Proline with or without Hydroxyproline Influences Collagen Concentration and Regulates Prolyl 4-Hydroxylase α (I) Gene Expression in Juvenile Turbot (Scophthalmus maximus L.)
ZHANG Kaikai, MAI Kangsen, XU Wei, ZHOU Huihui, LIUFU Zhiguo,ZHANG Yanjiao, PENG Mo, and AI Qinghui
?
Proline with or without Hydroxyproline Influences Collagen Concentration and Regulates Prolyl 4-Hydroxylase α (I) Gene Expression in Juvenile Turbot (L.)
ZHANG Kaikai, MAI Kangsen, XU Wei, ZHOU Huihui, LIUFU Zhiguo,ZHANG Yanjiao, PENG Mo, and AI Qinghui*
,,266003,
This study was conducted to investigate the effect of dietary proline (Pro), and Pro and hydroxyproline (Hyp) in combination on the growth performance, total Hyp and collagen concentrations of tissues, and prolyl 4-hydroxylase α(I) (P4H α(I)) gene expression in juvenile turbot feeding high plant protein diets. A diet containing 50% crude protein and 12% crude lipid was formulated as the basal and control, on which other two protein and lipid contents identical experimental diets were formulated by supplementing the basal with either 0.75% Pro (Pro-0.75) or 0.75% Pro and 0.75% Hyp (Pro+Hyp). Four groups of fish in indoor seawater recirculating systems, 35 individuals each, were fed twice a day to apparent satiation for 10 weeks. The results showed that dietary Pro and Hyp supplementation had no significant effect on growth performance and feed utilization of juvenile turbot (0.05). Total Hyp and collagen concentrations in muscle were significantly increased when dietary Pro and Hyp increased (0.05), and fish fed diet Pro+Hyp showed significantly higher free Hyp content in plasma than those fed other diets (0.05). The expression of P4H α(I) gene in liver and muscle was significantly up regulated in fish fed diet Pro-0.75 in comparison with control (0.05); however the gene was significantly down regulated in fish fed diet Pro+Hyp in muscle in comparison with fish fed diet Pro-0.75 (0.05). It can be concluded that supplement of crystal L-Pro and L-Hyp to high plant protein diets did not show positive effects on growth performance of juvenile turbot, but enhanced total collagen concentrations in muscle.
proline; hydroxyproline; juvenile turbot; high plant protein; collagen; prolyl 4-hydroxylase α(I)
1 Introduction
Proline (Pro) and hydroxyproline (Hyp) are unique amino acids both chemically and biochemically (Hu., 2008; Kaul., 2008). Continuously increasing evidences prove that Pro is a key regulator of multiple biochemical and physiological processes in cells (Wu., 2011). For example, Pro is a major nitrogenous substrate for the synthesis of polyamines (Wu., 2000; Wu., 2005). Pro and its metabolite pyrroline-5-carboxylate (P5C) are now known to regulate gene expression and cellular signaling pathways that are crucial for health and disease (Hu., 2008). Pro can scavenge free radicals (Kaul., 2008) by participating redox reactions in humans and animals (Phang., 2008, 2010). Pro may play a role in regulating the mammalian target of rapamycin (mTOR) activation pathway (van Meijl., 2010). While most mammals can synthesize Pro from arginine and glutamine/glutamate, the rate of endogenous synthesis is not adequate for neonates, birds and fish (Li., 2009; Wu., 2010). To date, several studies have demonstrated that Pro is essential for poultry (Graber., 1970; Baker, 2009), young mammals (Ball., 1986) and wounded mammals (Barbul, 2008). Therefore, Pro could be considered as a conditionally essential amino acid for mammalian, avian and aquatic species (Baker, 2009; Wu., 2010). Accordingly, it is valuable to investigate the effect of dietary Pro on the growth performance of fish.
Hyp is a post-translational metabolite of Pro in protein (primarily collagen) by vitamin C-dependent prolyl hydroxylase (Stanley, 1983) and free Hyp is generated from the degradation of collagens or other proteins containing hydroxylprolyl (Phang., 2008, 2010). Hyp is considered as a conditionally essential amino acid (Li., 2009), and its content in fish meal is much higher than that in plant protein sources (Li., 2011). Aksnes. (2008) found that dietary supplementation with crystalline Hyp to a plant protein-based diet enhances weight gain of salmon. Therefore, the possible impact of Hyp on growth performance has to be taken into account in formulating diets, especially high plant protein diets (Aksnes., 2006a, b; Kousoulaki., 2009). However, in our previous study, no significant difference was found in the growth performance of turbot fed high plant protein diets with graded contents of Hyp (Zhang., 2013). To date, it is not clear whether dietary Pro and Hyp has synergistic effect on the growth performance of fish fed high plant protein diets.
Pro and Hyp are major amino acids in collagen and they are vital for collagen biosynthesis, structure and strength (Barbul, 2008). Pro is an essential substrate for collagen synthesis (Gorres and Raines, 2010). Although Hyp can not be used as a substrate for the synthesis of collagen directly, it is essential for the folding of the newly synthesized procollagen polypeptide chains into stable triple-helical molecules (Myllyharju and Kivirikko, 2001). Prolyl 4-hydroxylase (P4H, EC 1.14.11.2) is the key modifying enzyme catalyzing the formation of 4-Hyp from Pro residues presenting in newly synthesized polypeptide chains of collagens (Kivirikko., 1990). P4H α(I) subunit contributes a major part to the catalytic site and are limiting in the formation of active P4Hs (Annunen., 1998). In our previous study, we found that total collagen concentration in muscle is significantly increased, while P4H α(I) gene expression in muscle is significantly decreased in turbot fed diets with increased Hyp (Zhang., 2013). It is unknown whether dietary Pro and Hyp have synergistic effect on the collagen concentration and P4H α(I) gene expression in fish fed high plant protein diets.
Turbot (L.) is an important commercial carnivorous fish species that has been widely farmed in Europe and East Asia because of its delicious meat and fast growth. Turbot has high dietary protein requirement (Lee., 2003; Cho., 2005) and fish meal is the main protein source in practical diets for turbot (Bonaldo., 2011). The main purpose of the present study was to investigate the effect of dietary Pro and the combination of Pro and Hyp on the growth performance, Hyp and collagen concentrations and P4H α(I) gene expression in juvenile turbot fed high plant protein diets.
