Induction of Antioxidant and Heat Shock Protein Responses During Torpor in the Gray Mouse Lemur,Microcebus murinus
Institute of Biochemistry&Department of Biology,Carleton University,Ottawa,ON KS 5B6,Canada
2UMR 7179 Centre National de la Recherche Scientifque,Muse′um National d’Histoire Naturelle,Brunoy 91800,France
3Department of Biology,Genetics Institute,University of Florida,Gainesville,FL 32611,USA
4Biochemistry Department,Schulich School of Medicine and Dentistry,Western University,London,ON N6A 5C1,Canada
5Chemistry and Chemical Engineering Department,Royal Military College Of Canada,Kingston,ON K7K 7B4,Canada
6Department of Surgery&Center for Engineering in Medicine,Massachusetts General Hospital&Harvard Medical
School,Charlestown,MA 02129,USA
Induction of Antioxidant and Heat Shock Protein Responses During Torpor in the Gray Mouse Lemur,Microcebus murinus
Cheng-Wei Wu1,3,#,a,Kyle K.Biggar1,4,#,b,Jing Zhang1,5,c, Shannon N.Tessier1,6,d,Fabien Piferi2,e,Martine Perret2,f, Kenneth B.Storey1,*,g
1Institute of Biochemistry&Department of Biology,Carleton University,Ottawa,ON K1S 5B6,Canada
2UMR 7179 Centre National de la Recherche Scientifque,Muse′um National d’Histoire Naturelle,Brunoy 91800,France
3Department of Biology,Genetics Institute,University of Florida,Gainesville,FL 32611,USA
4Biochemistry Department,Schulich School of Medicine and Dentistry,Western University,London,ON N6A 5C1,Canada
5Chemistry and Chemical Engineering Department,Royal Military College Of Canada,Kingston,ON K7K 7B4,Canada
6Department of Surgery&Center for Engineering in Medicine,Massachusetts General Hospital&Harvard Medical
School,Charlestown,MA 02129,USA
Heat shock proteins;
Antioxidant capacity;
Primate hypometabolism;
Stress response
A natural tolerance of various environmental stresses is typically supported by various cytoprotective mechanisms that protect macromolecules and promote extended viability.Among these are antioxidant defenses that help to limit damage from reactive oxygen species and chaperones that help to minimize protein misfolding or unfolding under stress conditions.To understand the molecular mechanisms that act to protect cells during primate torpor,the present study characterizes antioxidant and heat shock protein(HSP)responses in various organs of control(aroused)and torpid gray mouse lemurs,Microcebus murinus.Protein expression of HSP70 and HSP90α was elevated to 1.26 and 1.49 fold,respectively,in brown adipose tissue during torpor as compared with control animals,whereas HSP60 in liver of torpid animals was 1.15 fold of that in control (P<0.05).Among antioxidant enzymes,protein levels of thioredoxin 1 were elevated to 2.19 fold in white adipose tissue during torpor,whereas Cu–Zn superoxide dismutase 1 levels rose to 1.1 fold in skeletal muscle(P<0.05).Additionally,total antioxidant capacity was increased to 1.6 fold in liver during torpor(P<0.05),while remaining unchanged in the fve other tissues.Overall,our data suggest that antioxidant and HSP responses are modifed in a tissue-specifc manner during daily torpor in gray mouse lemurs.Furthermore,our data also show that cytoprotective strategies employed during primate torpor are distinct from the strategies in rodent hibernation as reported in previous studies.
Introduction
Survival in the face of unfavourable environmental conditions is a challenge for most animals.For instance,animal ftness is often limited by fuctuations in the availability of basic nutrients as well as by abiotic stresses(too hot,too cold,or too dry climate,low oxygen,etc.).When faced with environmental stresses,many animals exhibit adaptive responses that provide cytoprotection to combat potential damage to cells[1]. Changes in ambient temperature are among the most common stressors experienced by animals,which can often disrupt metabolic homeostasis.Such disruption can occur via a number of mechanisms including direct temperature effects on enzyme properties,protein conformation,and lipid fuidity, as well as secondary consequences such as changes in reactive oxygen species(ROS)generation.Many animals that must deal with extreme changes in temperature on a seasonal basis use strong metabolic rate depression to enter a torpid or dormant state when temperature is too cold(or too hot).They couple metabolic rate depression with enhanced cytoprotection,such as elevated levels of chaperones that help stabilize protein structure/function,as well as antioxidant defenses to deal with oxidative stress while in the hypometabolic state[2–4].
