Bio-amelioration of alkali soils through agroforestry systems in central Indo-Gangetic plains of India
Y.P. Singh · Gurbachan Singh · D.K. Sharma
ORIGINAL PAPER
Bio-amelioration of alkali soils through agroforestry systems in central Indo-Gangetic plains of India
Y.P. Singh · Gurbachan Singh · D.K. Sharma
Received: 2012-11-19; Accepted: 2013-05-01
? Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
A long-term field study was initiated during 1995 at Central Soil Salinity Research Institute, Regional Research Station, Lucknow (26°47′58′ N and 80°46′24′ E) to analyze the effect of agroforestry systems on amelioration of alkali soils. Three agroforestry systems (pastoral, silvipastoral and silvicultural) were compared with the control where no agroforestry system was introduced. Tree-based silvicultural and silvipastoral systems were characterized by tree species Prosopis juliflora and Acacia nilotica along with grass species Leptochloa fusca, Panicum maximum, Trifolium alexandrium and Chloris gayana. Growth of ten-year-old Prosopis juliflora and Acacia nilotica planted in combination with grasses was significantly higher over the silviculture system with the same species. Tree biomass yields of P. juliflora (77.20 t·ha-1) and A. nilotica (63.20 t·ha-1) planted under silvipastoral system were significantly higher than the sole plantation of (64.50 t·ha-1and 52.75 t·ha-1). Fodder yield under the pastoral system was significantly higher than the silvipastoral system during initial years but it was at par with that of silvipastoral systems after eight years of plantation. The microbial biomass carbon in the soils of silvipastoral systems was significantly higher than in soils under sole plantation of trees and control systems. The Prosopis-based silvipastoral system proved more effective in reducing soil pH, displacing Na+from the exchange complex, increasing organic carbon and available N, P and K. Improvement in soil physical properties such as bulk density, porosity, soil moisture and infiltration rate was higher in the Prosopis-based silvipastoral system than in the silviculture system or control. On the basis of biomass production and improvement in soil health due to tree + grass systems, silvipastoral agroforestry system could be adopted for sustainable reclamation ofhighly alkali soils.
agroforestry systems, alkali soils, biomass production, microbial biomass, soil amelioration
Introduction
Amelioration of alkali soils is essential for sustaining food, fuel, fodder, fiber, and timber production for an ever increasing human population (Garg and Jain 1992; Gill and Abrol 1991; Jain and Garg 1996; Singh, 1975). Geographically, the Indo-gangetic plains lies between 21° 55′ to 32° 39′ N and 73° 45′ to 88° 25′ E and is comprised of the states of Punjab, Haryana, U.P. and part of Bihar (North), West Bengal (south), and Rajsthan (north). Salt-affected soils of Indo-gangetic alluvium (2.7 million ha) constitute centuries old barren alkali soils without any land use system (NRSA & Associates 1996). A large tract of common lands (either government lands or village panchayat lands) are not in any productive use (Singh 2009). Alkali soils belonging to small and marginal farmers is unproductive because of high pH and exchangeable Na+percentage, which adversely affect physico-chemical and biological properties of these soils (Oster et al. 1996; Oster et al. 1999; Qadir et al. 1996; Qadir et al. 1997; Qadir et al. 2001). Presence of hard kankar (calcite) pan at a depth of about 90 cm of the soil profile is a major problem for planting of deep rooted plants (Garg 1998; Jain and Singh 1998; Singh 1989a). Tree growth in alkali soils is constrained mainly by the inability of roots to penetrate this calcite pan. Judicious use of these lands can substantially contribute to increasing demand for food, fodder, fuel and timber in India. Planting of trees and grasses on alkali soils provides an alternative to control further deterioration of these soils and to maintain soil health (Thorrold et al. 1997; Singh et al. 2008). Several studies have been conducted to measure the increase in N status of the soil under varying N2- fixing tree species (Acacia spp.). Because alkali soils are poor in organic carbon, the rates of organic carbon and N accumulation tend to be greatest in the first five years of plantation (Luken and Fonda 1983). Karnal grass (Leptochloa fusca) is suitable for pasture because it can tolerate extremelyalkaline conditions (Kumar 1996). Rhodes grass (Chloris gayana) and Gatton panic (Panicum maximum) are highly succulent, palatable and quick-growing grasses that have proven highly salt tolerant and produce abundant biomass in salt affected soils (Kumar and Abrol 1986; Bogdan 1977). Growing of these fodder species in combination with Prosopis juliflora and Acacia nilotica improved the soil health to such an extent that less tolerant but more palatable fodder species such as Trifolium alexandrium and Melilotus parviflora could be grown. Our study was initiated in 1995 to examine the potential of different agroforestry systems and to quantify their ameliorative effect on alkali soils. We focused on the evaluation of agroforestry systems for biomass production and soil ameliorating properties to meet the increasing demand for fuel wood and fodder due to lack of good quality arable land (Chaturvedi and Behl 1996; Hellden 1992; Swaminathan 1980; Toth 1981).
