (36%),decreased appetite (35%), cough (32%), nausea (29%), and constipation (24%).Only 6% of grade 3 or 4 toxicities are seen.Skin toxicity, including rash, pruritus, and vitiligo are the most commonly seen reactions (31% of the cases).Diarrhea was seen in 11% of cases and pneumonitis in 3%.Transaminitis was seen in 11% and thyroid abnormalities in 3%.Treatment of severe reactions consists of withdrawal of the drug and, if required,prednisone 1 to 2 mg/kg daily should be given until the patient is back at baseline and then tapered over a month86.
With pembrolizumab, 13% of patients experienced grade 3 or 4 drug-related adverse events.Common low grade reactions were fatigue, asthenia, fever, chills, myalgias and headaches.Pneumonitis was reported in 4% of cases and grade 3 transaminitis.Toxicities improved after pembrolizumab discontinuation and corticosteroid therapy.Only one case of grade 3 diarrhea was reported, which improved without steroids,and 8% of hypothyroidism which improved with thyroid hormone replacement72.
Other immunomodulatory and agonistic molecules
Other immune checkpoint antibodies are currently been studied as single agents, and more interestingly, in combination with other checkpoint inhibitors.The most relevant immune checkpoints currently under clinical investigation are lymphocyte activation gene -3 (LAG-3), KIR, OX 40, GITR,and 4-1BB.
As for LAG-3, preclinical studies have shown co-expression of LAG-3 and PD-1 on tumor infiltrating cells.Combinations of anti-LAG-3 antibodies with anti PD-1 antibodies showed decreased tumor growth and enhanced antitumor immunity,and also maintained immune homeostasis with decreased autoimmune responses87.There is currently an ongoing phase I trial testing anti-LAG-3 in advanced NSCLC (NCT01968109).
KIR is a molecule in the NK cell that binds HLA molecules to the surface of tumoral cells, inhibiting NK lymphocyte cytotoxic activity against malignant cells.If KIR is blocked, then NK cells are unblinded and can recognize and attack tumoral cells88.Lirilumab is an anti-KIR antibody currently in phase I trials in NSCLC (NCT01750580).
GITR affects regulatory T cells (Tregs) and also acts as a costimulatory receptor expressed after T cell activation that enhances T cell function and survival.Treatment with GITR agonistic antibody destabilizes intra-tumor Tregs allowing for more efficient cytolysis by CD8+T cells89.A phase I trial with anti-GITR antibody TRX-518 is ongoing in advanced solid tumors (NCT01239134).
4-1BB, also known as CD137 receptor, a member of the TNF family of receptors, is an immunomodulatory molecule expressed in immune cells.Urelumab (BMS 663513) is a CD137 agonist that has been tested in a phase I/II study with promising activity, although marked hepatic toxicities have been reported.A study of urelumab in combination with chemoradiotherapy for NSCLC has been terminated (NCT00461110)and the combination with nivolumab is being tested in solid tumors and B cell non-Hodgkin lymphoma (NCT02253992)90.There is an ongoing trial with 4-1BB agonist PF-05082566 plus PD-1 inhibitor MK-3475 in patients with solid tumors(NCT02179918)90.
OX40 promotes T cell survival and expansion.Preclinical studies showed that OX40 agonists increase anti-tumor immunity and improve disease free survival.Patients treated with one course of the antiOX40 mAb showed regression of at least one metastatic lesion in 12/30 patients and an acceptable toxicity profile91.
Cancer vaccines
Two types of vaccines, antigen specific and cell-based, are currently being studied in lung cancer.
Antigen specific vaccines
Several vaccines target tumor specific antigens, as those against melanoma-associated antigen-A3 (MAGE-3), MUC-1, EGFR,human telomerase reverse transcriptase (hTERT) or NYESO-1.
MAGE-A3: 35%-42% of NSCLCs have expression of MAGE-A3, which is presented to T cells.A phase II trial showed a correlation between the expression of a gene signature and immune related transcripts associated with better outcome when a MAGE A3 vaccine was used in NSCLC92.