One of the hallmark responses to high temperature stress is the induction of heat shock proteins(HSPs),a group of chaperone proteins that function to aid proteome stability[5]. However,HSPs are now well known to be induced by many abiotic stresses that disrupt the cellular proteome,such as hypoxia,ischemia,oxidative stress,heavy metals,UV radiation,and low temperature[6,7].HSP protein family members are named according to their molecular weight and the best known HSP proteinsincludeHSP27,HSP40,HSP60, HSP70,HSP90α,and HSP110.Moreover,the family now includes many other chaperone proteins[8,9].Although different HSPs respond to different cellular cues,their primary function is to maintain proteome stability,by guiding the folding of nascent proteins,re-folding misfolded proteins,preventing protein aggregation,and directing the degradation of unstable proteins[10,11].HSP induction is a known component of metabolic rate depression in many systems,supporting longterm survival in hypometabolic states including dormancy,torpor,aestivation,and diapause.For example,the expression of HSP10,60,90,and 110 was all upregulated in the hepatopancreas following 14 days of estivation in snails(Otala lactea)[7], whereas expression of HSP70 and HSP27(and its phosphorylated form)was upregulated in skeletal muscle of hibernating bats(Myotis lucifugus)[12,13].
Entry into hypometabolic states can also cause fuctuations to aerobic metabolism,leading to altered ROS production and potential oxidative damages[14].This is particularly prominent in mammalian hibernation,since two factors come into play.First,intermittent arousals from torpor necessitate a huge increase in oxygen uptake and consumption(with a proportional increase in ROS generation)to power the thermogenesis required to rewarm the body to euthermia. Second,to maintain fuidity of lipid fuel depots at the low body temperature(Tb)during hibernation requires an increase in their polyunsaturated fatty acid(PUFA)content,which is highly susceptible to lipid peroxidation[15,16].Hence,antioxidant defenses are necessary during the hibernation.These are provided by both low molecular weight metabolic antioxidants as well as antioxidant enzymes including superoxide dismutase (SOD),catalase,peroxiredoxin(PRX),thioredoxin(TRX), glutathione peroxidase,and other glutathione-linked enzymes [17,18].
Recent studies have presented the gray mouse lemur (Microcebus murinus)as a new model for the study of primate adaptation to environmental stress[19].These small primates utilize daily or multi-daily torpor,reducing their metabolic rate(a maximum of~80%reduction compare to resting metabolic rate recorded)in order to cope with unfavourable conditions during the dry season in Madagascar,when food and water are limited and ambient temperatures are reduced [19].Previous studies have shown that under short-day conditions combined with food restriction,gray mouse lemurs showed evidence of higher oxidative stress associated with increased torpor expression[20].To date,little is known about the cytoprotective responses of lemurs during torpor.We hypothesized that during torpor,lemurs activate endogenous defense mechanisms to alleviate cellular stress,potentially using similar mechanisms as observed during torpor in wellstudied mammalian hibernators(e.g.,bats and ground squirrels)[12,13,21–23].To test this hypothesis,we examined the expression of proteins involved in the heat shock response and antioxidant defense in lemurs during daily torpor to identify potential molecular mechanisms of the stress response in primate torpor.
Results
Expression of HSPs during torpor
We frst examined the expression of three major heat shock proteins(HSP60,HSP70,and HSP90α)in control(aroused)and torpid animals.Multiplex assay was employed to evaluate the protein expression in lemur tissues including the liver,muscle,heart,kidney,white adipose tissue(WAT),and brown adipose tissue(BAT).As shown inFigure 1,expression of HSP70 and HSP90α in BAT was signifcantly higher during torpor as compared to control animals;which was 1.27±0.08 fold and 1.49±0.14 fold,respectively(P<0.05)(Figure 1A).Signifcantly higher amount of HSP60 was only observed in the liver during torpor(1.15±0.02 fold,compared to control;P<0.05)(Figure 1D).Otherwise,the expression of HSPs were comparable between control and torpor states in the WAT(Figure 1B),kidney(Figure 1C),heart(Figure 1E), and skeletal muscle(Figure 1F).