Materials and methods
Experimental site
Our field experiment was initiated during 1995 at the research farm of Central Soil Salinity Research Institute, Regional Research Station, Lucknow, Uttar Pradesh (26°47′58′ N and 80°46′24′ E), on the Indo-Gangetic alluvial plains of India. The study site was representative of large areas of abandoned alkali soils of the Indo-Gangetic plains that have been classified as typical Natrustalfs with sandy loam on the surface, silty loam and clay loam in middle horizons, and sandy loam in lower horizons (Sharma et al. 2006). These soils displayed physical and nutritional problems due to poor soil water cover and soil-air relations caused by high bulk density (> 1.5 g·cm-3) and very poor infiltration. Soils were strongly alkaline (pH of 10.2) with electrolytic conductivity (EC) of 1.55 dS·m-1and exchangeable sodium percentage (ESP) of 89% in the upper 0?15 cm soil layer with a predominance of carbonate and bicarbonate of Na+(Table 1). The climate is semi-arid, subtropical and monsoonal with average annual rainfall of 817 mm. Mean climatological data for the study area covering 1995?2005 are given in Fig. 1.
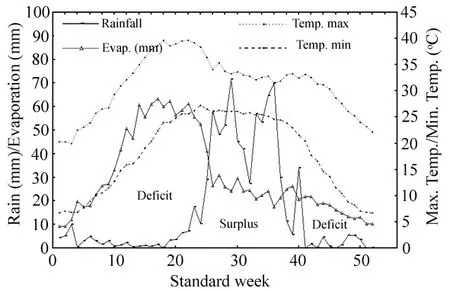
Fig. 1: Annual variations in rainfall, temperature and evaporation averaged during the period of 1995?2005.
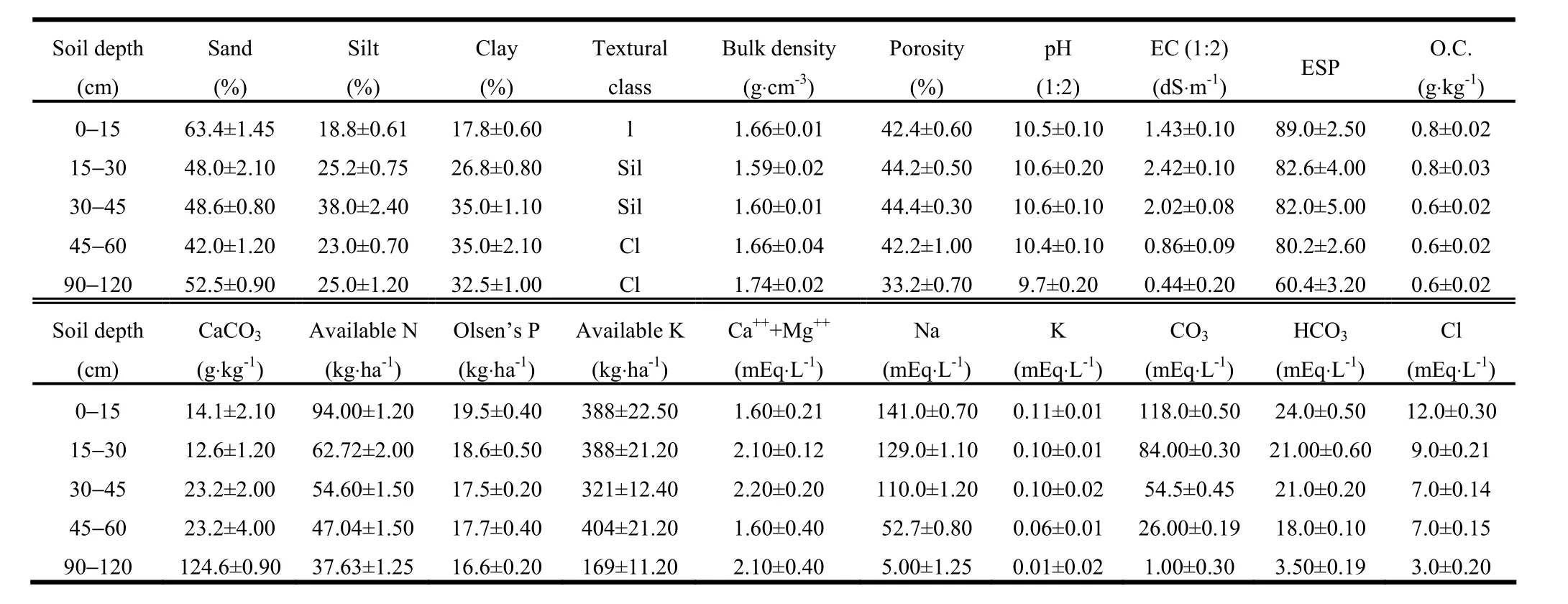
Table 1: Initial soil physico-chemical properties of the experimental site
Soil analysis