2 Materials and Methods
2.1 Experimental Diets
L-Pro (content>99%) and L-Hyp (content>99%) were obtained from Hengyuan Biotech. Co., Ltd (Shanghai, China). White fish meal (WFM), soybean meal (SBM), and wheat gluten meal (WGM) were used as the major protein sources, fish oil as the major lipid source and wheat flour as the carbohydrate source. Lysine-H2SO4, DL-Methionine, L-Threonine, L-Arginine, L-Isoleucine, L-Leucine, and L-Valine (crystalline amino acids) were supplemented to meet the essential amino acids requirements of juvenile turbot based on the whole body amino acid profile (Kaushik, 1998). The basal diet with 50% crude protein and 12% crude lipid was the same as that we formulated in our previous study (Zhang., 2013). The other two protein and lipid contents identical experimental diets were formulated by supplementing the basal diet with either 0.75% Pro or 0.75% Pro and 0.75% Hyp, which were named as Pro-0.75 and Pro+Hyp, respectively. The content of L-Alanine was adjusted in order to maintain an equal nitrogen concentration in all diets (Table 1). The final content of dietary Pro was 3.22%, 3.88% and 3.82%, respectively, while the final content of Hyp was 0.12%, 0.22%, and 0.67%, respectively. The amino acid composition of the experiment diets was shown in Table 2.
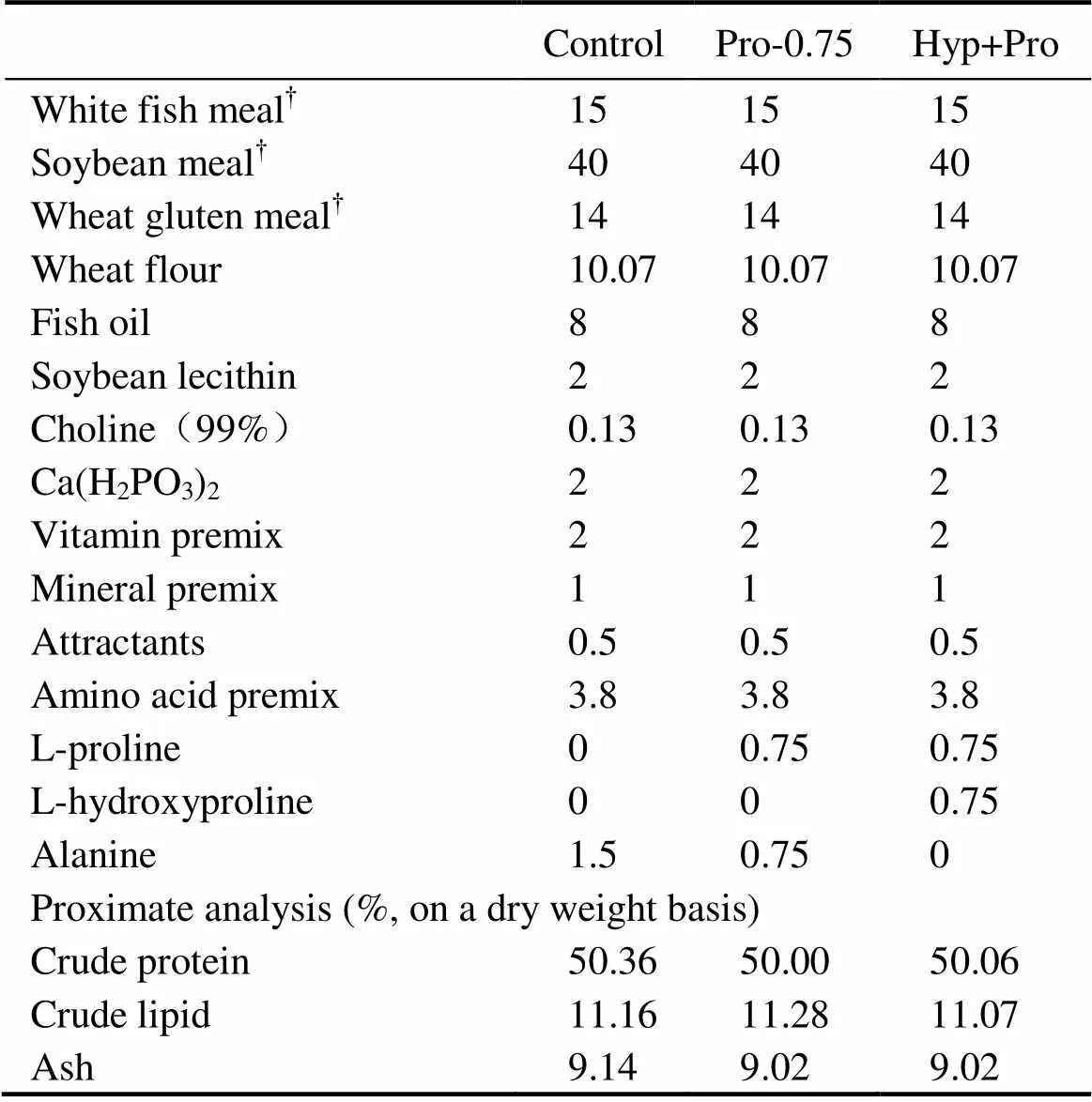
Table 1Formulation and proximate composition of the experimental diets (% dry matter)
Notes:?Supplied by Qihao Biotech. Co., Ltd. (Shandong, China); white fish meal, crude protein, 73.78%, crude lipid, 9.20%; soybean meal, crude protein, 50.34%, crude lipid 0.98%; wheat gluten meal, crude protein, 81.42%, crude lipid 2.00%. Vitamin premix (mgkg?1diet): retinyl acetate, 32; vitamin D3, 5; DL-α- tocopherol acetate, 240; vitamin K3, 10; thiamin, 25; riboflavin (80%), 45; pyridoxine hydrochloride, 20; vitamin B12(1%), 10; L-ascorbyl-2-monophosphate-Na (35%), 4000; calcium pantothenate, 60; nicotinic acid, 200; inositol, 800; biotin (2%), 60; folic acid, 20; ethoxyquin, 503; cellulose, 13970. Mineral premix (mgkg?1diet): MgSO4?7H2O, 1200; CuSO4?5H2O, 10; ZnSO4?H2O, 50; FeSO4?H2O, 80; MnSO4?H2O, 45; CoCl (1%), 50; Na2SeO3(1%), 20; Ca(IO3)2(1%), 60; calcium propionate, 1000; zoelite, 7485. Attractants: taurine:glycine:betaine=1:3:3. Amino acid premix (gkg?1diet): Lys-H2SO4, 8; DL-Methionine, 5; L-Threonine, 5; L-Arginine, 5; L-Isoleucine, 4; L-Leucine, 5; L-Valine, 6. L-proline and L-Hydroxyproline obtained from Hengyuan Biotech. Co., Ltd (Shanghai, China).
