UPLC-MS/MS for the determination of azilsartan in beagle dog plasma and its applicationin a pharmacokinetics study
Cheng Gong,Junfeng Wng,Yinghu Sun,*,Dwei Ding,Lu Zhong, Meng Zhu,Jin Sun,Xingrong Zhng,**
aShenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,ChinabShandong Weigao Pharm.Co.Ltd.,Weihai 264200,China
Short Communication
UPLC-MS/MS for the determination of azilsartan in beagle dog plasma and its application
in a pharmacokinetics study
Cheng Gonga,Junfeng Wangb,Yinghua Suna,*,Dawei Dinga,Lu Zhonga, Meng Zhua,Jin Suna,Xiangrong Zhanga,**
aShenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,ChinabShandong Weigao Pharm.Co.Ltd.,Weihai 264200,China
ARTICLEINFO
Article history:
Received 15 May 2014
Received in revised form
13 October 2014
Accepted 23 October 2014
Available online 20 December 2014
Azilsartan
The purpose of the study is to develop an ultra performance liquid chromatographytandem mass spectrometry(UPLC-MS/MS)to determinate the concentration of azilsartan in the dog plasma.After precipitated by methanol,the plasma sample containing azilsartan and diazepam(internal standard,IS)was determined by UPLC-MS/MS.The mobile phase consisted of acetonitrile-water was pumped at a f l ow rate of 0.3 ml/min in gradient elution.Kinetex 2.6 μ XB-C18 column(50×2.1 mm,100 ?;Phenomenex,USA) were used for LC separations.The column temperature was 30°C and the injection volume was 5 μl.The electrospray ionization(ESI)and multiple reaction monitoring(MRM)were applied at the transitions of m/z 457→279(azilsartan)and m/z 285→193(diazepam), respectively.The developed method was identif i ed a good linearity over a concentration range of 2.5-5000 ng/ml.The lower limit of quantitation(LLOQ)was 2.5 ng/ml.The intraday and inter-day precision(relative standard deviation,RSD%)were less than 10%and accuracy(relative error,RE%)was less than 5%at three quality control levels.The extraction recovery of azilsartan at three quality control levels were 82.41±0.68%, 98.66±11.00%,102.43±0.82%.And the recovery for IS(100 ng/ml)was 91.75±0.54%.A validated UPLC-MS/MS method was f i rstly developed for the quantif i cation of azilsartan in dog plasma and it was applied to the pharmacokinetics study.
?2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
Azilsartan,a new generation of angiotensin II receptor blocker(ARB),was successfully developed by Takeda in 2012 and the trade name is Azilva?.It is the main active metabolite of azilsartan medoxomil[1]which was approved as an antihypertention drug by US Food and Drug Administration (FDA)in 2011.Azilsartan selectively binds to the angiotensin II AT 1 receptor to inhibit the actions of angiotension II and results in lowering blood pressure(BP)[2].According to the drug label and literatures,azilsartan is principally absorbed in the upper parts of small intestine,and the best absorption sides are duodenum and jejunum[3-5].The volume of distribution of azilsartan is approximately 16 L and it is highly bound to human plasma proteins[6].Azilsartan is metabolized to two main metabolites by cytochrome P450 2C9 (CYP2C9).And the major pathways for the elimination of azilsartan are feces and urine[5,7].The recommended dose and maximum dose in adults are 20 mg and 40 mg taken orally per day.Compared with olmesartan and valsartan at maximal approved doses,azilsartan has superior eff i cacy at its maximal dose[8]and no signif i cant increases in adverse events(cardiovascular,cerebrovascular and renal events)are observed[9,10].Azilsartan shows sustaining reduction in BP even after 24 h administrated to rat and dog[11].What's more,azilsartan is well tolerated in patients with hypertension[12].
At present,most literatures about azilsartan or azilsartan medoxomil focus on its antihypertensive eff i cacy and safety [13-16].For future pharmacokinetics study,the method to quantify the concentration of azilsartan in plasma is needed. However,there is no reported UPLC-MS/MS method for determination of azilsartan in plasma.
In this study,methanol was added to the plasma sample to precipitate the protein,and the analyte and endogenous components were separated by the gradient elution.The UPLC-MS/MS methodwith simpleprocedure,high-sensitivity (LLOQ=2.5 ng/ml)and short analytical time(3.5 min per sample)was f i rst developed to assay the azilsartan in beagle dog plasma and was successfully applied to the pharmacokinetic study.