To characterize the physico-chemical properties of the experimental site, composite soil samples were taken to 120 cm depth from three soil profiles. These samples were mixed and homogenized. The samples were air dried, ground in a Willey mill and passed through a 2.0-mm sieve. The pH and EC of the soil were determined in 1:2 soil-water suspension using a digital pH meter and a digital conductivity meter following the procedure outlined by Singh et al. (1999). Organic carbon was quantified using the Walkley and Black rapid titration method as modified by Walkley (1947). Available N was determined by distillation of soil with KMnO4and NaOH (Subbiah and Asija 1956). ESP was calculated by the formula ESP = [exchangeable Na+(cmolc·kg-1) × 100/CEC (cmolc·kg-1)] (Richards 1954). The concentrations of Ca2+and Mg2+were estimated by an Inductivity Coupled PlasmaAnalyzer (Perkin Elmer), and Na+and K+were measured by Flame Photometer. Soil bulk density (BD) was determined by weighing a known volume of a core sample of 5.3 cm internal diameter (Wilde et al. 1964). Soil porosity was determined using the procedure described by Brady (1990). Soil samples for moisture analysis were taken at monthly interval from 50 cm away from tree trunks at 0, 0.15, 0.30, 0.60, 0.90 and 1.20 m soil depth and dried at 105°C for 24 hours. Double concentric infiltrometer cylinders with 60 cm outer and 30 cm inner diameters were used (Brechtel 1976; Yadav and Vasistha 1989) to measure the infiltration rate.