All ingredients were first ground to fine powder through a 180μm mesh. Pro and Hyp were blended as an amino acid premixture. The ingredients were then thoroughly mixed with fish oil, and water was added to produce stiff dough. The dough was then pelletized using an experimental feed mill (F-26(II), South China University of Technology, China) and dried for about 12h in a ventilated oven at 45℃, and were stored at ?20℃ until use. No difference in physical quality and sinking property was found between diets.

Table 2 Amino acid composition of the experimental diets (% dry matter)
2.2 Fish, Experimental Procedure and Conditions
The fish were fed exactly as what we have done early (Zhang., 2013). Juvenile turbotwere obtained from Jiaonan Guzhenying Turbot Farms (Shandong, China). Fish were transported to experiment station (Experimental Base of Ocean University of China, Qingdao, China) and stocked into indoor seawater recirculating systems (Fiberglass circular tanks with flat bottom, 400-L capacity filled to 300-L) to acclimatize for 2 weeks. During this period, fish were fed a commercial diet (Qihao Biotech. Co. Ltd., Shandong, China) twice a day to satiation. All rearing tanks were continuously aerated under natural photoperiod.
At the beginning of experiment, fish were fasted for 24h and weighed. Fish in similar sizes (8.12±0.01g) were randomly distributed into 12 tanks, 35 each tank and 4 tanks each diet. Fish were hand-fed to apparent satiation twice (08:00 and 18:00) a day with the consumption each tank recorded. Any uneaten feed was collected 1h after feeding, dried to constant weight at 70℃ and reweighed. Leaching loss in uneaten diet was estimated by leaving five samples each diet in tanks without fish for 1h, recovering, drying and reweighing. The feeding trial lasted for 10 weeks, during which water temperature varied between 15.0 and 18.0℃; salinity between 30 and 33; and pH between 7.5 to 8.0; and ammonia nitrogen was<0.1 mgL?1; nitrite<0.1mgL?1; and dissolved oxygen>6.0mgL?1.
2.3 Sample Collection
Ahead of experiment, ten fish individuals from the same population were randomly selected for determining the initial whole body proximate composition. At the end of experiment, fish were fasted for 24h and anaesthetized with eugenol (1:10000) (purity 99%, Shanghai Reagent, China) before sampling. Total number and mean body weight of fish each tank were measured. Four fish individuals each tank were randomly sampled and stored at ?20℃ for whole body composition analysis. Blood samples were taken from the caudal vein using heparinized syringes to obtain plasma samples after centrifugation (4000×g, 4℃, 10min) and immediately frozen in liquid nitrogen, and then stored at ?80℃ until analysis. Six fish individuals each tank were sampled for morphometric parameters. Individual body weight, body length, liver weight and visceral weight were recorded to calculate condition factor, hepatosomatic index and viserosomatic index. Liver and muscle samples were also frozen in liquid nitrogen, and then stored at ?80℃ for subsequent analysis of total Hyp and collagen contents and P4H α(I) gene expression.
2.4 Chemical Analysis
2.4.1 Body composition
Moisture, crude protein, crude lipid, and ash were analyzed for ingredients, experimental diets and fish samples (AOAC, 1995). Moisture was analyzed by drying the samples to constant weight at 105℃. Crude protein was determined using the Kjeldahl method and estimated by multiplying nitrogen by 6.25. Crude lipid was quantified by ether extraction using Soxhlet. Ash was examined by combustion in a muffle furnace at 550℃ for 16h. Duplicate analyses were conducted for each sample.
2.4.2 Amino acid content determination
Amino acids content were determined with the method of Xie. (2012). Samples of experimental diets were freeze-dried and 0.02g of samples was used for amino acid analysis. The samples were hydrolyzed with 15mL of 6molL?1HCl at 110℃ for 24h, then filtered and added to ultrapure water (from Milli-Q system, Millipore, Billerica, MA, USA) in a 50mL volumetric flask. Two milliliters of solution was transferred to a glass bottle and dried in a Binder Oven (VD23, Binder Company, Germany). Two milliliters of ultrapure water was added to the bottles and dried in the Binder Oven three times, and two milliliters of loading buffer was added to dissolve the remains. The supernatant was analyzed with ninhydrin method using an automatic amino acid analyzer (Biochrom 30, GE, Biochrom Ltd, Cambridge, UK) equipped with a sodium exchange column (μ-2345). The column temperature was 37–135℃. Ultraviolet detection was performed at a wavelength of 440nm (for proline) and 570 nm (for other amino acids).
2.4.3 Hyp content determination
The Hyp content in plasma and tissues was determined through the procedure reported by Reddy and Enwemeka (1996) with some modifications. Aliquots of 1mL standard Hyp (1–30μgmL?1; prepared from stock solution of Hyp (Sigma-Aldrich Corp., St. Louis, MO, USA): 1mg mL?1in 1mmolL?1HCl) or 100μL plasma samples were mixed with 2mL buffered chloramines T reagent (1.4gchloramines T dissolved in 20mL water, and then diluted with 30mL n-propanol and 50mL acetate-citrate buffer (pH 6.5); made fresh daily) and incubated for 20min at room temperature. Then, 2mL perchloric acid (27mL 70% perchloric acid diluted into 100mL volumetric flasks) was added and the mixture was incubated for a further 5min at room temperature before addition of 2mL P- DMAB solution (10% P-DMAB in n-propanol). The mixture was incubated at 60℃ for 20min to develop chromophore and was cooled down. Then the absorbance was read at 560nm using a spectrophotometer. The Hyp concentration was determined from a standard curve.
Approximately, 10–30mg tissue was hydrolyzed by 1mL 6molL?1hydrochloric acid at 130℃ for 3h. Before analysis, samples were diluted into 10mL volumetric flasks with ultra-pure water and mixed, filtered through 0.20mm filter. One milliliter of the solution was used to determine Hyp content with the same method as for plasma.
2.4.4 Vertebrae preparation
Vertebrae samples were prepared according to the method described by Aksnes. (2008) with some modifications. Four fish each tank were thawed overnight at about 15℃. The fish was gutted and filleted with head and tail remaining. The remaining meat was removed as much as possible by scraping the back bone with a small knife. The fish of the same tank were dipped in boiling water for 60s at a time with the remaining meat on the bones thoroughly removed using running cold water. Head and tail were removed from the vertebral column and side bones were cut at the vertebral base. Vertebrae free from surface water were weighed. The vertebral columns were lyophilized and the dried samples from each tank were pooled and ground into a fine powder to determine the Hyp content.
