Ovine fetal sex determination using circulating cell-free fetal DNA (ccffDNA) and cervical mucous secretions
R Asadpour, MH Asadi, R Jafari –Joozani, GH Hamidian
1Department of Clinical Science, Faculty of Veterinary Medicine, University of Tabriz, Iran
2Department of Basic science, Faculty of Veterinary Medicine, University of Tabriz, Iran
Ovine fetal sex determination using circulating cell-free fetal DNA (ccffDNA) and cervical mucous secretions
R Asadpour1*, MH Asadi1, R Jafari –Joozani1, GH Hamidian2
1Department of Clinical Science, Faculty of Veterinary Medicine, University of Tabriz, Iran
2Department of Basic science, Faculty of Veterinary Medicine, University of Tabriz, Iran
ARTICLE INFO
Article history:
Received 23 August 2014
Received in revised form 10 December 2014
Accepted 20 December 2014
Available online 20 March 2015
Cervical mucus
Blood plasma
Fetal sexing
Ovine
Objective: To use PCR to investigate the presence of fetal SRY gene in the ovine cervical secretions and maternal blood plasma, and to assess predict fetal sex at different times of gestation in the ewe. Methods: Fetal DNA was isolated from blood plasma and cervical secretions of 32 pregnant ewes during the 6 to 21 week of gestation. Overall, 15 male and 17 female fetuses were included in this study. After DNA extraction, the PCR amplified a 280 bp fragment from the X-chromosome and a 217 bp fragment from the Y-chromosome based on a sex-related polymorphism in the amelogenin locus. Results: The presence of fetal Y-chromosome was confirmed in 11 out of 15 cervical mucus and 12 out of 15 blood plasma samples collected from sheep with male fetuses. The sensitivity and specificity of tests were 70% with false negative results. Conclusion: This is the first report on validating the presence of fetal DNA material in the ovine cervical mucus and its potential usefulness for fetal sexing.
1. Introduction
Accurate diagnosis of fetal sex in domestic species has substantial commercial and research applications[1], particularly in the livestock industry[2,3]. Prediction of fetal sex in the ovine species could be useful in the management decisions such as sex selection in breeding programs, culling decisions, and decreasing the cost of progeny testing[4]. Trans-rectal ultrasonography and karyotyping by amniocentesis have been commonly used to determine fetal sex in pregnant ewes. In the livestock industry, transrectal ultrasonography (based on location of the genital tubercle) has been a traditional method of fetal sex determination. For example, transrectal ultrasonography on days 60 to 69 of pregnancy has been used to determine fetal sex in the ewe[5, 6]. However, fetal sexing by transrectal ultrasonography requires the breed and age of animals be taken into consideration and also needs expensive pieces of equipment. Fetal fluid sampling in pregnant animals is a prerequisite of both karyotyping and measurement of testosterone level which is an invasive procedure might result in termination of pregnancy[7]. Analysis of free fetal DNA in maternal circulation is a noninvasive and useful tool to determine fetal sex in the ewe. This fetal DNA, named circulating cell free fetal DNA (ccffDNA), has emerged as a valuable source for prenatal fetal sex determination and genetic evaluation. The ccffDNA is well known in human (with hemochorial placenta), and PCR analysis of Y-specific sequences such as DYZ1[8], DYZ3[9], and sex determining region (SRY)[10]. Molecular sexing of ovine had been described using the SRY and AMELX-AMELY genes[4].
Shettles[11] for the first time showed that, during pregnancy, the fetal cells are also shed from the regressing chorionic villi into the lower uterine pole in the woman and accumulate in the cervical mucus especially at the level of the internal os. To date, several methods have been used for sampling and detection of fetal genetic material in human cervical secretions resulting in the identification of fetalcells with a varying success rates[12]. To our knowledge, there is little published information regarding the presence or absence of the fetal genetic material in the ovine plasma and cervical secretions and its potential application for fetal sexing. Therefore this study was designed to evaluate the presence of fetal DNA in the maternal blood plasma and cervical mucus also the possibility of using it to sex the ovine fetus by PCR method.