Experiment
Five treatments comprised of T1- control (barren) where no agroforestry system was initiated and the plot was kept undisturbed; T2- pastoral system (Karnal grass (Leptochloa fusca (L.) for four years followed by Gatton panic grass (Panicum maximum) for six years (without amendments); T3- Only tree plantation (Prosopis juliflora (Swartz D.C.); T4- Only tree plantation (Acacia nilotica); T5- silvipastoral system (Prosopis juliflora + L. fusca for four years followed by Berseem (Trifolium alexandrium) for six years (without amendments) and T6- silvipastoral system (Acacia nilotica + L. fusca) for four years followed by Rhodes grass (Chloris gayana) Kunth (L.) (T. Mannetie & Kertsen S.M.M. 1992) for six years without amendments. Natural grasses were allowed to grow in T1, T2and T3. The natural grasses such as Sporobolus marginatus, Cynodon dactylon, Aristida abscendens, Launnacea procumbens, Verimonia cineria and Cyprus difformis that regenerated under the trees and control treatments were harvested annually. L. fusca planted under pastoral and silvipastoral systems for four years was harvested quarterly and used as fodder for cattle. Similarly, P. maximum and C. gayana planted after four years as sole and in combination with trees respectively were harvested quarterly. T. alexandrium a seasonal fodder grass was harvested five times during the cropping season (October–April) after which the plot was allowed to grow natural grasses. All these grass species were used as fodder for cattle.
Planting methodology
Nine month old saplings of tree species viz. P. juliflora and A. nilotica (L) of almost uniform height, collar diameter and phenotype raised in the state government forest department nursery in normal soil were planted during September 1995 in auger holes of 45 cm diameter at the surface, 20 cm at the base and 120 cm deep in a Randomized Block Design (RBD). Three rows of twelve plants each (36 plants) were replicated four times, keeping a distance of 5 m and 4 m between the rows and plants, respectively. Each augerhole was filled with a uniform mixture of original soil + 4 kg gypsum + 10 kg FYM+ 20 kg river sand before planting. The amount of amendment was determined by volume of soil extracted from the augerhole and the alkalinity of the soil. No fertilizer was applied to the plants during the study period. For establishment of saplings, three irrigations with 10 cm depth of water were applied monthly during the first year after planting and thereafter once annually in June.
Root slips of Karnal grass (L. fusca) were planted under pastoral and silvipastoral systems at 50 cm × 30 cm plant to plant and row to row spacing without application of any amendment for four years. After four years, L. fusca was replaced with P. maximum a perennial and shade tolerant grass, T. alexandrium and C. gayana. Root slips of P. maximum and C. gayana were planted in 1999 during the rainy season at 50 cm × 30 cm plant to plant and row to row spacing. However, T. alexandrium, an annual grass, was sown every year during October at a seeding rate of 25 kg·ha-1.
Tree growth and biomass
Tree growth parameters including plant height, diameter at breast height (DBH, at 1.30 m above ground level), diameter at stump height (DSH, at 0.65 m above ground level), crown diameter and pruned biomass were measured five and ten years after planting. To measure the pruned biomass and to stimulate growth of trees and inter-planted grasses (Singh et al. 1989), side branches up to one third of the total stem length were pruned during the dormant period. The pruned biomass of the trees was used as fuel. Four randomly selected trees representing the range of size variations were harvested ten years after planting by cutting at the ground surface and each plant component was weighed separately on air dry basis for biomass estimation using a stratified sampling procedure. Stump root and lateral roots of the sample plants were excavated and cleaned by applying a gentle jet of water at the site. Four trees of each diameter class were marked for the annual increments. In this study, we used the Gompertz non-linear growth model using curve expert version 1:3 for calculating the annual increment (Draper and Smith 1998): where, Y is the biomass yield (t·ha-1), a, b and c are the regression constants, x is the age of the plant, and e is the constant.

The biomass yield of natural grasses regenerated under control, silvicultural and silvipastoral systems was estimated at the termination of the growing season and measured on annual dry weight basis. The data were analyzed statistically using standard analysis of variance in M STAT-C software. The treatment comparisons were made using a t-test at 5% level of significance.
Microbial biomass
The microbial biomass carbon was estimated by the fumigation extraction method (Vance et al. 1987). Sieved soil samples at 40% water holding capacity were fumigated with alcohol free chloroform in vacuum dessicators and stored in the dark for 24 hours. After removing the fumigant the soil samples were extracted with 0.5 M K2SO4for 30 min on a shaker. The extracts of fumigated and unfumigated samples were analyzed for determining the organic carbon using the acid dichromate method.