2.4.5 Calculation of collagen content
The collagen content in muscle and vertebrae were estimated by multiplying the Hyp content (% of sample) by 8 according to AOAC method 990.26 (AOAC, 2000), considering that collagen in connective tissue contains 12.5% Hyp if the nitrogen-to-protein factor is 6.25.
2.4.6 RNA extraction and real-time quantitative PCR analysis of P4H α(I) gene expression
Total RNA from liver and muscle of 3 fish individuals each tank was extracted using Trizol Reagent (Invitrogen, USA). The RNA was separated through a 1.2% denature-ing agarose gel to check the integrity. The RNA was treated with Recombinant DNase I (RNase-free) (Takara, Japan) to remove possible DNA contaminant according to the manufacturer’s instructions. The quantity and quality of total RNA were assessed using Nano Drop?ND-1000 spectrophotometer (Nano-Drop Technologies, Wilmington, DE, USA). The ratio of 260/280 absorbances of all tissues ranged from 1.81 to 1.97, indicating a satisfactory purity of RNA extracted. Purified RNA was subjected to reverse transcription to cDNA with PrimeScriptTMRT reagent Kit (Takara, Japan) following the instruction from supporter. The primers for real-time PCR were designed using Primer Premier 5.00 based on nucleotide sequences of P4H α(I) gene of turbot (JX863890). Real-time PCR was carried out in a quantitative thermal cycler (Mastercycler ep realplex, Eppendorf, German) in a final volume of 25μL containing 12.5μL 2×SYBR?Premix Ex TaqTM(Perfect Real Time) (Takara, Japan), 0.5μL of primers (each 10μmolL?1), 2μL of cDNA mix. P4H α(I) Gene- specific primers P4H α(I) F (5’-GAC ACC ACT GAT GGG TTT ATT TCC-3’), P4H α(I) R (5’-TTC ACG CCA GGT AGG TCT CC-3’) were applied to evaluate the mRNA abundance of P4H α(I) gene in liver and muscle. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (DQ848904) (F: 5’-TCC AAT GTT TGT CAT GGG AGT T-3’; R: 5’-CCA GAG GAG CCA GGC AGT T-3’) was used as internal reference. The real-time PCR began with 2min at 95℃, followed by 40 cycles of 10s at 95℃, 10s at 58℃, and 20s at 72℃. No template controls were run for each PCR assay. A four-fold serial dilution was used to assess PCR efficiencies for each assay, quantifying five concentrations (in triplicate). The primer amplification efficiency was analyzed according to the following equation E=10(–1/Slope)?1, the value was 0.9904 for P4H α(I) and 0.9717 for GAPDH in liver and 0.9763 for P4H α(I) and 1.010 for GAPDH in muscle. The absolute ΔCTvalue (P4H α(I) CT?GAPDH CT) of the slope is 0.0465 for liver and 0.0814 for muscle, which indicated that the ΔΔCTcalculation for the relative quantification of P4H α(I) could be used. The expression contents of P4Hα(I) was calculated by 2-ΔΔCTmethod, and the value stood for n-fold difference relative to the calibrator (Livak and Schmittgen, 2001).
2.5 Calculations and Statistical Analysis
The following variables were calculated:
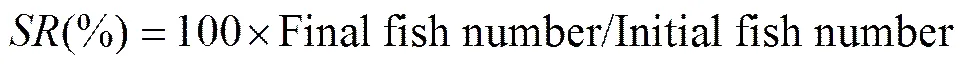
,
,
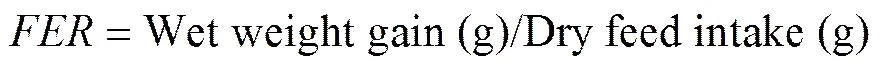
,
,
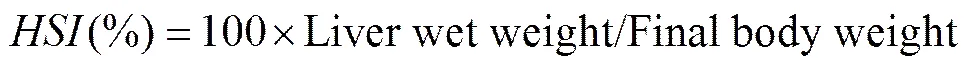
,
whereis survival rate,is thermal-unit growth coefficient,is feed intake,is feed efficiency rate,is protein efficiency ratio,is condition factor,is hepatosomatic index, andis viscerosomatic index. The Software SPSS 17.0 was used for all statistical evaluations. All data were subjected to one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test. Differences were regarded as significant when<0.05. All data were expressed as means ± standard error.
3 Results
3.1 Survival rate and Growth Performance
during this feeding trial ranged from 99.05% to 100% and was independent of dietary treatments (>0.05, Table 3). There was no significant difference in((1.08–1.10)×1000),(1.29%–1.30% per day),(1.36–1.38), or(2.65–2.74) of juvenile turbot fed different diets (>0.05, Table 3).
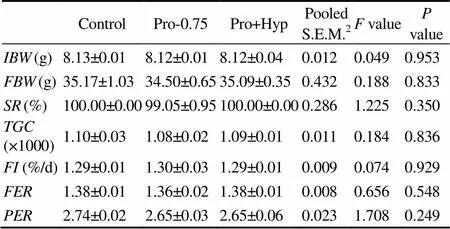
Table 3Survival rate and growth performance of turbot fed diets with different contents of Pro and/or Hyp
Notes: Data are expressed as means ± SE (=4). Values in the same column with the same superscript or absence of superscripts are not significant different (>0.05); S.E.M.: standard error of means; IBW: initial body weight; FBW: final body weight; SR: survival rate; TGC: thermal-unit growth coefficient;FI: feed intake; FER: feed efficiency rate; PER: protein efficiency ratio.
3.2 Body Composition
There was no significant difference in moisture (76.96%–77.06%), crude protein (15.11%–15.20%), or crude lipid (3.87%–4.03%) contents of whole body among fish fed experimental diets (0.05, Table 4).However, fish fed diets Pro-0.75 and Pro+Hyp showed significantly increased crude ash content of whole body compared to that of control (0.05, Table 4).