2. Materials and methods
2.1. Blood sampling and plasma separation
This study was performed on Gezel sheep (Tabriz, Iran). Thirty–two pregnant ewes in gestational week 6 to 21 were divided in two groups: less than 3 months and more than 3 months of pregnancy. In addition, three normal nonpregnant ewes and three normal rams were used as control animals. As a source of ccffDNA, peripheral blood samples were obtained from the animals. Tubes containing EDTA and 10 mL of blood samples were centrifuged at 664 × g for 10 minutes to separate plasma from packed cells and buffy coat.
Pregnant uteri were collected from 32 Gezel sheep after slaughter in a local abattoir. The uteri were rinsed by sterile saline solution, and then a smear was prepared from secretions at the external os using a sterile swab. Afterward an incision was made on the uterine wall at the level of internal os and another smear was prepared from secretions at this area. The prepared smears were stained with Eosin-Nigrosin dyes (Merck?, Germany) and studied using light microscope to make sure that none of cervical specimens were contaminated with sperm cells (to avoid false positive results in case ewes are mounted by rams shortly before slaughter). Next, the cervical lumen was exposed completely using a pair of sterile surgical scissors and cervical secretions were totally collected into capped sterile 1.5 mL microtubes. All samples were kept at ?20 ℃ until further molecular analysis.
2.2. DNA extraction from maternal blood plasma
Twelve hundred microliters of maternal blood plasma and an equal volume of TRIS- ethylenediamine tetraacetic acid buffer (pH 8) were mixed in a Falcon tube. Then, 15 mL proteinase K (20 mg/mL) solution was added and the mixture was digested at 56 ℃ for 3 hours. Next, 2.5 mL of equilibrium phenol and chloroform/isoamylalcohol were added. The tubes were centrifuged at 5 095 g for 10 minutes and the supernatants were transferred to fresh tubes. This process was repeated again and then a half volume of supernatant, ammonium acetate (7 M), and 2 volumes of supernatant, 100% ethanol and 10mL of glycogen (20 mg/mL) were added and the mixtures were stored at -20 ℃ overnight. Then, the tubes were centrifuged at 5 095×g for 15 minutes at room temperature (approximately 18 ℃-24 ℃). The supernatant was discarded, and DNA was deposited with 70% ethanol and then dried in air. The amount and quality of DNA were determined using spectrophotometry. Only DNA of sufficient purity, having an absorbance ratio (at 260/280 nm) of 1.7 to 2 was considered for PCR analysis.
2.3. PCR analysis
We employed the sequence length polymorphisms between the amelogenin X and amelogenin Y genes (AMLX/Y) as markers for sexing the ovine fetus. The oligonucleotide sequence of the primers (Cinnagen Co. Tehran, Iran) used in the current study were (SE 47); 5′-CAGCCAAACCTC CCTCTGC-3′ and (SE 48); 5′-CCCGCTTGGTCTTGTCTGTTGC-3′. This primer set was designed to amplify a single fragment of 280 bp on the X-chromosome (female fetuses) and two fragments of 280 and 217 bp on X and Y chromosomes, respectively (male fetuses). Therefore, our sexing method was based on the presence (male fetus) or absence (female fetus) of the fetal Y-chromosome in the cervical or serum samples. The PCR reaction mixture (15μL/tube) consisted of 1.2 U Taq DNA polymerase, 1.5 mM MgCl2, 0.1 mM of each dNTPs, 0.4 μM male-specific primers, and 2.4 μL template DNA. The PCR condition consisted of an initial denaturing at 94 ℃ for 5 min followed by 37 cycles of 94 ℃ for 45 s., 64.7 ℃ for 45 s., 72 ℃ for 30 s., and after the last cycle the samples were kept at 72 ℃ for 5 min for the final extension, and then the PCR procedure was completed. The PCR products were analyzed with electrophoresis in 2.2 % (w/v) TAE-based agarose gel stained with ethidium bromide (0.5μg/mL). All samples were tested at least in duplicate
2.4. PCR controls
A no-template control (NTC) was included in each run for all PCR techniques. The negative control was composed of a standard female DNA. The positive control was a mixture of female DNA containing 2% male DNA. Fetal sex was confirmed after foaling. Accuracy, sensitivity, and specificity of molecular sex determination were calculated as described [13].