Results and discussion
Tree growth
Survival percentage and growth of plants ten years after planting did not differ significantly (Table 2). Higher values were, however, recorded in T5and T6. The highest survival rate was recorded in T5followed by T6, T3and T4. Similarly, plant height at ten years in T5and T6was higher than in T3and T4. This was because of improvement in soil fertility, higher nutrient and water availability and suitable hydrothermal conditions that developed in the soil and stimulated plant growth (Singh et al. 1987). DBH, DSH and crown diameter in T5and T6were higher than in T3and T4.
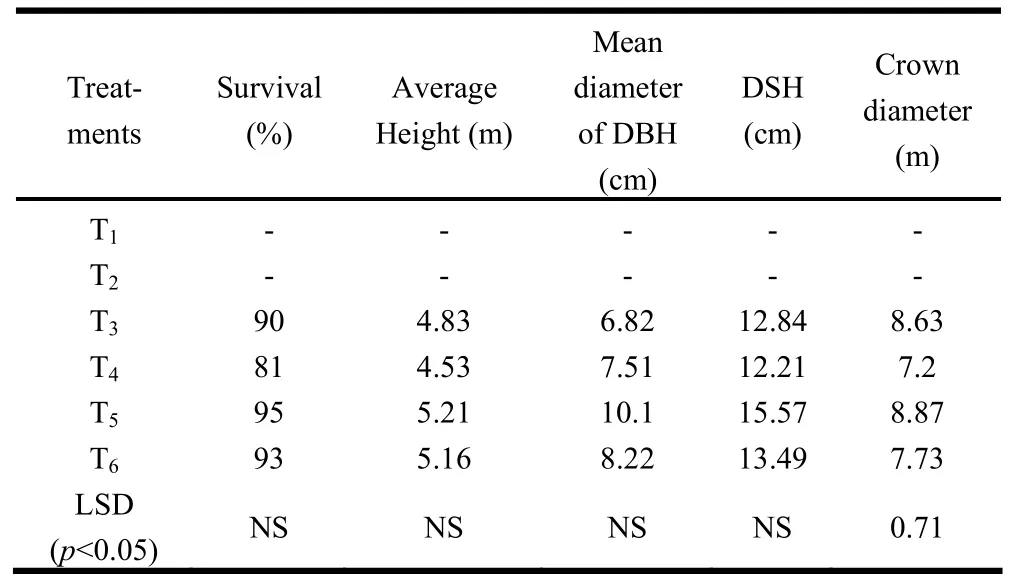
Table 2: Performance of trees under different agroforestry systems 10 years after planting
Tree biomass production
We estimated average pruned biomass production during the ten-year period (Table 3). Maximum pruned biomass (3.35 t·ha-1) was recorded in T4and minimum pruned biomass (2.56 t·ha-1) was recorded in T6. The P. juliflora silvipastoral system (T5) produced significantly greater pruned biomass than the Acacia silvipastoral system (T3). Singh et al. (1989a) reported that the plant height of P. juliflora increased significantly with pruning of trees in highly alkali soils. Pruning of side branches of trees helps the trees grow better and also improve the forage yield of inter-planted grasses (Singh et al. 1989). The dry biomass production was estimated by uprooting the trees ten years after planting (Table 3). Biomass was greatest in T5and least in T4 (Table 3). The P. juloflora based silvipastoral system (T5) produced significantly drier biomass than T3, T4and T6. This was because of higher availability of moisture, nutrients and fixation of N through N2fixing grass species planted in the treatment. Malik et al. (1986) and Singh et al. (1987) reported higher tree growth and biomass yields with nitrogen fixing leguminous intercrops. Annual increments in biomass yield in T6(6.83 t·ha-1) and T5(6.73 t·ha-1) during years 0?5 of tree growth were similar both were greater than in T3and T4. However, during years 5?10 of tree growth, annual increments in T5and T3were significantly greater than in T6and T4(Table 4). The greater annual increment under silvipastoral systems was due to improvement in soil fertility (Singh et al. 2010).