2.Materials and methods
2.1.Materials
Azilsartan(99.8%)was kindly provided by Weigao Pharm. Co.Ltd.(Shandong,China).Diazepam(internal standard, IS>99.0%purity)was purchased from Baoyuan Pharm.Co. Ltd.(Shanxi,China).HPLC-grade acetonitrile and methanol were obtained from Fisher Scientif i c(Pittsburgh,PA,USA). HPLC-grade Formic acid was purchased from Dikma(Richmond Hill,NY,USA).Double distilled water was used throughout the study.The azilsartan tablets(Azilva?)were purchased from Takeda Pharm.Co.Ltd.(Tokyo,Japan).The azilsartan test tablets were prepared in the laboratory.
2.2.Instrumentation
The UPLC-MS/MS system consisted of a Waters ACQUITY UPLC system and a Tandem Quadrupole(TQ)Detector with an electrospray ionization(ESI)interface(Waters Corp.,USA).All data were processed by MassLynx 4.1 software with the QuanLynx program(Waters Corp.,USA).
2.3.UPLC/MS/MS conditions
The analytical column used was Kinetex 2.6 μ XB-C18 column (50×2.1 mm,100 ?;Phenomenex,USA).The column was maintained at 30°C.The mobile phase was composed of solvent A(acetonitrile with 0.2%Formic acid)and solvent B(0.2% Formic acid).The gradient program started with 40%solvent A for 1.0 min.The percentage of solventA was increased linearly to 80%from 1.0 min to 2.5 min and was decreased linearly to 40%from 2.51 min to 3.0 min.Then the percentage of solvent A maintained 40%from 3.0 min to 3.5 min and the gradient program was completed.The f l ow was always at 0.3 ml/min in the gradient program.
For MS detection,the electrospray ionization source was operated in a positive mode(ESI+).Cone voltage and collision energy were 20 V,14 V for azilsartan and 30 V,25 V for diazepam(internal standard,IS),respectively.The temperature and gas f l ow of de-solvent were 350°C and 650 L/h.To analyze the drugs in plasma,multiple reaction monitoring(MRM)was used at the transitions of m/z 457→279 for azilsartan and m/z 285→193 for diazepam,respectively,with a scantime of0.2 s.
2.4.Preparation of standard and quality control(QC) samples
The stock solutions of azilsartan and IS were prepared in methanol at 100 μg/ml and 1 mg/ml,respectively.Then they were diluted to 10 μg/ml for azilsartan and 100 ng/ml for IS with methanol-water(80:20,v/v)to obtain standard solutions. The standard solutions of azilsartan were used for preparing the calibration curve and QC samples.The calibration curve was prepared by spiking 200 μl of blank plasma with 200 μl of appropriate azilsartan standard solution and 200 μl IS standard solution.Then 400 μl of methanol was added to the sample.Finally,the calibration curve was prepared at the concentration of 2.5,5,10,20,50,200,500,1000,4000 and 5000 ng/ml.And the QC samples for azilsartan at the concentration of 5,200,4000 ng/ml was prepared as the same process.
2.5.Plasma sample preparation
200 μl of IS standard solution(100 ng/ml)and 200 μl of methanol-water(80:20,v/v)were added to 200 μl plasma.The mixture was vortex-mixed for 3 min.400 μl of methanol was added to the sample to precipitate the protein and then the mixture was vortex-mixed for 3 min again.After that,the sample was centrifuged at 13,000 rpm for 10 min.At the end, 5 μl supernatant was injected into the UPLC-MS/MS system.
2.6.Method validation
2.6.1.Selectivity
The selectivity of the method was to distinguish and quantify the analyte in the plasma samples.To conf i rm the selectivity of the method,the chromatograms of six different batches of blank plasma and the corresponding spiked samples were compared.
2.6.2.Calibration curve and lower limit of quantitation
The calibration curve was determined by plotting the peak area ratio(A,area of analyte/area of IS)versus the concentration using weighted(1/C2)least square linear regression. The lower limit of quantitation(LLOQ),10 times greater than the ratio of signal-to-noise,was determined.
2.6.3.Precision and accuracy
The precision and accuracy expressed as relative standard deviation(RSD)and relative error(RE),respectively,were calculate by analyzing the QC samples at low(5 ng/ml),medium(200 ng/ml)and high(4000 ng/ml)concentration on three validation days.
2.6.4.Extraction recovery and matrix effect
Theextractionrecoveryofazilsartanwasassessedby comparing the peak areas of the extracted QC samples with those of the samples which mixed corresponding standard solutions with the extracted blank plasma.The matrix effect of azilsartan was determined by comparing the peak areas of the analyte at low,medium and high concentration in the extracted blank plasma with those of the correspondingstandard solutions.The recovery and matrix effect of diazepam(IS)were also determined.