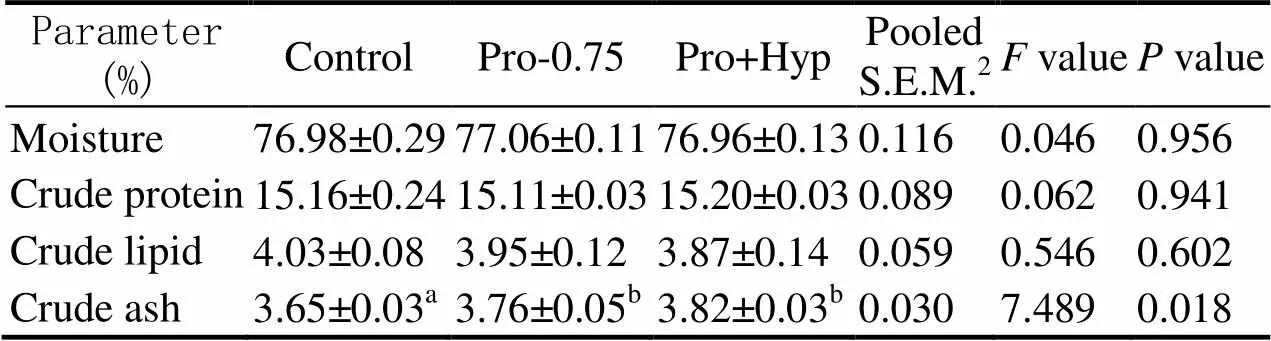
Table 4 Proximate composition (% wet weight) in whole body of turbot fed diets with different contents of Pro and/or Hyp
Notes: Data are expressed as means ± SE (=4).Values in the same column with the same superscript or absence of superscripts are not significant different (>0.05); S.E.M.: standard error of means.
3.3 Condition Factor, Hepatosomatic Index, and Viscerosomatic Index
No significant difference in K (3.63%–3.79%), HSI (0.93%–1.03%), and VSI (5.54%–5.61%) was found among diets (0.05, Table 5).
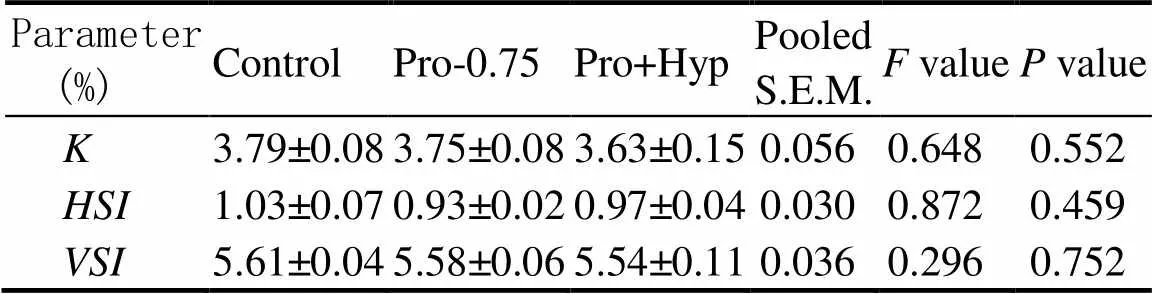
Table 5Condition factor (K), Hepatosomatic (HSI), and viscerosomatic (VSI) index of juvenile turbot fed diets with different contents of Pro and/or Hyp
Notes: Data are expressed as means ± SE (=4). Values in the same column with the same superscript or absence of superscripts are not significant different (>0.05). S.E.M.: standard error of means., Condition factor;, Hepatosomatic index;, Viscerosomatic index.
3.4 Hyp Content in Liver, Plasma, Muscle and Vertebrae
Total Hyp content in liver (0.260–0.285gkg?1) was not significantly affected by diets (>0.05, Table 6). Fish fed diet Pro+Hyp showed significantly higher free Hyp con- tent in plasma compared to fish fed the other diets (0.05). Total Hyp concentration in muscle was significantly increased as dietary Pro and/or Hyp increased (0.05), and fish fed diet Pro+Hyp showed the significantly highest total Hyp concentration in muscle (0.05).
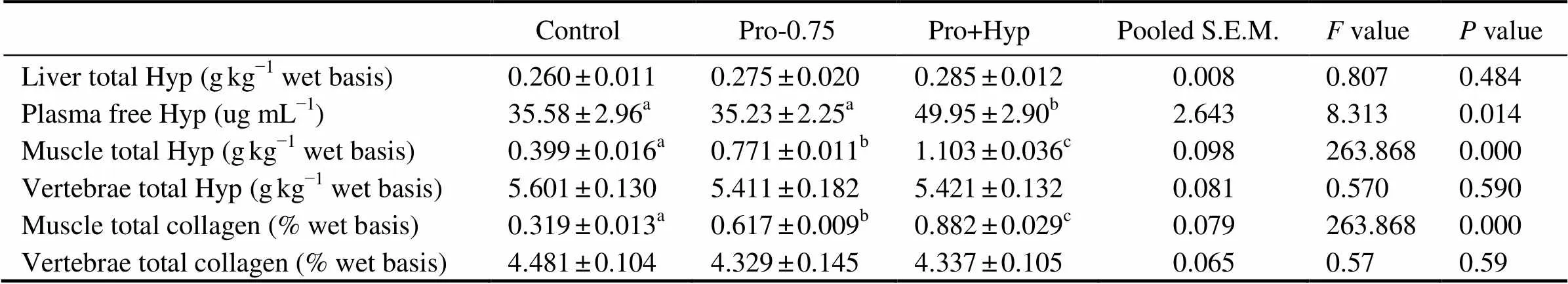
Table 6Liver total Hyp, plasma free Hyp, muscle and vertebrae total Hyp, muscle and vertebrae total collagen concentrations of juvenile turbot fed diets with different contents of Pro and/or Hyp
Notes: Data are expressed as means ± SE (=4). Values in the same column with the same superscript or absence of superscripts are not significant different (>0.05); S.E.M.: standard error of means.
Total Hyp concentration in vertebrae (5.411–5.601gkg?1) was much higher than that in muscle (0.399–1.103gkg?1) and liver (0.260–0.285gkg?1) of fish, but no significant difference was found among diets (0.05, Table 6).
3.5 Muscle and Vertebrae Collagen Concentration
Total collagen concentration in muscle of fish showed the same trend as total Hyp content in muscle, which was significantly enhanced as dietary Pro and/or Hyp increased (<0.05, Table 6). Fish fed diet Pro+Hyp showed the significantly highest total collagen concentration in muscle compared to other diets (<0.05).
Total collagen concentration in vertebrae of fish fed experiment diets ranged from 4.329% to 4.481%, which were much higher than those in muscle (0.319%–0.882%, Table 6), but no significant difference was observed among diets (0.05).