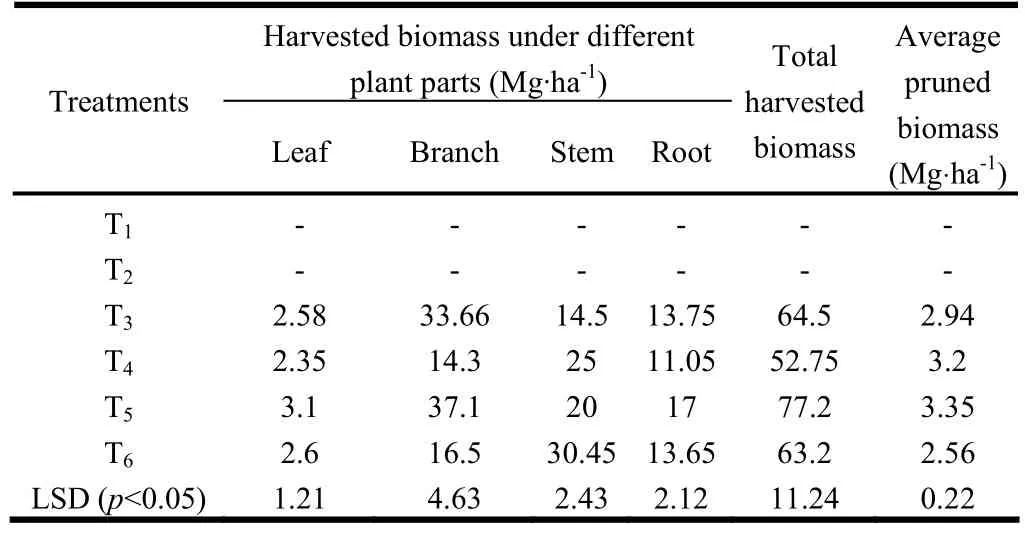
Table 3: Total dry biomass production and allocation in to stem, branch and root components ten years of plantation in alkali soils
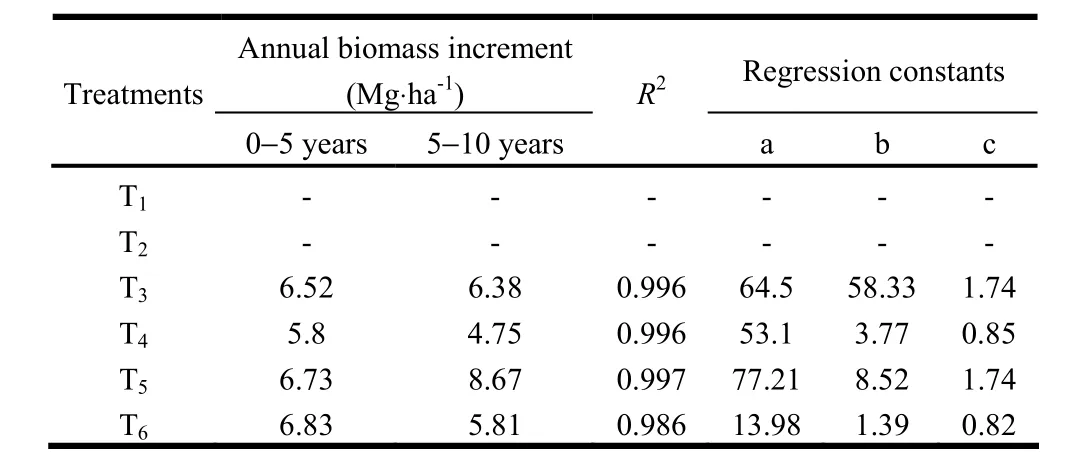
Table 4: Annual increment and r2 values for tree biomass under different agroforestry systems
Productivity of fodder grasses
The fodder productivity of naturally regenerated grasses under all treatments increased with increasing age of plants. Four years after planting, the productivity of fodder grass under T2was significantly greater than in T1, T3, T4, T5and T6(Fig. 2). A similar trend was observed six and eight years after planting because of high tolerance of L. fusca to alkalinity as reported from Australia (Lazarides 1970), India (Ashok 1988a; Singh and Dagar 2005), Pakistan (Sandhu et al. 1981; Qureshi et al. 1982; Kumar 1996), and Zambia (Verboon and Brunt 1970). Ten years after planting, T5produced significantly more fodder than T1, T3, T4and T6. This might have been because of improvement in soil health due to the combined effect of P. juliflora and T. alexandrium nitrogen fixing grass species. Kumar et al. (1990?1991) found that T. alexandrium yield in salt-affected soils was less affected by tree canopy species than were other fodder crops. Pruning of side branches of trees during dormant period helps to improve the forage yield of inter-planted grasses. When large canopy-forming trees such as Prosopis and Acacia are pruned of side branches, light penetrates through the openings and increases the productivity of understory vegetation. Maximum annual increment in fodder yield under all treatments was recorded at stand ages of four to six years (Table 5). This was probably because the yield of L. fusca declined from the third year onwards when soil con-ditions improved (Kumar and Abrol 1983; Kumar 1988b). After six years, annual increments in fodder yields were significantly higher in T5and T6than in T1, T2,T3and T4because of more soil amelioration and improvement in fertility of alkali soils.