2.5. Statistical analysis
Comparisons were made between two groups of ewes, at less than and more than 3 months of pregnancy, using the independent-sample t test by the SPSS-19.0 package (SPSS Inc., New York, NY, USA). All results are shown as mean ±SEM and differences were considered significant at P<0.05.
3. Results
3.1. Plasma samples
The extracted DNA from blood plasma of ewes with a male fetus showed one band at 280 bp, whereas there was no band after amplification of extracted DNA from ewes bearing a female fetus. Combining the results of the use of PCR analyses on male and female pregnancies, the overall test accuracy for correct sex determination using plasma samples was equal to 78±1 (95 % confidence interval) [12/15 (80 %) cases from known male pregnancies and 13/17 (76%) cases from known female pregnancies]
3.2. Cervical samples
Gel electrophoresis results of PCR analyses on the cervical samples taken from female and male pregnancies are shown in Figures 1 and 2. The estimated age and sex of the sampled fetuses are summarized in Table 1. Light microscopic examination of prepared smears showed no sperm contamination in any cervical samples. Therefore, the possibility of obtaining false positive results in the male pregnancies due to the contamination of samples with Y bearing spermatozoa was excluded. Two expected PCR product sizes of 280 bp fragment from the X-chromosome and a 217 bp fragment from the Y-chromosome (with fetal origin) were produced in eleven out of 15 cervical mucus samples collected from pregnant ovine with male fetuses as well as for positive control sample Collected from control ram (Figure 2). A noticeable reduction in the sharpness of Y product (217 bp) relative to X product (280 bp) was observed in the positive samples collected from male fetuses compared to normal male (Figure 2). Combining the results of the use of PCR analyses on male and female pregnancies, the overall test accuracy for correct sex determination using cervical samples was equal to 71.87±1 (95% confidence interval) [11/15 (73%) cases from known male pregnancies and 12/17 (70%) cases from known female pregnancies].
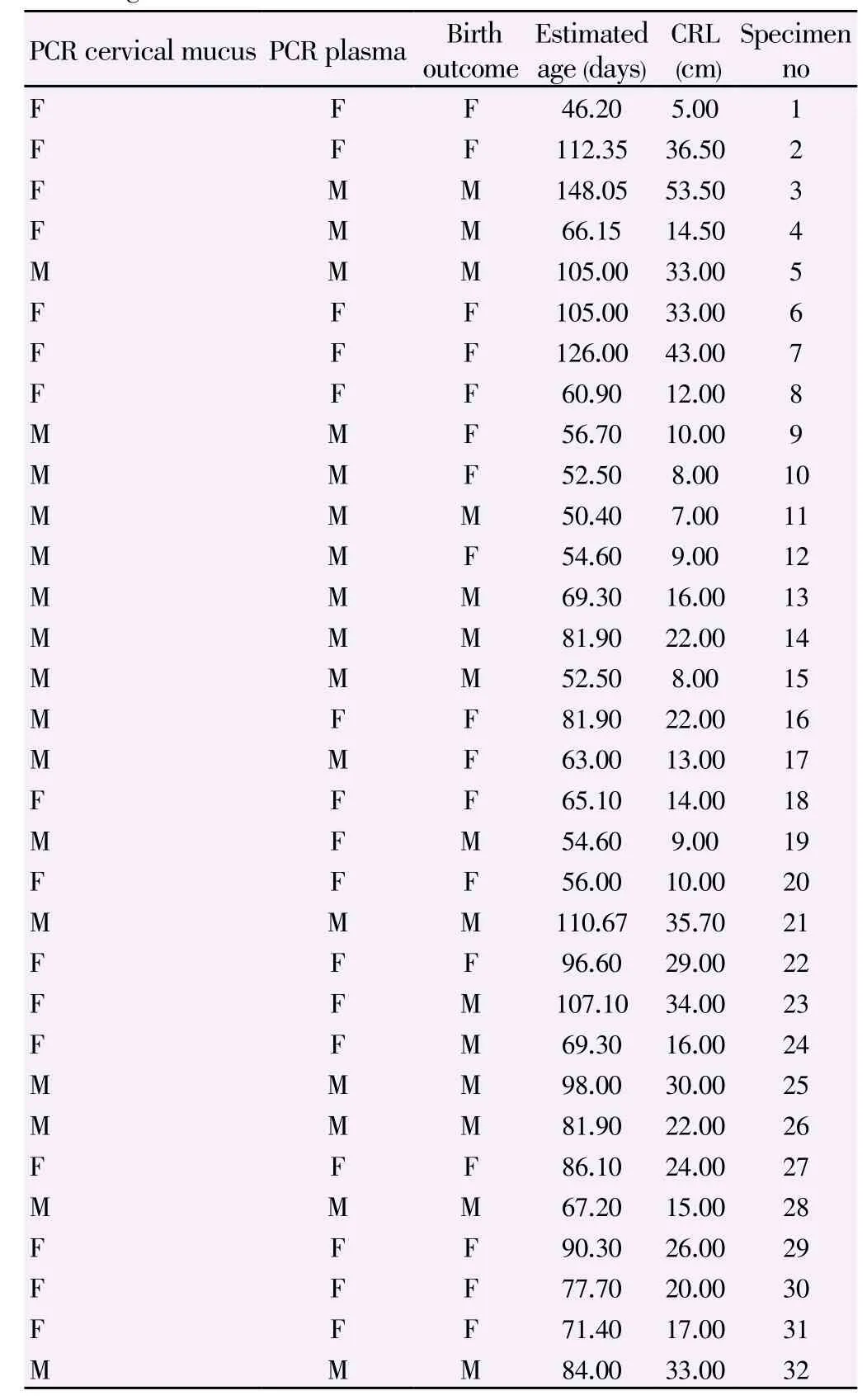
Table 1 Fetal sex predication by PCR analysis of cell –free fetal DNA in maternal blood plasma and cervical mucus of 32 pregnant ewes using the SRY gene.
4. Discussion
The current study investigated the presence of the fetal DNA in the plasma and maternal cervical mucus and also evaluated the possible application of it for ovine fetal sexing. For years, ultrasonic technology has been the method of choice for determining fetal sex in domestic animals. In ewe, fetal sex is determined during days 60 to 69 of pregnancy using the transrectal ultrasonography method[6]. However, this technique bears some disadvantages, because it requires extensive experience on the part of the operator, and reaching the fetus becomes difficult as gestation ensues, making increasingly difficult or sometimes impossible to predict fetal sex during the later stages of gestation[14].