Total antioxidant defense during torpor
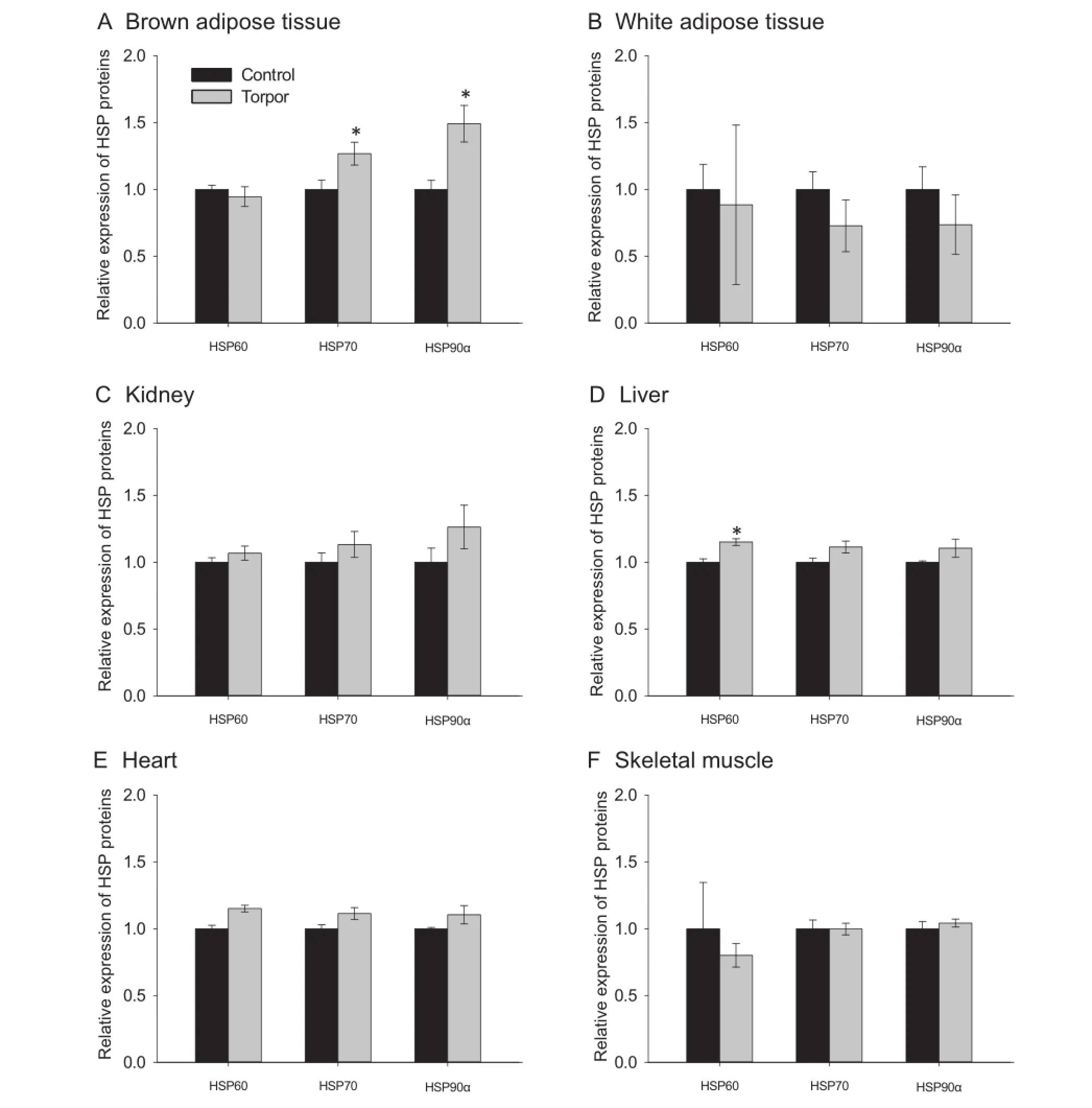
Figure 1 HSP expression in gray mouse lemurs during daily torpor
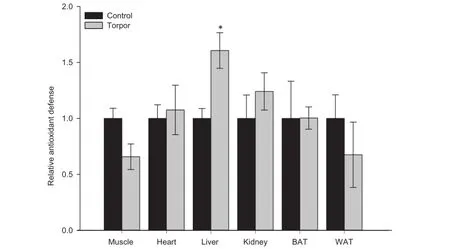
Figure 2 Total antioxidant capacity in gray mouse lemurs during daily torpor
We then evaluated the total antioxidant capacity in six lemur tissues comparing control and torpor conditions(Figure 2). The antioxidant assay kit measures the cumulative antioxidant capacity supplied by a variety of cellular antioxidant molecules including vitamin C,vitamin E,glutathione,bilirubin,albumin,and uric acid.This is accomplished by measuring the rate at which these cellular antioxidant molecules inhibit the metmyoglobin-catalyzed oxidation of 2,2′-azino-bis(3-ethyl benzthiazoline-6-sulfonic acid(ABTS)to its radical cation form.A signifcant change in tissue antioxidant capacity was observed only in liver,which is 1.61±0.16 fold in liver of torpid lemurs relative to control(aroused)animals(P<0.05). Totalantioxidantcapacity did notchange signifcantly between control and torpid lemurs in any of the other tissues, although antioxidant capacity in skeletal muscle tended to be lower during torpor.