2.6.5.Stability
The stability test was regard as an important part of the method validation.The stability of azilsartan in the plasma samples was evaluated under various conditions,including at ambient temperature(25°C)for 12 h,post-processed for 12 h, freeze-thaw cycles for 3 times and long-term stability(-20°C for 20 days).All stability tests were analyzed by QC samples (n=3)at low,medium and high concentration.
2.7.Pharmacokinetics application
The above-mentioned validated method was successfully used to analyze plasma samples for a pharmacokinetic study of azilsartan.The animal protocols in the study were approved by the Shenyang Pharmaceutical University Animal Care and Use Committee.
Six healthy beagle dogs(weight 10-12 kg)were segmented into two groups randomly,and study in a crossover experimental design.The washout period is a week.Dogs were fasted for a night before the experiment,but have free access to water.Each group was administrated orally with commercial tablets(Azilva?,Reference)and self-made tablets(Test), respectively.The dosage of administration is 20 mg.
Blood samples(2 ml)were collected in heparinized tubes from forearm vein of the dog before drug administration and at 0.25,0.5,0.75,1,1.5,2,2.5,3,4,6,8,10,14 and 24 h postdosing.Then the plasma was separated by centrifugation at 3000 rpm for 10 min and kept in the refrigerator at-20°C until analysis.
The main pharmacokinetic parameters were calculated by the DAS 2.0 software(Mathematical Pharmacology Professional Committee of China,Shanghai,China).
3.Results and discussion
3.1.Optimization of LC-MS/MS condition
Both azilsartan and diazepam(IS)are polar compounds.The electrospray ionization source was set to a positive mode (ESI+)in order to offer higher signal intensity for them.The cone voltage and collision energy of azilsartan and IS were developed for the best response of the parent and daughter ions.The protonated molecular ions[M+H]+with greatest response in the daughter-ion mass spectrum were m/z 279 for azilsartan and m/z 193 for IS as shown in Fig.1.Various mobile phases were attempted in this study.For the sake of obtaining good peak shapes with short retention time,the mobile phase consistedof 0.2%formicacid and acetonitrilewith0.2%formic acid.The gradient elution in this method was developed to ensure that the assay was not interfered by endogenous substances.
3.2.Method validation
3.2.1.Specif i city
The specif i city of the analytical method was evaluated by comparing the representative MRM chromatograms of(A) blank plasma,(B)blank plasma spiked with azilsartan(at the concentration of LLOQ)and IS(100 ng/ml),(C)plasma from a dog after single oral administration of 20 mg azilsartan in Fig.2.And no endogenous interference for azilsartan or IS was observed.The analytical time was 3.50 min for each sample. The retention times of azilsartan and IS were 1.99 min and 2.11 min,respectively.
3.2.2.Linearity and sensitivity
The calibration curves over the concentration range of 2.5-5000 ng/ml showed a good linearity.The typical regressionequationwasshownasthefollowingequation: A=0.130049C+0.110229(correlation coeff i cient,r=0.9971), where A stands for the ratio of the peak area of analyte to the peakareaofISand C standsfortheconcentrationofazilsartan in the plasma.All correlation coeff i cients exceeded 0.9950 on three validation days.
LLOQ was determined as 2.5 ng/ml and the precision(RSD %)and accuracy(RE%)values were 6.75%and-10.80%, respectively,within the acceptable range of±20%.
3.2.3.Precision and accuracy
The intra-and inter-day precision and accuracy for the determination of azilsartan were listed in Table 1.From the results,it indicated that the intra-day precision and accuracy were all less than 6%while the inter-day precision and accuracy were all less than 9%.All the results were within the acceptable range of±15%.
3.2.4.Extraction recovery and matrix effect
The extraction recoveries for azilsartan at low(5 ng/ml),medium(200 ng/ml)and high(4000 ng/ml)concentrations were 82.41±0.68%,98.66±11.00%,102.43±0.82%.The recovery for IS(100 ng/ml)was 91.75±0.54%(n=6).
The matrix effects for azilsartan at three concentration levels(5,200 and 4000 ng/ml)were 105.43±8.16,99.41±3.43, and 104.37±1.99%,respectively.The matrix effects of IS was 93.66±3.76%.All the ratios of matrix effects for azilsartan and IS were within the acceptable range of±15%.The resultsshowed that no signif i cant matrix effects for azilsartan and IS were discovered in this study.