3.6 Expression of P4H α(I) Gene in Liver and Muscle
Expression of P4H α(I) gene in liver and muscle was significantly affected by dietary Pro and/or Hyp content (<0.05, Fig.1). Fish fed diet Pro-0.75 showed the highest hepatic P4H α(I) gene abundance (1.36±0.04), which was significantly higher than that in control (<0.05), but no significant difference was found between fish fed diet Pro-0.75 and Pro+Hyp (0.05). P4H α(I) gene abundance in muscle was significantly higher in fish fed diet Pro-0.75 and Pro+Hyp compared to fish fed the control diet (<0.05), and fish fed diet Pro+Hyp showed significantly lower P4H α(I) gene abundance in muscle compared to fish fed diet Pro-0.75 (<0.05).
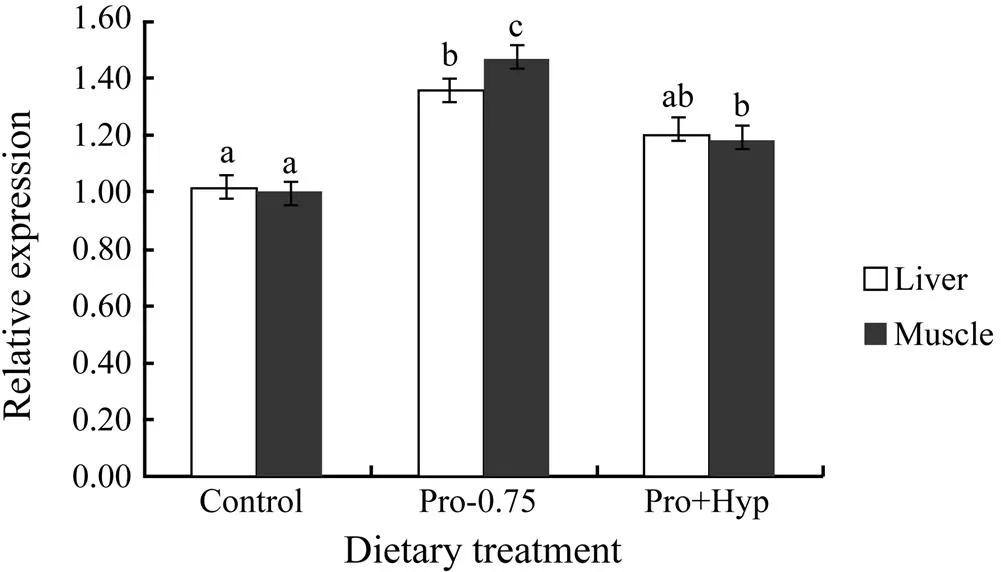
Fig.1 Relative mRNA expression of P4H α(I) in the liver and muscle of turbot fed diets with different contents of Pro and/or Hyp for 10 weeks.It was evaluated by real-time quantitative PCR. Values are means ± S.E.M. (n=4). Bars of the same tissue with same letters are not significantly different by Tukey’s test (P>0.05).
4 Discussion
Pro and Hyp are unique amino acids for maintaining cell structure and function. They are now considered conditionally essential for mammalian, avian and aquatic species (Zhang., 2006; Baker, 2009; Li., 2009; Wu., 2010). The requirement for Pro as a nutrient for poultry (Graber., 1970; Baker, 2009), young mammals (Ball., 1986), and wounded mammals (Barbul, 2008) were determined already. For example, dietary supplementation of Pro dose-dependently improved daily weight gains of young chicken (Graber., 1970) and young pig (Kirchgessner., 1995). Also supplementing 1% Pro to a corn- and soybean meal-based diet enhanced the growth performance of weanling pig (Wu., 1996). Additionally, supplementing 0.07%, 0.14%, and 0.28% crystalline Hyp in high plant protein diets enhanced weight gain of Atlantic salmon, but no significant effect was observed for dietary Pro addition (Aksnes., 2008). However, in the present study, dietary Pro and Hyp supplementation had no significant effect on growth performance and feed utilization of juvenile turbot, which was inconsistent with the documented but was in agreement with the findings of Albrektsen. (2010) and Kousoulaki. (2010) who found that growth performance of Atlantic salmon was not significantly affected by dietary supplementation of free/bone Hyp and crystalline Hyp. Also in a recent study on turbot, Zhang. (2013) have found that dietary inclusion of L-Hyp had no positive effect on growth performance. The different responses were probably due to species difference, dietary amino acid composition, and/or experimental duration.
In the present study, there was no significant difference in moisture, crude protein, and crude lipid contents of whole body among fish fed the experimental diets, but fish fed diets Pro-0.75 and Pro+Hyp showed significantly higher crude ash content compared to control. Due to lack of comparable studies on the effect of dietary Pro and/or Hyp on body composition of fish, it is difficult to compare the results with those of other studies, except for general nutritional knowledge. As the major amino acids in collagen, Pro and Hyp are vital for collagen biosynthesis, structure and strength (Barbul, 2008). They may affect tissues rich in Pro and Hyp, such as bone and skin, which contain about 50% and 25% of total collagen, respectively (Bollet, 1994). During bone growth, the organic matrix which is mainly comprised of collagen fibrosis first forms as osteoid, and subsequently mineralized (Meunier, 2002). Therefore, the effect of dietary Pro and Hyp on crude ash contents of whole body may be due to the influence on bone mineralization. However, further studies are needed to investigate the exact mechanism involved in this process.
In the present study, fish fed diets with increased Pro and/or Hyp showed significantly higher total Hyp concentration in muscle, and free Hyp content in plasma was significantly increased in fish fed diet Pro+Hyp compared to control. Some work have confirmed that Hyp content in tissues and plasma was significantly enhanced as dietary Hyp increased (Kousoulaki., 2009; Albrektsen., 2010; Zhang., 2013). Because Hyp locates almost exclusively in collagen thus being essential for stabilizing the triple helical structure of collagen (Brinckmann., 2005), its content in tissues and plasma is thought to be a reliable indicator of collagen metabolism (Kindt., 2003).
In the present study, total collagen concentration in muscle was significantly enhanced as dietary Pro and/or Hyp increased and fish fed diet Pro+Hyp showed the significantly highest total collagen concentration in muscle. The results suggested that dietary Pro and Hyp have synergistic effects on collagen biosynthesis in muscle. Collagen, the most abundant protein of the extracellular matrix, plays many important structural and functional roles in tissues and influences the texture property, and functional and tensile strength of flesh (Aidos., 1990; Gordon and Hahn, 2010). Therefore, the increased dietary Pro and/or Hyp content may consequently improve the property of muscle.