Expression of antioxidant enzymes during torpor
The protein expression levels of fve antioxidant enzymes were measured using a Human Oxidative Stress Luminex panel in the six lemur tissues,comparing control(aroused)and torpor states.The fve antioxidant enzymes measured in this study are involved in the detoxifcation of ROS molecules and are crucial to the oxidative stress response.These enzymes include Cu/Zn-SOD1(the cytoplasmic form),Mn-SOD2(the mitochondrial form),catalase,thioredoxin 1(TRX1),and peroxiredoxin 2 (PRX2).Interestingly,expression of the majority of enzymes werecomparablebetweentorporand arousalin most of the tissues examined(Figure 3).Among them,SOD1 the protein expression of cytoplasmic SOD1 was signifcantly higher in both BAT and skeletal muscle during torpor (Figure 3A and F),which was 1.9±0.47 and 1.1±0.02 fold as compared to control,respectively(P<0.05).In addition, the expression of TRX1 was signifcantly higher during torpor(2.19±0.37 fold as compared with control)in WAT (Figure 3B;P<0.05).
Discussion
To survive in challenging environments,animals often need to display considerable phenotypic plasticity at a metabolic level to adjust their energy demands to the realities of fuel/energy availability in the environment.To date,it has been well documented that coordinated reductions in energy expenditures on nonessential metabolic processes and a shift toward an altered metabolism that includes multiple cytoprotective mechanisms are hallmarks of stress-induced hypometabolism[1].
The present study focuses on these two classes of cytoprotective proteins to analyze their roles in lemur torpor.Interestingly,wefoundthatexpression ofHSPswasmostly unchanged during torpor,with signifcant upregulation of selected HSPs observed only in BAT and liver(Figure 1).Elevated expression of HSP70 and HSP90α in BAT is particularly interesting,since this tissue produces heat through nonshivering thermogenesis(NST)to rewarm animals during arousal back to euthermia[24–27].Previous studies have shown that gene and protein expression of HSP70 was upregulated in BAT of Sprague–Dawley rats during cold exposure, in parallel with the induction of uncoupling protein 1 (UCP1),suggesting a specifc role for HSPs in this thermogenic organ[28].Thermogenesis in BAT arises from uncoupling ATP synthesis from the electron transport chain in the mitochondria,which requires the expression of UCP1[25–28].In lemurs,expression of UCP1 is also upregulated in BAT to support NST during torpor and/or arousal[25].HSP70,alongwith HSP90α,also functions as a molecular chaperone in the mitochondria to promote translocation and folding of mitochondrialproteins[29,30].Upregulation ofHSP70and HSP90α could contribute to ensuring proper folding of UCP1 in the mitochondria,as an aid to NST during torpor in the lemur[28,29],and/or aid overall maintenance of the active conformations of proteins in the face of rapidly-rising temperatures in BAT during active NST.
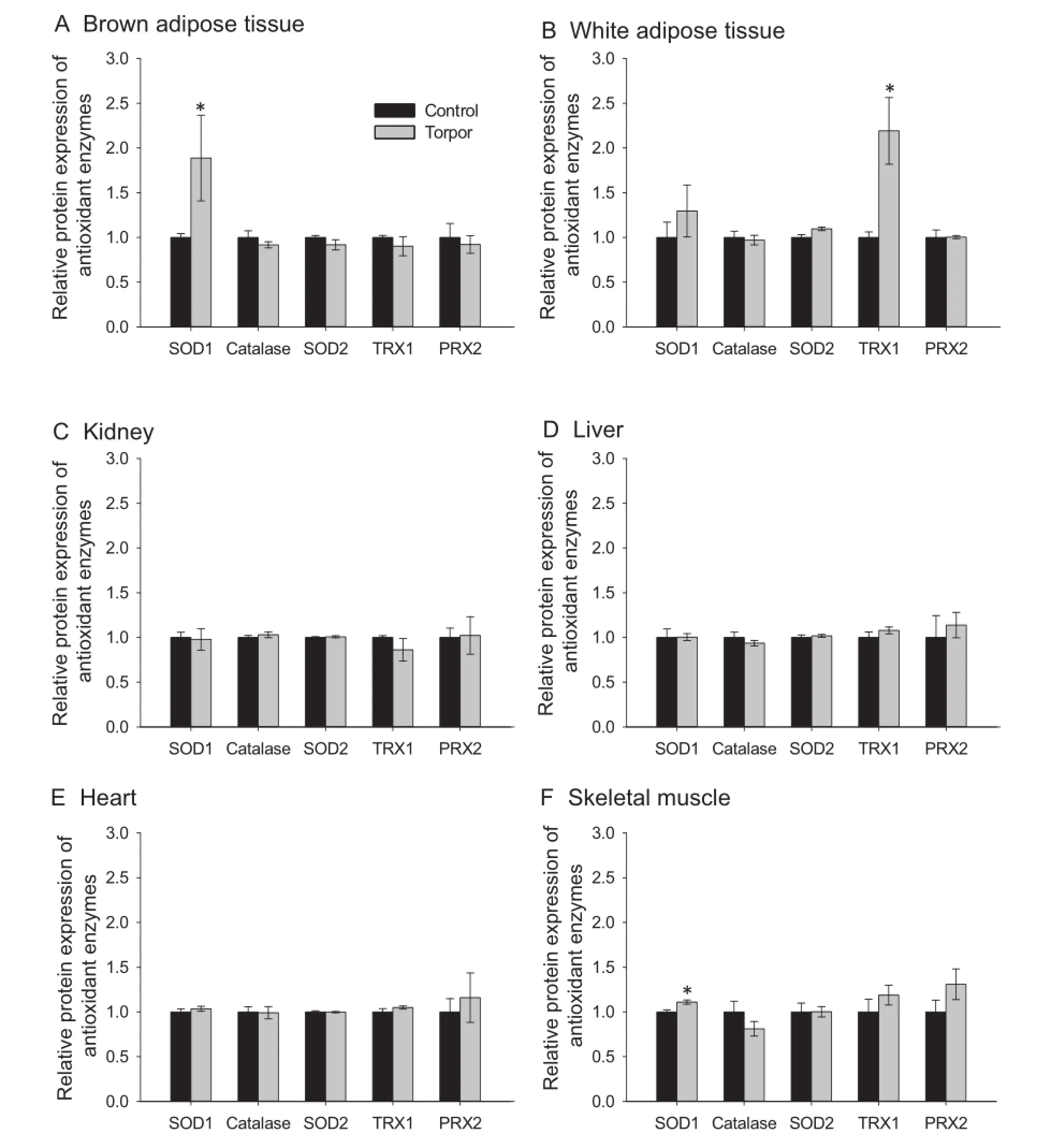
Figure 3 Expression of antioxidant enzymes in gray mouse lemurs during daily torpor