The PCR was used to co-amplify a sex-based polymorphism in the amelogenin locus (AMLX and AMLY). Different sets of amelogenin gene primers have been confirmed in several studies as a reliable molecular marker for sex determination with many domesticated animals such as cattle, pigs, goats and sheep[15]. In the present study, we demonstrated the presence of fetal derived Y chromosome in eleven out of 15 cervical mucus samples taken from pregnant cows with male fetuses and four of cervical samples from female fetuses were positive for Y-chromosome. We believe that differences may by due to the contamination, accuracy techniques. Also the amount of fetal DNA present in the cervical samples can affect the detection risk of the PCR. Based on studies carried out in human medicine, percentage of cervical samples with fetal cells is highly dependent on the sampling approach, skill of individual operator and molecular technique which is employed[12]. Several approaches have been used for retrieving cervical samples such as using swabs, endocervical or uterine lavage, using cytobrush and aspiration[16]. Our method is comparable with using cytobrush or aspiration for collecting cervical mucus. In one study, the endocervical mucus was collected by a simple aspiration technique by means of Pipelle catheters[16]. PCR analysis with amelogenin gene primers, documented the presence of fetal cells in 11/15 (80 %) of samples obtained from mothers with male fetuses and four sample from female fetuses was found to be Y-positive.
It should be noticed that in humans, after completion of implantation, the growing embryo is entirely covered with an endometrial layer named “Decidua capsularis”. Nonetheless, fetal cells can pass it and enter the uterine lumen. This layer does not form in ovine. This is due to non-invasive pattern of embryonic attachment to the maternal endometrium in this species[17]. Therefore, fetal cells are directly in contact with uterine lumen from where they can reach the cervical secretions. Our preliminary results from the cervical mucus indicate that the age of the fetus might not be a contributing factor in obtaining positive results. Divar et al. [18] indicated that amplification of Y-chromosome segments from cervical mucus of pregnant cows after 70 days of pregnancy is highly specific for presence of a male fetus, but that this approach currently lacks enough sensitivity for it to be considered as a reliable fetal sexing method.