L-BLP25 (stimuvax) targets the peptide MUC1 which is overexpressed in lung cancer and associated with poor prognosis93.Early trials with L-BLP25 were promising but the phase III trial did not meet its primary endpoint of OS94.Two studies are currently ongoing to evaluate L-BLP25, one in combination with bevacizumab for inoperable patients with stage III-IV NSCLC following chemo/radiotherapy(NCT00828009), and another in Asian patients is pending results (NCT01015443).
rEGF: 40%-80% of NSCLCs express EGFR.Therefore a vaccine composed of humanized recombinant EGF is being studied.Two trials were started but terminated to initiate a new phase III with a biomarker to enrich the population.Median OS was 11.7 months for patients with anti-EGF antibody response vs.3.6 months for non-responders95.A phase III trial is now planned (NCT01444118).
Anti-telomerase-based vaccine GV1001 showed clinical benefit in a phase I and II trial, with PFS improvement in inoperable NSCLC after chemoradiation (19 months in responders vs.3.5 months in non-responders, P=0.001).Responders were those who developed GV1001-specific T cell memory responses and had IFN-γ (high)/IL-10(low)/IL-4(low)cytokine profiles96.
An NY-ESO-1 vaccine achieved antibody responses in 9 of 10 patients.Of these 10, 2 patients with lung cancer and 1 with esophageal cancer showed stable disease97.A clinical trial in cancer patients with tumors expressing NY-ESO-1 tested the vaccine with or without sirolimus (NCT01522820).
Cell based vaccines
Belagenpumatucel-L (Lucanix) is composed of five transforming growth factor B2 (TGF-B2) antisense gene-modified allogenic NSCLC cell lines.In a phase III clinical trial for those patients that were included after completing 12 weeks of chemotherapy,survival analysis showed improvement in overall survival (20.7 vs.13.4 months, P=0.8) and for those patients treated with radiotherapy, median survival was 40 vs.10 months (P=0.036)98.
Tergenpumatucel-L consists of genetically modified allogeneic NSCLC tumor cells lines with the alpha-(1,3)-galactosyltransferase (αGal) moiety on the cell surfaces which generates an innate immune reaction, killing the foreign NSCLC tumor cells.In a phase II clinical trial, 28 patients with metastatic NSCLC or recurrent disease received the vaccine with a median OS of 11.3 months and a long stabilization in 8 of 28 patients with one patient alive after 50 months of follow-up.Patients who received salvage chemotherapy after progressing on tergenpumatucel-L had a better OR to subsequent chemotherapy treatments than patients who had not received prior tergenpumatucel-L99.
Peptide vaccines have been also tested in solid tumors, such as the peptide vaccine against the indoleamine 2,3-dioxygenase(IDO) enzyme.In a phase I trial in 15 advanced NSCLC,HLA-A2 positive patients, one patient developed a partial response after 1 year of treatment with the vaccine, and longlasting stable disease was demonstrated in further six patients.Median OS was 25.9 months.Furthermore, expression of IDO was detected in nine of ten tumor biopsies by IHC.In longterm analyses of two clinically responding patients, the ratio of kynurenine/tryptophan in serum (Kyn/Trp) remained stable100.
Conclusion
New strategies to treat lung cancer, inducing durable responses to therapy, are currently being developed.The incorporation of immunotherapy to the arsenal of drugs for treatment of lung cancer is a promising approach to reach these goals, with low rates of side effects.
Personalized medicine currently offers the best profile in terms of side effects and efficacy in different cancers, and combination with immune checkpoint blockers could enhance its activity.
In view of promising results reported, now the challenge is finding those biomarkers that can help us select the best treatment approach for every patient.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Finn OJ.Cancer immunology.N Engl J Med 2008;358:2704-2715.