Compared to the other tissues studied,expression of HSP60 was signifcantly upregulated only in the liver during torpor, albeit to a minor extent(Figure 1).HSP60 is a mitochondrial chaperone and plays a crucial role in regulating cell survival inresponse to increased levels of iron-dependent oxidative stress [31].Previous studies showed that peroxide levels were elevated in HSP60-depleted cells,while elevated expression of HSP60 led to greater cellular resistance against H2O2and superoxide anions[31].Additionally,recent studies have also shown that upregulation of HSP60 expression is linked to chemicallyinduced ROS elevation inDrosophila,as well as type-2 diabetes associated oxidative stress in HeLa cells[32,33]. Therefore,the upregulation of HSP60 in lemur torpor could function similarly in regulating ROS resistance.The expression of HSP60 is also elevated during hibernation of ground squirrels,with previous microarray screening studies showing putative up-regulation of HSP40,HSP60,and HSP70 in liver during torpor[34].Although the exact role of HSP60 in regulating oxidative stress is not fully understood,this link is not surprising due to the role of HSP60 in regulating mitochondrial protein import and folding[35].
To better understand the state of oxidative stress in lemur tissues during torpor,the total antioxidant capacity of six tissues was measured,along with expression levels of fve antioxidant enzymes.An increase in total antioxidant capacity was observed in liver during torpor,but there were no signifcant changes in other tissues including BAT.The increase in liver antioxidant capacity may be indicative of a potential increase in oxidative stress during torpor.Interestingly,such increased antioxidant capacity was correlated with the elevated HSP60 expression,which was also seen in liver during torpor. Recent studies have also shown that the protein expression of SOD1 and catalase are elevated in some of tissues during hibernation of ground squirrels as compared to euthermic controls[21,36,37].We observed limited changes in the protein levels of antioxidant enzymes across the six tissues during lemur torpor,with signifcant upregulation observed only for TRX1 in WAT and SOD1 in skeletal muscle and BAT.The general lack of change in the protein expression levels of antioxidant enzymes was intriguing;however,it should be noted that the antioxidant capacity of the tissue is the real measure of their functionality during torpor.
Conclusion
The data presented in this study show that selected similarities in cytoprotective mechanisms occur between primate and rodent torpor,for example,activation of HSPs such as HSP60 in BAT and HSP70 in liver.However,in terms of antioxidant response,it appears that the transcriptional activation and increased synthesis of antioxidant enzymes are not the major responsive events in lemur torpor.This is in contrast to previous fndings in ground squirrel torpor,with evidence of upregulation of PRX in the BAT and the heart during torpor, catalase in the skeletal muscle,and both SOD1 and SOD2 in BAT in response to torpor[21,38].It is likely that the difference in duration and depth of torpor could differentially infuence the transcriptional responses observed between torpid lemurs and hibernating ground squirrels.Ground squirrel torpor bouts can last for 3–25 days during the hibernation season,whereas lemur average daily torpor is just 8–15 h [19,39].The shorter length of metabolic depression in lemur torporcould suggestthatothermorerapidly-activated mechanisms may be adapted in torpor.Such mechanisms of adaptation may include posttranslational modifcations to proteins/enzymes,as is also known for reversible protein phosphorylation in rodent hibernation[40].In conclusion, our study provides an initial insight into the molecular profles of the stress response during primate torpor and provides a basis for the future exploration into the cellular mechanisms that are utilized primates to coordinate either daily torpor or seasonal hibernation.