An alternative method for embryo sexing could be a molecular technique based on fetal DNA obtained from the maternal circulation. Several studies have reported the extraction of DNA from maternal blood plasma for fetal sexing in various animals. Kadokawa et al. [19] tried for the first time to extract fetal DNA from bovine maternal blood and reported its absence in cows during early to late gestation. Similarly, Wang et al. [20] successfully used fetal DNA in cow blood plasma for prediction of fetal sex. They confirmed an overall accuracy rate of 100% for male and 91% for female fetuses. Likewise, de Leon et al. [21] used the SRY gene to determine fetal sex from extracted ccffDNA in blood plasma of pregnant mares. Our study is the report of the presence of fetal DNA in blood plasma of pregnant ewes. We analyzed the blood plasma of 15 ewes with male and 17 ewes with female fetuses during 6 to 21 weeks of gestation and found three false negative cases. Negative PCR results may be explained by the presence of PCR inhibitors that are co extracted from the serum samples and dramatically reduce the sensitivity and amplification efficiency of PCR. Protein contamination of the extracted DNA can lead to PCR failure.
da Cruz et al.[22] showed a strong relationship between the probability of correctly predicting fetal sex and the stage of gestation in cattle. In our study, we were not able to demonstrate relationship between pregnancy age and fetal DNA. This finding is also in contrast with the study of Lo et al. [23] who identified human fetal DNA in the blood plasma of women Lo et al.[23]. They speculated that fetal DNA could be detected in maternal serum as early as the seventh week of gestation and its concentration increased as pregnancy progressed. The mechanism of fetal DNA leakage to maternal circulation is not completely understood. However, cell lysis resulting from physical and immunologic damage and developmentally regulated apoptosis of fetal tissuescould allow fetal DNA to cross the placental membrane[24]. Although ovine placenta with synepitheliochorial structure is anticipated to prevent transplacental cell leakage, previous studies in animals such as cow and horse with the same placental type, suggested transfer of fetal DNA through the placenta. In the present study, we also demonstrated the presence of free fetal DNA in the maternal circulation which is indicative of the fetal DNA leakage in ewes. However, more studies are needed to elucidate how this DNA leakage occurs in the absence of direct contact between the placenta and maternal blood.
In conclusion, this study demonstrates a novel opportunity for non-invasive assessment of pregnancy, and possible to achieve fetal sex determination using ccffDNA and maternal mucosal cervix in ovine.
Conflict of interest statement
All authors declared no conflict of interests.
Acknowledgment
This research was supported by University of Tabriz.
[1] Peippo J, Huhtinen M, Kotilainen T. Sex diagnosis of equine preimplantation embryos using the polymerase chain reaction. Theriogenology 1995; 44: 619-627.
[2] Han SH, Yang BC, Ko MS, Oh HS, Lee SS. Length difference between equine ZFX and ZFY genes and its application for molecular sex determination. J Assist Reprod Genet 2010; 27: 725-728.
[3] Bucca S. Equine fetal gender determination from mid-to advancedgestation by ultrasound. Theriogenology 2005; 64:568 -571.
[4] Kadivar A, Hassanpour H, Mirshokraei P, Azari M , Hosseini KH, Karami A. Detection and quantification of cell-free fetal DNA in ovine maternal plasma; use it to predict fetal sex. Theriogenology 2013; 79: 995-1000.
[5] Coubrough CA, Castell MC. Fetal sex determination by ultrasonically locating the genital tubercle in ewes. Theriogenology 1998; 50: 263-277.
[6] de Freitas Neto LM, dos Santos MH, de Aguiar Filho CR, de Almeida Irm?o JM, Caldas EL, Neves JP, et al. Ultrasonographic fetal sex identification in pregnant sheep derived from natural mating and embryo transfer. J Reprod Dev 2010; 56: 347-350.
[7] Makondo K, Amiridis GS, Jeffcoate IA, O’Shaughnessy PJ, Boyd JS, Paterson C, et al. Use of polymerase chain reaction to sex the bovine fetus using cells recovered by ultrasound-guided fetal fluid aspiration. Anim Reprod Sci 1997; 49: 125-133.