2.Roithmaier S, Haydon AM, Loi S, Esmore D, Griffiths A, Bergin P, et al.Incidence of malignancies in heart and/or lung transplant recipients: a single-institution experience.J Heart Lung Transplant 2007;26:845-849.
3.Anagnostou VK, Brahmer JR.Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer.Clin Cancer Res 2015;21:976-984.
4.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al.Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion.Nat Med 2002;8:793-800.
5.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al.Programmed death ligand-1 expression in nonsmall cell lung cancer.Lab Invest 2014;94:107-116.
6.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ.Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses.Immunity 2007;27:111-122.
7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC,McDermott DF, et al.Safety, activity, and immune correlates of anti-PD-1 antibody in cancer.N Engl J Med 2012;366:2443-2454.
8.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al.Phase I study of single-agent anti-programmed death-1(MDX-1106) in refractory solid tumors: safety, clinical activity,pharmacodynamics, and immunologic correlates.J Clin Oncol 2010;28:3167-3175.
9.Lenschow DJ, Walunas TL, Bluestone JA.CD28/B7 system of T cell costimulation.Annu Rev Immunol 1996;14:233-258.
10.Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, et al.Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4.Cell 1992;71:1093-1102.
11.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al.Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer.J Clin Oncol 2010;28:105-113.
12.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al.Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in nodepositive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98.J Clin Oncol 2013;31:860-867.
13.Fridman WH, Pages F, Sautes-Fridman C, Galon J.The immune contexture in human tumours: impact on clinical outcome.Nat Rev Cancer 2012;12:298-306.
14.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al.Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer.N Engl J Med 2003;348:203-213.
15.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK,Ledbetter JA.CTLA-4 is a second receptor for the B cell activation antigen B7.J Exp Med 1991;174:561-569.
16. Thompson CB, Allison JP.The emerging role of CTLA-4 as an immune attenuator.Immunity 1997;7:445-450.
17.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ,Green JM, et al.CTLA-4 can function as a negative regulator of T cell activation.Immunity 1994;1:405-413.
18.Kearney ER, Walunas TL, Karr RW, Morton PA, Loh DY,Bluestone JA, et al.Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4.J Immunol 1995;155:1032-1036.
19.Krummel MF, Allison JP.CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation.J Exp Med 1995;182:459-465.
20.Krummel MF, Sullivan TJ, Allison JP.Superantigen responses and co-stimulation: CD28 and CTLA-4 have opposing effects on T cell expansion in vitro and in vivo.Int Immunol 1996;8:519-523.
21.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA,Sharpe AH.Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4.Immunity 1995;3:541-547.
22.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al.Role of LAG-3 in regulatory T cells.Immunity 2004;21:503-513.
23.Gandhi MK, Lambley E, Duraiswamy J, Dua U, Smith C, ElliottS, et al.Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigenspecific CD8+ T-cell function in Hodgkin lymphoma patients.Blood 2006;108:2280-2289.
24.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ,et al.The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity.Nat Immunol 2005;6:1245-1252.
25.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A,Zheng XX, et al.Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance.Nat Immunol 2003;4:1102-1110.
26.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, et al.TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression.PLoS One 2012;7:e30676.
27.Zhuang X, Zhang X, Xia X, Zhang C, Liang X, Gao L, et al.Ectopic expression of TIM-3 in lung cancers: a potential independent prognostic factor for patients with NSCLC.Am J Clin Pathol 2012;137:978-985.
28.Zhou P, Shaffer DR, Alvarez Arias DA, Nakazaki Y, Pos W, Torres AJ, et al.In vivo discovery of immunotherapy targets in the tumour microenvironment.Nature 2014;506:52-57.
29.Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, et al.VISTA regulates the development of protective antitumor immunity.Cancer Res 2014;74:1933-1944.
30.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A,Lee KP, et al.Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4.Science 1995;270:985-988.
31.Chambers CA, Sullivan TJ, Allison JP.Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells.Immunity 1997;7:885-895.
32.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK,Anderson AC.Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity.J Exp Med 2010;207:2187-2194.