Materials and methods
Animal treatments
A total of 8 female mouse lemurs(2–3 years of age)were used in the experiment.Animals were born in an authorized breeding colony at the National Museum of Natural History (Brunoy,France;European Institution Agreement No.D91-114-1).Protocols used for animal experiments were as described previously,and were carried out by Dr.Martine Perret and the Adaptive Mechanisms and Evolution Team [24,25].Detailed animal protocols can be found in[41].
Total protein lysate preparation
Sample lysates were prepared according to the manufacturer’s protocol for the assay panels used(Luminex,Toronto ON, Canada).Briefy,tissue samples of~50 mg were homogenized 1:2(w/v)with a Dounce homogenizer using the supplied lysis buffer with the addition 1:100(v/v)protease inhibitor cocktail (Catalog No.PIC003.1,Bioshop,Burlington ON,Canada). Supernatants containing soluble proteins were removed after centrifugation at 4500×gfor 15 min,and protein concentrations were determined by the Bradford assay.Lysates were normalized to the same concentration and diluted with manufacturer’s assay buffer to a fnal concentration of 0.6 μg/μl for the oxidative stress panel and to 4.5 ng/μl for the heat shock protein panel.
Luminex multiplex assay
The multiplex immunoassays utilized for this study included the Human Oxidative Stress Magnetic Bead Panel(Catalog No.H0XSTMAG-18K,Millipore,Etobicoke ON,Canada) and the Heat Shock Protein Magnetic Bead 5-Plex Kit (Catalog No.48-615MAG,Millipore).Luminex assays were performed as instructed by the manufacturer’s protocol,which were described in detail by Biggar et al.[41]in this special issue.
Antioxidant capacity assay
Total antioxidant capacity was measured in control and torpid lemurs using an Antioxidant Assay kit(Catalog No.709001, Cayman Chemicals,Ann Arbor,MI,USA).This assay determines total cellular antioxidant levels by measuring the rateatwhich antioxidantsin each sampleinhibitthe metmyoglobin-catalyzed oxidation of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid(ABTS)to its radical cation form.Frozen tissue samples were homogenized at 1:4(w/v) in chilled antioxidant assay buffer with the addition of protease inhibitor as per manufacturer’s instructions.Sampleswere then centrifuged at 10,000×gfor 10 min at 4°C.The resulting supernatants were collected and soluble protein was determined using the Bradford assay.All samples were standardized to the same protein concentration for the following assays.The antioxidant assays were initiated by addition of sample lysate along with metmyoglobin and chromogen as per the manufacturer’s protocol.Antioxidant capacity was then measured at 750 nm and converted to Trolox equivalents (mM/mg wet mass)using a Trolox antioxidant assay standard curve.Total Trolox equivalents were subsequently converted to relative antioxidant levels,by standardizing against the frst control lemur sample.
Statistical analysis
Data were presented as mean±SEM(n=4).All statistical and graphing analyses were performed using Sigmaplot 12.0 software.Statistical test was performed using the two-tailed Student’st-test,with a signifcance level ofP<0.05.
Authors’contributions
All authors contributed to the conception and design of the project and to the editing of the manuscript.MP and FP carried out the animal experiments;CWW,KKB,SNT,and JZ conducted biochemical assays.Data analysis and assembly of the draft manuscript was carried out by KBS,CWW,and KKB.All authors read and approved the fnal manuscript.
Competing interests
The authors have declared no competing interests.
Acknowledgments
We thank Janet M.Storey for editorial review of the manuscript and Laurine Haro and Philippe Guesnet for technical and material assistance in the preparation of the lemur tissue samples.This work was supported by a Discovery grant from the Natural Sciences and Engineering Research Council (NSERC)of Canada(Grant No.6793)and a grant from the Heart and Stroke Foundation of Canada(Grant No.G-14-0005874)to KBS.KBS holds the Canada Research Chair in Molecular Physiology;CWW,KKB,and SNT all held NSERC postgraduate scholarships.
[1]Storey KB,Storey JM.Metabolic rate depression in animals: transcriptional and translational controls.Biol Rev Camb Philos Soc 2004;79:207–33.
[2]Joanisse DR,Storey KB.Oxidative damage and antioxidants inRana sylvatica,the freeze-tolerant wood frog.Am J Physiol 1996;271:R545–53.