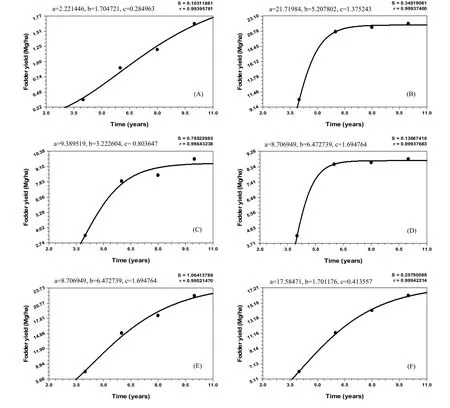
Fig. 2: Fodder yield over the years under (A) control (B) pastoral (C and D) Prosopis and Acacia based silvicultural and (E and F) Prosopis and Acacia based silvipastoral systems.
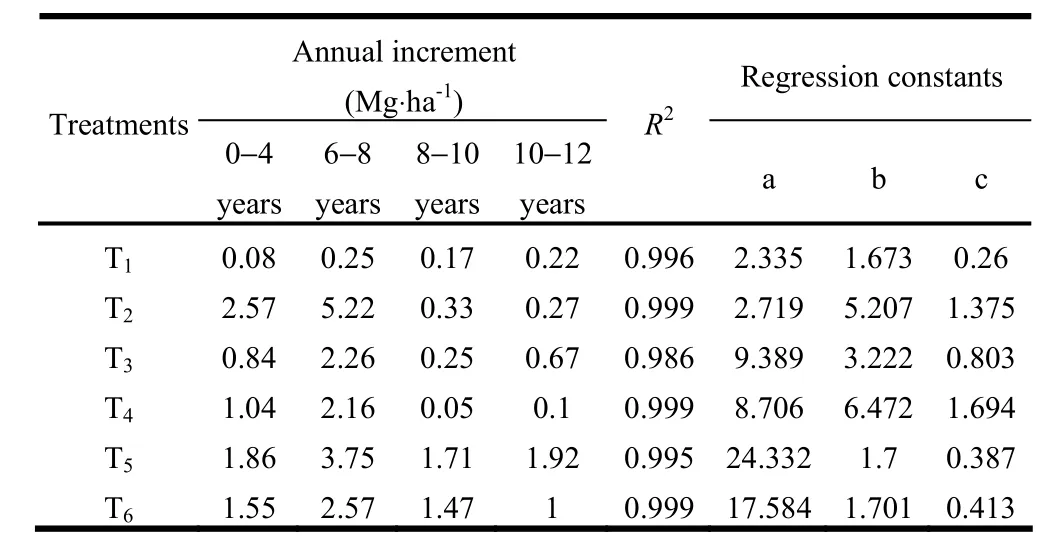
Table 5: Annual increments, R2and regression constants for fodder yields under different agroforestry systems
Microbial biomass
Microbial biomass carbon in alkali soils was greater in agroforestry systems (Table 6). Microbial biomass carbon in surface (0?7.5 cm) and sub-surface (7.5?15 cm) soils in T1measured 43 and 23 μg·g-1, respectively, ten years after planting. In T2where grasses were planted, microbial biomass carbon was 76 and 32 μg·g-1, respectively. In T3(plantation of P. juliflora only), the microbial biomass carbon in surface and sub-surface soil layers was 81 and 43 μg·g-1, respectively, which was higher than in the pastoral system. In T4(Acacia tree plantation), microbial biomass carbon was 63 and 26 μg·g-1, respectively, lower than in T3and T2. In T5and T4, microbial biomass carbon at 0?7.5 and 7.5?15 cm soil depths was 132 and 61 μg·g-1, respectively, and 123 to 51 μg·g-1, respectively, which was significantly higher than in other treatments. This was due to the ameliorative effects of trees and grasses on soil conditions, and the increase in organic carbon content in the soil. Microbial biomass carbon was significantly affected by soil depth. Significantly higher amounts of biomass carbon in surface soils might have been due to higher amounts of readily available organic compounds such as sugars, amino acids and organic acids from the roots that play important roles in the maintenance energy of microbial populations (Bowen and Rovira 1991).