33.Pardoll DM.The blockade of immune checkpoints in cancer immunotherapy.Nat Rev Cancer 2012;12:252-264.
34.Karwacz K, Bricogne C, MacDonald D, Arce F, Bennett CL,Collins M, et al.PD-L1 co-stimulation contributes to ligandinduced T cell receptor down-modulation on CD8+ T cells.EMBO Mol Med 2011;3:581-592.
35.Chen YB, Mu CY, Huang JA.Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study.Tumori 2012;98:751-755.
36.Chen YY, Wang LB, Zhu HL, Li XY, Zhu YP, Yin YL, et al.Relationship between programmed death-ligand 1 and clinicopathological characteristics in non-small cell lung cancer patients.Chin Med Sci J 2013;28:147-151.
37.Lee SY, Choi HK, Lee KJ, Jung JY, Hur GY, Jung KH, et al.The immune tolerance of cancer is mediated by IDO that is inhibited by COX-2 inhibitors through regulatory T cells.J Immunother 2009;32:22-28.
38.Srivastava MK, Andersson A, Zhu L, Harris-White M, Lee JM, Dubinett S, et al.Myeloid suppressor cells and immune modulation in lung cancer.Immunotherapy 2012;4:291-304.
39.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al.Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer:results from a randomized, double-blind, multicenter phase II study.J Clin Oncol 2012;30:2046-2054.
40.Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R,et al.Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial.Ann Oncol 2013;24:75-83.
41.Zatloukal P, Heo SD, Park K, Kang J, Butts C, Bradford D, et al.Randomized phase II clinical trial comparing tremelimumab (CP-675,206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC).J Clin Oncol 2009;27:abstr 8071.
42.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK,Antonia SJ, et al.Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced,refractory squamous non-small-cell lung cancer (CheckMate 063):a phase 2, single-arm trial.Lancet Oncol 2015;16:257-265.
43.Ramalingam SS, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al.Phase II study of nivolumab (anti-PD-1,BMS-936558, ONO-4538) in patients with advanced, refractory squamous non-small cell lung cancer: metastatic non-small cell lung cancer.Int J Radiat Oncol Biol Phys 2014;90:1266-1267.
44.Rizvi NA, Shepherd FA, Antonia SJ, Bahmer JR, Chow LQ,Goldman J, et al.First-line monotherapy with nivolumab (Anti-PD-1; BMS-936558, ONO-4538) in advanced non-small cell lung cancer (NSCLC): safety, efficacy, and correlation of outcomes with PD-L1 status.Int J Radiat Oncol Biol Phys 2014;90:S31.
45.Brahmer JR, Horn L, Antonia SJ, Gandhi L, Sequist LV, Sankar V, et al.Survival and long-term follow-up of the phase I trial of nivolumab (Anti-PD-1; BMS-936558; ONO-4538) in patients(pts) with previously treated advanced non-small cell lung cancer(NSCLC).J Clin Oncol 2013;31:abstr 8030.
46.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA,et al.Long-term survival, clinical activity, and safety of nivolumab(Anti-PD-1; BMS-936558, ONO-4538) in patients (Pts) with advanced non-small cell lung cancer (NSCLC).Int J Radiat Oncol Biol Phys 2014;90:S34.
47.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE,Poddubskaya E, et al.Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer.N Engl J Med 2015;373:123-135.
48.Paz-Ares L, Horn L, Borghaei H, Spigel DR, Steins M, Ready N,et al.Phase III, randomized trial (CheckMate 057) of nivolumab(NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC).J Clin Oncol 2015;33:LBA109.
49.Antonia SJ, Brahmer JR, Gettinger S, Chow LQ, Juergens R,Shepherd FA, et al.Nivolumab (Anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy(PT-DC) in advanced non-small cell lung cancer (NSCLC).Int J Radiat Oncol Biol Phys 2014;90:S2.