Collagens form more than 90% of the organic mass of bone and provide most of the biomechanical properties essential for the function of bone (Gelse., 2003), thus slight difference may cause significant biological effect. Although total collagen concentration in vertebrae was much higher than that in muscle, the vertebrae total collagen concentration was independent of dietary Pro and Hyp contents, which was inconsistent with the findings of Aksnes. (2008), who found that fish fed diets with increasing dietary Hyp content showed slight but significantly higher Hyp content in vertebrae. As Hyp locates mainly in collagen, the increased Hyp content indicated an increased concentration of collagen. The different responses were postulated to be due to different mechanism of collagen metabolism in vertebrae among species, and/or the different experimental duration and culture condition. The results in the present study also suggested that diet containing 3.22% Pro and 0.12% Hyp may be enough to maintain normal vertebrae collagen formation of juvenile turbot.
Collagen biosynthesis involves a large number of cotranslational and post-translational modifications in the polypeptide chains that affect the quality and stability of collagen molecule. P4H is one of the key enzymes required for the synthesis of collagens (Kivirikko., 1990) and P4H activity is controlled mainly by regulating the expression of P4H α subunit gene. In the present study,the expression of P4H α(I) gene in liver and muscle was significantly up regulated in fish fed diet Pro-0.75 in comparison with control; however the gene expression was significantly down regulated in fish fed diet Pro+Hyp in muscle in comparison with fish fed diet Pro-0.75 (< 0.05). Therefore, it could be concluded that to some extent the increased dietary Pro content enhanced the activity of P4H (I)in liver and muscle. This may have consequently resulted in higher collagen synthesis ability of fish fed diets with increased Pro content, and the reason was postulated to be due to the increased Pro availability for collagen biosynthesis. However, the increased dietary Hyp content may decrease the activity of P4H (I) in muscle which is in agreement with the recent study on turbot (Zhang., 2013). The improved collagen concentration in muscle of turbot fed diet with increased Hyp could be due to the suppression of collagen degradation.It should be noted that the composition and amount of collagen depend not only on the great number of posttranslational modification steps but also on the fractional degradation of collagen (Laurent, 1987). The results of the present study suggested that the improved collagen concentrations in muscle of fish fed diet with the combination of Pro and Hyp could be due to the increased synthesis and decreased degradation of collagen by dietary Pro and Hyp, respectively.
It can be concluded from the present study that dietary supplementation of L-Pro and L-Hyp to high plant protein diets did not show positive effect on growth performance of juvenile turbot, but significantly improved total collagen concentration in muscle which may be due to the increased synthesis and decreased degradation of collagen, respectively.
Acknowledgements
This study was financially supported by the China Agriculture Research System (CARS-50-G08), Agricultural Scientific and Technological Achievements into Capital (2010GB23600673) and the National Natural Science Foundation of China (Grant Nos. 31072222 and 30901 108).
Aidos, I., Lie, ?., and Espe, M., 1990. Collagen content in farmed Atlantic salmon (L.)., 47: 1440-1444.
Aksnes, A., Hope, B., and Albrektsen, S., 2006a. Size-fraction- ated fish hydrolysate as feed ingredient for rainbow trout () fed high plant protein diets. II: Flesh quality, absorption, retention and fillet levels of taurine and anserine., 261: 318-326.
Aksnes, A., Hope, B., H?stmark, ?., and Albrektsen, S., 2006b. Inclusion of fractionated fish hydrolysate in high plant protein diets for Atlantic cod,., 261: 1102-1110.
Aksnes, A., Mundheim, H., Toppe, J., and Albrektsen, S., 2008. The effect of dietary hydroxyproline supplementation on salmon (L.) fed high plant protein diets., 275: 242-249.
Albrektsen, S., Sirnes, E., Aksnes, A., and Hagen, ?., 2010. Impacts of dietary hydroxyproline on growth, muscle firmness, collagen and PYD cross-links formation in Atlantic salmon (). In:. Qingdao, China, 79.
Annunen, P., Autio-Harmainen, H., and Kivirikko, K. I., 1998. The novel type II prolyl 4-hydroxylase is the main enzyme form in chondrocytes and capillary endothelial cells, whereas the type I enzyme predominates in most cells., 273: 5989-5992.
Association of Official Analytical Chemists (AOAC), 1995.. 16th edition. Association of Official Analytical Chemists, Arlington, VA, 1-45.
Association of Official Analytical Chemists (AOAC), 2000.990.26, 985.14, 992.23, 991.36923.03,. 17th edition. Gaithersburg, Association of Official Analytical Chemists, MD.
Baker, D. H., 2009. Advances in protein-amino acid nutrition of poultry., 37: 29-41.
Ball, R. O., Atkinson, J. L., and Bayley, H. S., 1986. Proline as an essential amino acid for the young pig., 55: 659-668.
Barbul, A., 2008. Proline precursors to sustain mammalian collagen synthesis., 138: 2021S-2024S.
Bollet, A. J., 1994. Nutrition and diet in rheumatic diseases, In:,. 8th edition. Shills, M. E.,., eds., Lea and Febiger, Philidelphia, 1362-1374.
Bonaldo, A., Parma, L., Mandrioli, L., Sirri, R., Fontanillas, R., Badiani, A., and Gatta, P. P., 2011. Increasing dietary plant proteins affects growth performance and ammonia excretion but not digestibility and gut histology in turbot () juveniles., 318: 101-108.
Brinckmann, J., Notbohm, H., and Müller, P. K., 2005. Collagen,. Springer, Berlin, 247pp.
Cho, S. H., Lee, S. M., Lee, S. M., and Lee, J. H., 2005. Effect of dietary protein and lipid levels on growth and body composition of juvenile turbot (L.) reared under optimum salinity and temperature conditions., 11: 235-240.
Gelse, K., P?schl, E., and Aigner, T., 2003. Collagens–structure, function, and biosynthesis., 55: 1531-1546.
Gordon, M. K., and Hahn, R. A., 2010. Collagens., 339: 247-257.
Gorres, K. L., and Raines, R. T., 2010. Prolyl4-hydroxylase. Critical., 45: 106-124.
Graber, G., Allen, N. K., and Scott, H. M., 1970. Proline essentiality and weight gain., 49: 692-697.
Hu, C. A., Khalil, S., Zhaorigetu, S., Liu, Z., Tyler, M., Wan, G., and Valle, D., 2008. Human Δ1-pyrroline-5-carboxylate synthase: Function and regulation., 35: 665-672.
Kaul, S., Sharma, S. S., and Mehta, I. K., 2008. Free radical scavenging potential of L-proline: Evidence fromassays., 34: 315-320.
Kaushik, S. J., 1998. Whole body amino acid composition of European seabass (), gilthead seabream () and turbot () with an estimation of their IAA requirement profiles., 11: 355-358.