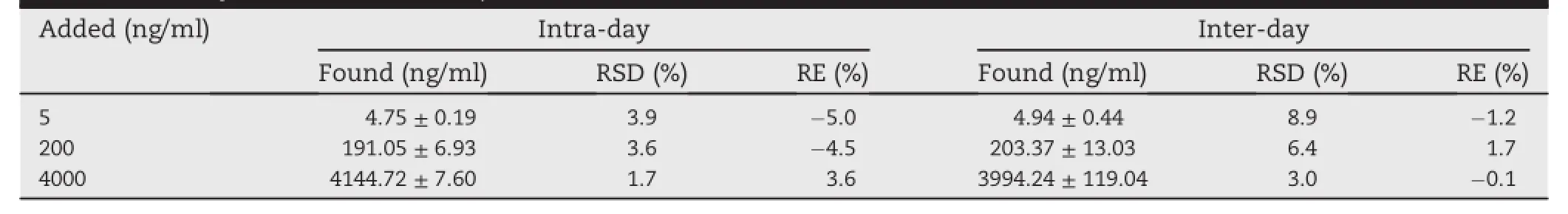
Table 1-Precision and accuracy for the determination of azilsartan in dog plasma samples by UPLC-n=18;mean±SD). MS/MS(intra-day, 6;inter-day,n=
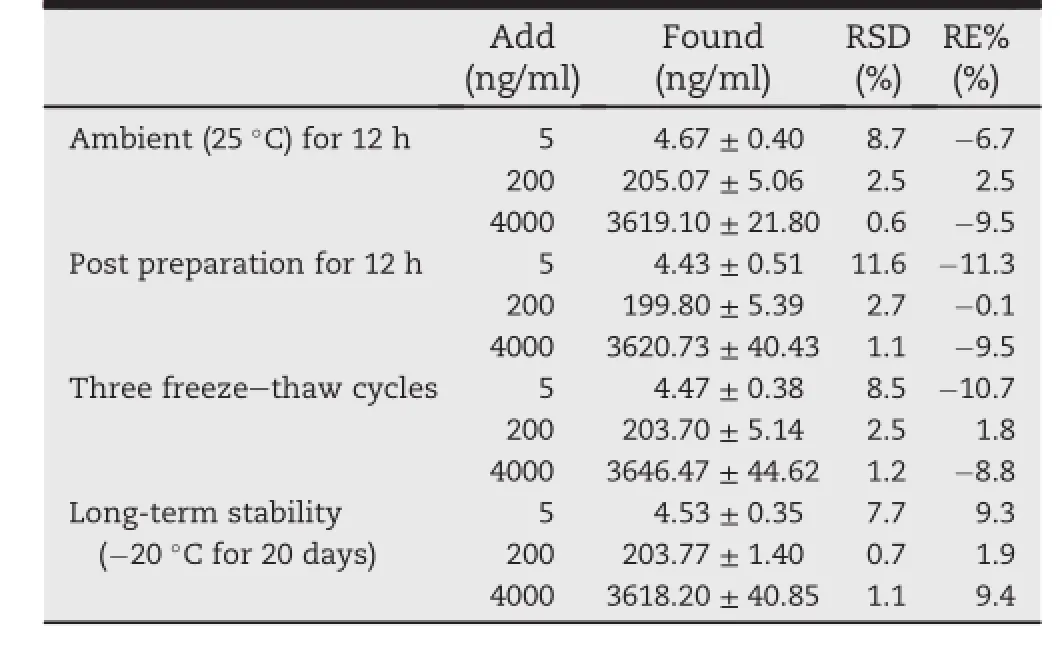
Table 2-Stability of azilsartan in beagle dog plasma under various conditions(mean±SD,n=3).
3.2.5.Stability
The stability test was performedby analyzing replicates(n=3) of QC samples at low,medium and high concentrations under different conditions.All the data of the stability test were shown in Table 2.It suggested that the azilsartan showed a good stability in the dog plasma during the analytical procedure.
3.3.Pharmacokinetic studies
ThevalidatedUPLC-MS/MSmethodwasf i rstusedforthestudy of pharmacokinetic behaviors of azilsartan in beagle dogs.The mean plasma concentration-time curves for azilsartan marketed tablets(Reference)and self-made tablets(Test)after oral administration to six healthy beagle dogs are shown in Fig.3. The Tmaxand Cmaxwere obtained directly from the curves.It was clearly indicated that Cmaxwere 955.33±272.39 ng/ml for Referece and 939.68±335.41 ng/ml for Test,respectively,while theTmaxwere0.75±0.09hand1.50±0.28h.AUC0-24hvaluesfor ReferenceandtheTeswerefoundtobe3387.36±866.96ng/ml·h and 3121.05±770.54 ng/ml·h,respectively.The data were shown as mean±standard error(n=6).The relative bioavailability of Test compared with Reference was 108.53%.