50.Gettinger S, Chow LQ, Borghaei H, Shen Y, Harbison C, Chen AC, et al.Safety and response with nivolumab (Anti-PD-1;BMS-936558, ONO-4538) plus erlotinib in patients (Pts) with epidermal growth factor receptor mutant (EGFR MT) advanced non-small cell lung cancer (NSCLC).Int J Radiat Oncol Biol Phys 2014;90:S34-S35.
51.Rizvi NA, Chow LQ, Borghaei H, Shen Y, Harbison C, Alaparthy S, et al.Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC.J Clin Oncol 2014;32:abstr 8022.
52.Antonia SJ, Gettinger SN, Chow LQ, Juergens RA, Borghaei H,Shen Y, et al.Nivolumab (anti-PD-1; BMS-936558, ONO-4538)and ipilimumab in first-line NSCLC: Interim phase I results.J Clin Oncol 2014;32:abstr 8023.
53.Antonia SJ, Bendell JC, Taylor MH, Calvo E, Jaeger D, De Braud FG, et al.Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer(SCLC): CA209-032.J Clin Oncol 2015;33:abstr 7503.
54.Garon EB, Balmanoukian A, Hamid O, Hui R, Gandhi L, Leighi N, et al.Preliminary safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer(NSCLC).Annual Congress of the American Society of Clinical Oncology (ASCO) 2014.
55.Rizvi NA, Garon EB, Patnaik A, Gandhi L, Leighl NB,Balmanoukian AS, et al.Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer(NSCLC).J Clin Oncol 2014;32:abstr 8007.
56.Ott PA, Maria Elez-Fernandez ME, Hiret S, Kim DW, Moss RA,Winser T, et al.Pembrolizumab (MK-3475) in patients (pts)with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028.J Clin Oncol 2015;33:abstr 7502.
57.Patnaik A, Socinski MA, Gubens MA, Gandhi L, Stevenson J,Bachman RD, et al.Phase 1 study of pembrolizumab (pembro;MK-3475) plus ipilimumab (IPI) as second-line therapy for advanced non-small cell lung cancer (NSCLC): KEYNOTE-021 cohort D.J Clin Oncol 2015;33:abstr 8011.
58.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P,et al.Safety and activity of anti-PD-L1 antibody in patients with advanced cancer.N Engl J Med 2012;366:2455-2465.
59.Segal NH, Antonia SJ, Brahmer JR, Maio M, Blake-Haskins A, Li X, et al.Preliminary data from a multi-arm expansion study of MEDI4736,an anti-PD-L1 antibody.J Clin Oncol 2014;32:abstr 3002^.
60.Khleif S, Lutzky J, Segal N, Antonia S, Blake-Haskins A, Stewart R, et al.MEDI4736, an anti-PD-L1 antibody with modified Fc domain: Preclinical evaluation and early clinical results from a phase 1 study in patients with advanced solid tumors.Eur J Cancer 2013;49:abstr 802.
61.Soria JC, Cruz C, Bahleda R, Delord JP, Horn L, Herbst RS, et al.Clinical activity, safety, and biomarkers of a PD-L1 blockade in non-small cell lung cancer (NSCLC): additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1).Eur J Cancer 2013;49:abstr 3408.
62.Spira AI, Park K, Mazières J, Vansteenkiste JF, Rittmeyer A,Ballinger M, et al.Efficacy, safety and predictive biomarker results from a randomized phase II study comparing atezolizumab vs docetaxel in 2L/3L NSCLC (POPLAR).J Clin Oncol 2015;33:abstr 8010.
63.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al.Overall survival and long-term safety of nivolumab(Anti-programmed death 1 antibody, BMS-936558, ONO-4538)in patients with previously treated advanced non-small-cell lung cancer.J Clin Oncol 2015;33:2004-2012.
64.Garon EB, Gandhi L, Rizvi N, Hui R, Balmanoukian AS, Patnaik A,et al.Antitumor activity of pembrolizumab (pembro; MK-3475)and correlation with programmed death ligand 1 (PD-l1) expression in a pooled analysis of patients (pts) with advanced non–small cell lung carcinoma (NSCLC).Ann Oncol 2014;25:v1-v41.