[3]Carey HV,Sills NS,Gorham DA.Stress proteins in mammalian hibernation.Am Zool 1999;39:825–35.
[4]Carey HV,Frank CL,Seifert JP.Hibernation induces oxidative stress and activation of NK-kappaB in ground squirrel intestine.J Comp Physiol B 2000;170:551–9.
[5]Taylor RP,Benjamin IJ.Small heat shock proteins:a new classifcation scheme in mammals.J MolCellCardiol 2005;38:433–44.
[6]Kregel KC.Heat shock proteins:modifying factors in physiological stress responses and acquired thermotolerance.J Appl Physiol 1985;92:2177–86.
[7]Storey KB,Storey JM.Heat shock proteins and hypometabolism: adaptive strategy for proteome preservation.Res Rep Biol 2011;2:57–68.
[8]Kampinga HH,Hageman J,Vos MJ,Kubota H,Tanguay RM, Bruford EA,et al.Guidelines for the nomenclature of the human heat shock proteins.Cell Stress Chaperones 2009;14:105–11.
[9]Morano KA,Grant CM,Moye-Rowley WS.The response to heat shock and oxidative stress inSaccharomyces cerevisiae.Genetics 2012;190:1157–95.
[10]Bukau B,Horwich AL.The Hsp70 and Hsp60 chaperone machines.Cell 1998;92:351–66.
[11]Walter S,Buchner J.Molecular chaperones––cellular machines for protein folding.Angew Chem Int Ed Engl 2002;41:1098–113.
[12]Lee K,Park JY,Yoo W,Gwag T,Lee JW,Byun MW,et al. Overcoming muscle atrophy in a hibernating mammal despite prolonged disuse in dormancy:proteomic and molecular assessment.J Cell Biochem 2008;104:642–56.
[13]Eddy SF,McNally JD,Storey KB.Up-regulation of a thioredoxin peroxidase-like protein,proliferation-associated gene,in hibernating bats.Arch Biochem Biophys 2005;435:103–11.
[14]Brown JC,Chung DJ,Belgrave KR,Staples JF.Mitochondrial metabolic suppression and reactive oxygen species production in liver and skeletal muscle of hibernating thirteen-lined ground squirrels. Am J Physiol Regul Integr Comp Physiol 2012;302:R15–28.
[15]Frank CL,Storey KB.The optimal depot fat composition for hibernation by golden-mantled ground squirrels(Spermophilus lateralis).J Comp Physiol B 1995;164:536–42.
[16]Frank CL,Karpovich S,Barnes BM.Dietary fatty acid composition and the hibernation patterns in free-ranging arctic ground squirrels.Physiol Biochem Zool 2008;81:486–95.
[17]Hermes-Lima M,Zenteno-Savin T.Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp Biochem Physiol C:Toxicol Pharmacol 2002;133:537–56.
[18]Hall A,Karplus PA,Poole LB.Typical 2-Cys peroxiredoxins––structures,mechanisms and functions.FEBS J 2009;276:2469–77.
[19]Schmid J.Daily torpor in the gray mouse lemur(Microcebus murinus)in Madagascar:energetic consequences and biological signifcance.Oecologia 2000;123:175–83.
[20]Giroud S,Perret M,Gilbert C,Zahariev A,Goudable J,Le Maho Y,et al.Dietary palmitate and linoleate oxidations,oxidative stress,and DNA damage differ according to season in mouse lemurs exposed to a chronic food deprivation.Am J Physiol Regul Integr Comp Physiol 2009;297:R950–9.
[21]Vucetic M,Stancic A,Otasevic V,Jankovic A,Korac A,Markelic M,et al.The impact of cold acclimation and hibernation on antioxidant defenses in the ground squirrel(Spermophilus citellus): an update.Free Radic Biol Med 2013;65:916–24.
[22]Storey KB.Mammalian hibernation:transcriptional and translational controls.In:Roach RC,Wagner PD,Hackett PH,editors. Hypoxia through the lifecycle.New York:Kluwer/Plenum Academic;2003.p.21–38.
[23]Yan J,Burman A,Nichols C,Alila L,Showe LC,Showe MK, et al.Detection of differential gene expression in brown adipose tissue of hibernating arctic ground squirrels with mouse microarrays.Physiol Genomics 2006;25:346–53.
[24]Giroud S,Blanc S,Aujard F,Bertrand F,Gilbert C,Perret M. Chronic food shortage and seasonal modulation of daily torpor and locomotor activity in the grey mouse lemur(Microcebus murinus).Am J Physiol 2008;294:R1958–67.
[25]Ge′nin F,Nibbelink M,Galand M,Perret M,Ambid L.Brown fat and nonshivering thermogenesis in the gray mouse lemur(Micro-cebus murinus).Am J Physiol Regul Integr Comp Physiol 2003;284:R811–8.