[8] Zhao Y, Zou L. Application of fetal DNA in maternal plasma in noninvasive prenatal diagnosis. J Huazhong Univ Sci Technolog Med Sci 2004; 24: 59-61.
[9] Honda H, Miharu N, Ohashi Y, Ohama K. Successful diagnosis of fetal gender using conventional PCR analysis of maternal serum. Clin Chem 2001;47: 41-46.
[10] Zhong XY, Holzgreve W, Hahn S. Detection of fetal Rhesus D and sex using fetal DNA from maternal plasma by multiplex polymerase chain reaction. BJOG 2000; 107: 766-769.
[11] Shettles LB. Use of the Y chromosome in prenatal sex determination. Nature 1971; 5288: 52-53.
[12] Imudia AN, Kumar S, Diamond MP, DeCherney AH, Armant DR. Transcervical retrieval of fetal cells in the practice of modern medicine: are view of the current literature and future direction. Fertil Steril 2010; 93(6):1725-1730.
[13] Kastelic JP. Critical evaluation of scientific articles and other sources of information; an introduction to evidence-based veterinary medicine. Theriogenology 2006; 66: 534-542.
[14] Ali A. Effect of gestational age and fetal position on the possibility and accuracy of ultrasonographic fetal gender determination in dairy cattle. Reprod Domest Anim 2004; 39: 190-194.
[15] Quirino CR, Leal SR, daSilva Fontes R, Marques VCL, Matos LF, Filho GADS. In: 9th World Congresson Genetics Applied to Livestock Production(WCGALP), Leipzig, Germany 2010; August1-6. [16] Adinolfi M, Sherlock J. First trimester prenatal diagnosis using transcervical cells: an evaluation. Hum Reprod Update 1997; 4: 383-392.
[17] Wooding P, Burton G. Comparative placentation: structures, functions and evolution. Berlin: Springer Verlag; 2008.
[18] Divar MR, Sharifiyazdi H, Kafi M. Application of polymerase chain reaction for fetal gender determination using cervical mucous secretions in the cow. Vet Res Commun 2012; 36: 215-220.
[19] Kadokawa H, Takusari N, Minezawa M, Takahashi H, Kariya T. Absence of fetal cells in bovine jugular and uterine vein blood at a level of 1 in 10 000. J Reprod Dev 1996; 42: 205-208.
[20] Wang G, Cui Q, Cheng K, Zhang X, Xing G, Wu S. Prediction of fetal sex by amplification of fetal DNA present in cow plasma. J Reprod Dev 2010; 56(6): 639-642.
[21] de Leon PM, Campos VF, Dellagostin OA, Deschamps JC, Seixas FK, Collares T. Equine fetal sex determination using circulating cell-free fetal DNA ccffDNA). Theriogenology 2012; 77(3): 694-698.
[22] da Cruz AS, Silva DC, Costa EOA, De M-Jr P, da Silva CC, Silva DM, et al. Cattle fetal sex determination by polymerase chain reaction using DNA isolated from maternal plasma. Anim Reprod Sci 2012; 131: 49-53.
[23] Lo YMD, Tein MSC, Lau TK, Haines CJ, Leung TN, Poon PMK, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet 1998; 62: 768-775.
[24] Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet 1997; 350(9076): 485-487.
*Corresponding author: Reza Asadpour, Department of Clinical Science, Faculty of Veterinary Medicine, University of Tabriz, Iran.
Tel: +984192377
E-mail: r_asadpour@tabrizu.ac.ir
 Asian Pacific Journal of Reproduction2015年1期
Asian Pacific Journal of Reproduction2015年1期
- Asian Pacific Journal of Reproduction的其它文章
- Molecular dysregulation of renal development: Congenital anomalies of the kidney and urinary tract
- A new treatment for premature ejaculation? Case series for a desensitizing masturbation aid
- Reproductive status of Camelus bactrianus during early breeding season in India
- Effect of frequency of collection on seminal characteristics of White Pekin duck
- Evaluation of norgestomet Crestar? on oestrus synchronization and reproductive performance of dairy cows in Algeria
- Metabolic syndrome among infertile women with polycystic ovary syndrome