65.Lutzky J, Antonia SJ, Blake-Haskins A, Li X, Robbins PB,Shalabi AM, et al.A phase 1 study of MEDI4736, an anti–PD-L1 antibody, in patients with advanced solid tumors.J Clin Oncol 2014;32:abstr 3001^.
66.Spigel DR, Gettinger SN, Horn L, Herbst RS, Gandhi L, Gordon MS, et al.Clinical activity, safety, and biomarkers of MPDL3280A,an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC).J Clin Oncol 2013;31:abstr 8008.
67.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS,et al.Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients.Nature 2014;515:563-567.
68.Gandhi L, Balmanoukian A, Hui R, Hamid O, Rizvi NA, Leighl N, et al.Abstract CT105: MK-3475 (anti-PD-1 monoclonal antibody) for non-small cell lung cancer (NSCLC): Antitumor activity and association with tumor PD-L1 expression.Cancer Res 2014;74:CT105.
69.Gettinger SN, Shepherd FA, Antonia SJ, Brahmer JR, Quan Man Chow L, Juergens RA, et al.First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety,efficacy, and correlation of outcomes with PD-L1 status.J Clin Oncol 2014;32:abstr 8024.
70.Brahmer JR, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al.Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced non-small-cell lung cancer (NSCLC):Survival and clinical activity by subgroup analysis.J Clin Oncol 2014;32:abstr 8112^.
71.Brahmer JR, Rizvi NA, Lutzky J, Khleif S, Blake-Haskins A,Li X, et al.Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC.J Clin Oncol 2014;32:abstr 8021^.
72.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al.Pembrolizumab for the treatment of non-small-cell lung cancer.N Engl J Med 2015;372:2018-2028.
73.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al.Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy.Clin Cancer Res 2014;20:5064-5074.
74.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ,Robert L, et al.PD-1 blockade induces responses by inhibiting adaptive immune resistance.Nature 2014;515:568-571.
75.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK.Nonsmall-cell lung cancers: a heterogeneous set of diseases.Nat Rev Cancer 2014;14:535-546.
76.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al.BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma.Clin Cancer Res 2013;19:1225-1231.
77.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH,Christensen CL, et al.Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors.Cancer Discov 2013;3:1355-1363.
78.D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Landi L, Minuti G, et al.PD-L1 and PD-1 expression in molecularly selected non-small-cell lung cancer (NSCLC) patients.J Thorac Oncol 2014;9:S7-S52.
79.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al.Ipilimumab plus dacarbazine for previously untreated metastatic melanoma.N Engl J Med 2011;364:2517-2526.
80.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al.Immunologic correlates of the abscopal effect in a patient with melanoma.N Engl J Med 2012;366:925-931.
81.Kong YC, Flynn JC.Opportunistic autoimmune disorders potentiated by immune-checkpoint inhibitors anti-CTLA-4 and Anti-PD-1.Front Immunol 2014;5:206.
82.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al.Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma.N Engl J Med 2015;373:23-34.
83.McDermott D, Haanen J, Chen TT, Lorigan P, O’Day S.Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial(MDX010-20).Ann Oncol 2013;24:2694-2698.
84.Danielli R, Ridolfi R, Chiarion-Sileni V, Queirolo P, Testori A, Plummer R, et al.Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy.Cancer Immunol Immunother 2012;61:41-48.
85.Yousaf N, Davidson M, Goode E, Thomas C, Hung R, Gore M,et al.The cost of ipilimumab toxicity: a single-centre analysis.Melanoma Res 2015;25:259-264.
86.McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD,Smith DC, et al.Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab.J Clin Oncol 2015;33:2013-2020.
87.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al.Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape.Cancer Res 2012;72:917-927.
88.Benson DM Jr, Cohen AD, Jagannath S, Munshi NC, Spitzer G,Hofmeister CC, et al.A phase I trial of the anti-KIR antibody IPH2101 and lenalidomide in patients with relapsed/refractory multiple myeloma.Clin Cancer Res 2015;21:4055-4061.