4.Conclusion
In this study,a rapid,selective and sensitive UPLC-MS/MS method was developed for the determination of azilsartan in beagle dog plasma.The precision,extraction recovery,matrix effect and stability of this method have been validated.It has been successfully used for the pharmacokinetic study of azilsartan in beagle dog plasma for the f i rst time.
REFERENCES
[1]Perry M.Azilsartan medoxomil.Clin Drug Invest 2012;32:621-639.
[2]Ojima M,Igata H,Tanaka M,et al.In vitro antagonistic properties of a new angiotensin type 1 receptor blocker, azilsartan,in receptor binding and function studies. J Pharmacol Exp Ther 2011;336:801-808.
[3]Kawaguchi N,Ebihara T,Takeuchi T,et al.Absorption of TAK-491,a new angiotensin II receptor antagonist,in animals.Xenobiotica 2013;43:182-192.
[4]Angeli F,Verdecchia P,Pascucci C,et al.Pharmacokinetic evaluation and clinical utility of azilsartan medoxomil for the treatment of hypertension.Expert Opin Drug Met 2013;9:379-385.
[5]U.S.Food and Drug Administration.Edarbi(azilsartan medoxomil)tablets.Available from::http://www.accessdata. fda.gov/drugsatfda_docs/label/2014/200796s006lbl.pdf.
[6]Jones D,Jackson H,Agboton C,et al.Azilsartan medoxomil (Edarbi):the eighth angiotensin II receptor blocker.Pharm Ther 2011;36:634.
[7]Baker WL,White WB.Azilsartan medoxomil:a new angiotensin II receptor antagonist for treatment of hypertension.Ann Pharmacother 2011;45:1506-1515.
[8]White B,Weber A,Sica D,et al.Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension.Hypertension 2011;57:413-420.
[9]Zaiken K,Cheng M.Azilsartan medoxomil:a new angiotensin receptor blocker.Clin Ther 2011;33:1577-1589.
[10]Taylor A,Siragy H,Nesbitt S.Angiotensin receptor blockers: pharmacology,eff i cacy,and safety.J Clin Hypertens 2011;13:677-686.
[11]Kusumoto K,Igata H,Ojima M,et al.Antihypertensive, insulin-sensitising and renoprotective effects of a novel, potent and long-acting angiotensin II type 1 receptor blocker, azilsartan medoxomil,in rat and dog models.Eur J Clin Pharmacol 2011;669:84-93.
[12]Sica D,White B,Weber A,et al.Comparison of the novel angiotensin II receptor blocker azilsartan medoxomil vs valsartan by ambulatory blood pressure monitoring.J Clin Hypertens 2011;13:467-472.
[13]Rakugi H,Enya K,Sugiura K,et al.Comparison of the eff i cacy and safety of azilsartan with that of candesartan cilexetil in Japanese patients with grade I-II essential hypertension:a randomized,double-blind clinical study.Hypertens Res 2012;35:552-558.
[14]Bakris L,Sica D,Weber M,et al.The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure.J Clin Hypertens 2011;13: 81-88.
[15]Bakris L,Sica D,White B,et al.Antihypertensive eff i cacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil.Am J Med 2012;125.1229.e1-1229. e10.
[16]Cushman C,Bakris L,White B,et al.Azilsartan medoxomil plus chlorthalidone reduces blood pressure more effectively than olmesartan plus hydrochlorothiazide in stage 2 systolic hypertension.Hypertension 2012;60:310-318.
*Corresponding author.Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Tel./fax:+86 24 23986325.
**Corresponding author.Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Tel./fax:+86 24 23986521.
E-mail addresses:sunyinghua77@aliyun.com(Y.Sun),xrzhxr@126.com(X.Zhang).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.10.004
1818-0876/?2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
UPLC-MS/MS
Pharmacokinetics study
 Asian Journal of Pharmacentical Sciences2015年3期
Asian Journal of Pharmacentical Sciences2015年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- Design and comparative in-vitro and in-vivo evaluation of starch-acrylate graft copolymer based salbutamol sulphate sustained release tablets
- Characterization of recrystallized itraconazole prepared by cooling and anti-solvent crystallization
- Enhanced bioavailability of rebamipide nanocrystal tablets:Formulation and in vitro/in vivo evaluation
- Liposomes for systematic delivery of vancomycin hydrochloride to decrease nephrotoxicity: Characterization and evaluation
- Chlorogenic acid loaded chitosan nanoparticles with sustained release property,retained antioxidant activity and enhanced bioavailability