[26]Scantlebury M,Lovegrove BG,Jackson CR,Bennett NC, Lutermann H.Hibernation and non-shivering thermogenesis in the Hottentot golden mole(Amblysomus hottentottus longiceps).J Comp Physiol B 2008;178:887–97.
[27]Barger JL,Barnes BM,Boyer BB.Regulation of UCP1 and UCP3 in arctic ground squirrels and relation with mitochondrial proton leak.J Appl Physiol 2006;101:339–47.
[28]Matz JM,LaVoi KP,Moen RJ,Blake MJ.Cold-induced heat shock protein expression in rat aorta and brown adipose tissue. Physiol Behav 1996;60:1369–74.
[29]Altieri DC.Hsp90 regulation of mitochondrial protein folding: from organelle integrity to cellular homeostasis.Cell Mol Life Sci 2013;70:2463–72.
[30]Deshaies RJ,Koch BD,Werner-Washburne M,Craig EA, Schekman R.A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature 1988;332:800–5.
[31]Cabiscol E,Bell?′G,Tamarit J,Echave P,Herrero E,Ros J. Mitochondrial Hsp60,resistance to oxidative stress,and the labile iron pool are closely connected inSaccharomyces cerevisiae.J Biol Chem 2002;277:44531–8.
[32]Singh MP,Reddy MM,Mathur N,Saxena DK,Chowdhuri DK. Induction of hsp70,hsp60,hsp83 and hsp26 and oxidative stress markers in benzene,toluene and xylene exposedDrosophila melanogaster:role of ROS generation.Toxicol Appl Pharmacol 2009;235:226–43.
[33]Hall L,Martinus RD.Hyperglycaemia and oxidative stress upregulate HSP60 and HSP70 expression in HeLa cells.Springerplus 2013;2:431.
[34]Storey KB.Mammalian hibernation:transcriptional and translational controls.Adv Exp Med Biol 2003;543:21–38.
[35]Cheng MY,Hartl FU,Martin J,Pollock RA,Kalousek F, Neupert W,et al.Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria.Nature 1989;337:620–5.
[36]Morin Jr P,Ni Z,McMullen DC,Storey KB.Expression of Nrf2 and its downstream gene targets in hibernating 13-lined ground squirrels,Spermophilustridecemlineatus.MolCellBiochem 2008;312:121–9.
[37]Wu CW,Storey KB.FoxO3a-mediated activation of stress responsive genes during early torpor in a mammalian hibernator. Mol Cell Biochem 2014;390:185–95.
[38]Morin P,Storey KB.Antioxidant defense in hibernation:cloning and expression of peroxiredoxins from hibernating ground squirrels,Spermophilustridecemlineatus.Arch Biochem Biophys 2007;461:59–65.
[39]Carey HV,Andrews MT,Martin SL.Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature.Physiol Rev 2003;83:1153–81.
[40]Storey KB,Wu CW.Stress response and adaptation:a new molecular toolkit for the 21st century.Comp Biochem Physiol A: Mol Integr Physiol 2013;165:417–28.
[41]Biggar KK,Wu CW,Tessier SN,Zhang J,Pifferi F,Perret M, Storey KB.Primate torpor:regulation of stress-activated protein kinases during daily torpor in the gray mouse lemur,Microcebus murinus.Genomics Proteomics Bioinformatics 2015;13:81–90.
Received 13 February 2015;accepted 24 March 2015
Available online 17 June 2015
Handled by Jun Yu
*Corresponding author.
E-mail:kenneth_storey@carleton.ca(Storey KB).
#Equal contribution.
aORCID:0000-0001-6370-429x.
bORCID:0000-0002-1204-3329.
cORCID:0000-0002-6076-7321.
dORCID:0000-0003-2373-232x.
eORCID:0000-0001-9316-1935.
fORCID:0000-0002-3801-0453.
gORCID:0000-0002-7363-1853.
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences and Genetics Society of China.
http://dx.doi.org/10.1016/j.gpb.2015.03.004
1672-0229?2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
This is an open access article under the CC BY license(http://creativecommons.org/licenses/by/4.0/).
 Genomics,Proteomics & Bioinformatics2015年2期
Genomics,Proteomics & Bioinformatics2015年2期
- Genomics,Proteomics & Bioinformatics的其它文章
- Genomics,Proteomics&Bioinformatics
- Special Issue on‘‘Biomarkers for Diseases’’
- Special Issue on‘‘Computational Cardiology’’
- Special Issue on‘‘Metagenomics of Marine Environments’’
- Acknowledgments to Reviewers 2014
- Cytokine and Antioxidant Regulation in the Intestine of the Gray Mouse Lemur (Microcebus murinus)During Torpor