89.Clouthier DL, Zhou AC, Watts TH.Anti-GITR agonist therapy intrinsically enhances CD8 T cell responses to chronic lymphocytic choriomeningitis virus (LCMV), thereby circumventing LCMV-induced downregulation of costimulatory GITR ligand on APC.J Immunol 2014;193:5033-5043.
90.Kobayashi T, Doff BL, Rearden RC, Leggatt GR, Mattarollo SR.NKT cell-targeted vaccination plus anti-4-1BB antibody generates persistent CD8 T cell immunity against B cell lymphoma.Oncoimmunology 2015;4:e990793.
91.Lei F, Song J, Haque R, Haque M, Xiong X, Fang D, et al.Regulation of A1 by OX40 contributes to CD8(+) T cell survival and anti-tumor activity.PLoS One 2013;8:e70635.
92.Batchu RB, Gruzdyn O, Potti RB, Weaver DW, Gruber SA.MAGE-A3 with cell-penetrating domain as an efficient therapeutic cancer vaccine.JAMA Surg 2014;149:451-457.
93.Ohgami A, Tsuda T, Osaki T, Mitsudomi T, Morimoto Y,Higashi T, et al.MUC1 mucin mRNA expression in stage I lung adenocarcinoma and its association with early recurrence.AnnThorac Surg 1999;67:810-814.
94.Mitchell P, Thatcher N, Socinski MA, Wasilewska-Tesluk E, Horwood K, Szczesna A, et al.Tecemotide in unresectable stage III non-smallcell lung cancer in the phase III START study: updated overall survival and biomarker analyses.Ann Oncol 2015;26:1134-1142.
95.Rodriguez PC, Neninger E, Garcia B, Popa X, Viada C, Luaces P, et al.Safety, immunogenicity and preliminary efficacy of multiple-site vaccination with an epidermal growth factor (EGF) based cancer vaccine in advanced non small cell lung cancer (NSCLC) patients.J Immune Based Ther Vaccines 2011;9:7.
96.Brunsvig PF, Kyte JA, Kersten C, Sundstrom S, Moller M, Nyakas M,et al.Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial.Clin Cancer Res 2011;17:6847-6857.
97.Kakimi K, Isobe M, Uenaka A, Wada H, Sato E, Doki Y, et al.A phase I study of vaccination with NY-ESO-1f peptide mixed with Picibanil OK-432 and Montanide ISA-51 in patients with cancers expressing the NY-ESO-1 antigen.Int J Cancer 2011;129:2836-2846.
98.Giaccone G, Bazhenova L, Nemunaitis J, et al.A phase III study of belagenpumatucel-L therapeutic tumor cell vaccine for non-small cell lung cancer.2013 Europen Cancer Congress 2013:Abs LBA2.
99.Morris JC, Rossi GR, Harold N, Tennant L, Ramsey WJ, Vahanian N, et al.Potential chemo-sensitization effect of tergenpumatucel-L immunotherapy in treated ptients with advanced non-small cell lung cancer 8NSCLC).J Clin Oncol 2013;31:abstr 8094.
100.Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, et al.Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase.Clin Cancer Res 2014;20:221-232.
 Cancer Biology & Medicine2015年3期
Cancer Biology & Medicine2015年3期
- Cancer Biology & Medicine的其它文章
- Acute coronary syndrome: a rare case of multiple endocrine neoplasia syndromes with pheochromocytoma and medullary thyroid carcinoma
- Quantitative proteomic analysis for high-throughput screening of differential glycoproteins in hepatocellular carcinoma serum
- Development of targeted therapies in treatment of glioblastoma
- Fueling the engine and releasing the break: combinational therapy of cancer vaccines and immune checkpoint inhibitors
- Reality of evidence-based practice in palliative care
- Single-cell analyses of circulating tumor cells