Kindt, E., Gueneva-Boucheva, K., Rekhter, M. D., Humphries, J., and Hallak, H., 2003. Determination of hydroxyproline in plasma and tissue using electrospray mass spectrometry., 33: 1081-1092.
Kirchgessner, M., Fickler, J., and Roth, F. X., 1995. Effect of dietary proline supply on N-balance of piglets. 3. Communication on the importance of nonessential amino acids for protein retention., 73: 57-65.
Kivirikko, K. I., Helaakoski, T., Tasanen, K., Vuori, K., Myllyl?, R., Parkkonen, T., and Pihlajaniemi, T., 1990. Molecular biology of prolyl 4-hydroxylase., 580: 132-142.
Kousoulaki, K., Albrektsen, S., Langmyhr, E., Olsen, H. J., Campbell, P., and Aksnes, A., 2009. The water soluble fraction in fish meal (stickwater) stimulates growth in Atlantic salmon (L.) given high plant protein diets., 289: 74-83.
Kousoulaki, K., Olsen, H. J., Albrektsen, S., Langmyhr, E., Campbell, P., and Aksnes, A., 2010. Fractionation of stickwater (SW) by micro-, ultra- and nano-filtration. Effect of different SW fractions and supplemented hydroxyproline and taurine on Atlantic salmon (L.) performance fed very low fish meal diets., 167.
Laurent, G. J., 1987. Dynamic state of collagen: Pathways of collagen degradationand their possible role in regulation of collagen mass., 1: C1-C9.
Lee, J. K., Cho, S. H., Park, S. U., Kim, K. D., and Lee, S. M., 2003. Dietary protein requirement for young turbot (L.)., 9: 283-286.
Li, P., Mai, K. S., Trushenski, J., and Wu, G. Y., 2009. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds., 37: 43-53.
Li, X. L., Rezaei, R., Li, P., and Wu, G. Y., 2011. Composition of amino acids in feed ingredients for animal diets., 40: 1159-1168.
Livak, K. J., and Schmittgen, T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2? ΔΔCT method., 25: 402-408.
Meunier, F. J., 2002. Skeleton. In:. Panfili, J.,., eds., Ifremer-IRD co edition, Brest, 65-87.
Myllyharju, J., and Kivirikko, K. I., 2001. Collagens and collagen-related diseases., 33: 7-21.
Phang, J. M., Donald, S. P., Pandhare, J., and Liu, Y. M., 2008. The metabolism of proline, a stress substrate, modulates carcinogenic pathways., 35: 681-690.
Phang, J. M., Liu, W., and Zabirnyk, O., 2010. Proline metabolism and microenvironmental stress., 30: 441-463.
Reddy, G. K., and Enwemeka, C. S., 1996. A simplified method for the analysis of hydroxyproline in biological tissues., 29: 225-229.
Stanley, D. W., 1983. Relation of structure to physical properties of animal materials. In:. Peleg, M., and Bagley, E. B., eds., Westport, Avi Publish. Comp., Inc., Connecticut, 157-206.
van Meijl, L. E., Popeijus, H. E., and Mensink, R. P., 2010. Amino acids stimulate Akt phosphorylation, and reduce IL-8 production and NF-kappaB activity in HepG2 liver cells., 55: 1568-1573.
Wu, G. Y., Meier, S. A., and Knabe, D. A., 1996. Dietary glutamine supplementation prevents jejuna atrophy in weaned pigs., 126: 2578-2584.
Wu, G. Y., Flynn, N. E., and Knabe, D. A., 2000. Enhanced intestinal synthesis of polyamines from proline in cortisol- treated piglets., 279: E395-E402.
Wu, G. Y., Bazer, F. W., Hu, J. B., Johnson, G. A., and Spencer, T. E., 2005. Polyamine synthesis from proline in the developing porcine placenta., 72: 842-850.
Wu, G. Y., Bazer, F. W., and Burghardt, R. C., 2010. Functional amino acids in swine nutrition and production. In:. Doppenberg, J.,., eds., Wageningen Academic Publishers, The Netherlands, 69-98.
Wu, G. Y., Bazer, F. W., Burghardt, R. C., Johnson, G. A., Kim, S. W., Knabe, D. A., Li, P., Li, X. L., McKnight, J. R., Satterfield, M. C., and Spencer, T. E., 2011. Proline and hydroxyproline metabolism: implications for animal and human nutrition., 40: 1053-1063.
Xie, F. J., Ai, Q. H., Mai, K. S., Xu, W., and Wang, X. J., 2012. Dietary lysine requirement of large yellow croaker (, Richardson 1846) larvae., 43: 917-928.
Zhang, K. K., Ai, Q. H., Mai, K. S., Xu, W., Liufu, Z. G., Zhang, Y. J., and Peng, M., 2013. Effects of dietary hydroxyproline on growth performance, body composition, hydroxyproline and collagen concentrations in tissues in relation to prolyl 4-hydroxylase α(I) gene expression of juvenile turbot,L. fed high plant protein diets., 404-405: 77-84.
Zhang, Y., Dabrowski, K., Hliwa, P., and Gomulka, P., 2006. Indispensable amino acid concentrations decrease in tissues of stomachless fish, common carp in response to free amino acid- or peptide-based diets., 31: 165-172.
(Edited by Qiu Yantao)
10.1007/s11802-015-2436-0
(July 8, 2013; revised December 5, 2013; accepted January 8, 2015)
.Tel: 0086-532-82031943 E-mail: qhai@ouc.edu.cn
ISSN 1672-5182, 2015 14 (3): 541-548
? Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
 Journal of Ocean University of China2015年3期
Journal of Ocean University of China2015年3期
- Journal of Ocean University of China的其它文章
- Research on China’s Aquaculture Efficiency Evaluation and Influencing Factors with Undesirable Outputs
- Sustainability Evaluation of Different Systems for Sea Cucumber (Apostichopus japonicus) Farming Based on Emergy Theory
- Tolerance, Oxygen Consumption and Ammonia Excretion of Ophiopholis sarsii vadicola in Different Temperatures and Salinities
- Effect of Shrimp (Litopenaeus vannamei) Farming Waste on the Growth, Digestion, Ammonium-Nitrogen Excretion of Sea Cucumber (Stichopus monotuberculatus)
- Larval and Juvenile Growth Performance of Manila Clam Hybrids of Two Full-Sib Families
- Cloning, Expression and Activity Analysis of a Novel Fibrinolytic Serine Protease from Arenicola cristata