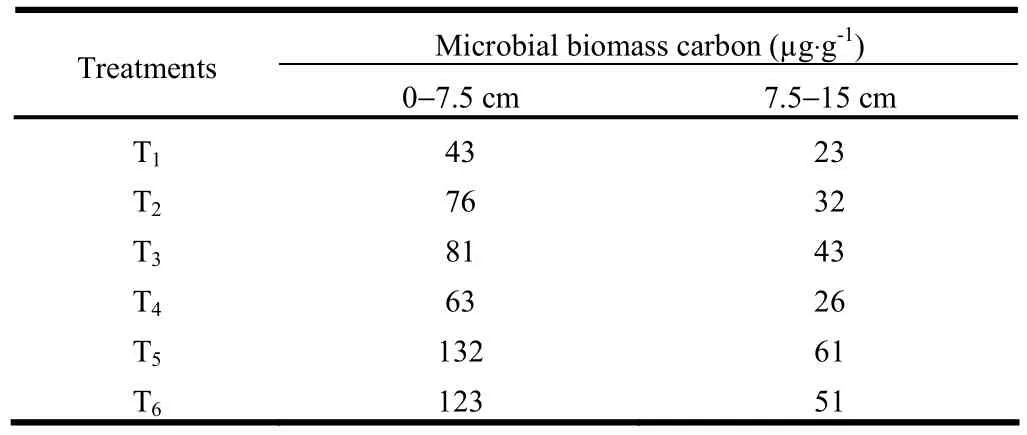
Table 6: Soil microbial biomass carbon under different agroforestry systems in alkali soils
Soil amelioration
After ten years of experimentation, the maximum reduction in soil pH was in T5(Table 7). This confirms earlier studies (Abrol and Prasad 1985; Singh et al. 1993; Singh 1995). This resulted from larger root surface area (Jain and Garg 1996) and high replacement of exchangeable Na+by Ca2+and subsequent leaching of Na+. In soil containing plant roots, the presence of CO2enhances CaCO3dissolution, resulting in measurable replacement of adsorbed Na+(ibid.). EC and ESP declined significantly over their initial levels of 1.43 dS·m-1and 89%. The influence of trees, grasses and a combination of both was more pronounced in the surface layer and it followed an increasing trend with soil depth. The significant effect of agroforestry systems on ESP was recorded up to 120 cm of soil depth in comparison with the control (T1) where there was no significant change in ESP throughout the soil profile. This might have been due to increasing CO2concentration in the soil, which, on dissolution in water, produces carbonic acid (H2CO3), which reduces soil pH and dissolves Ca (HCO3)2by increasing the solubility of CaCO3(Garg and Jain 1996). A fourfold increase in organic carbon content was recorded in T5and T6, and about a threefold increase in T3and T4ten years after plantation (Fig. 3). However, in T1, the organic carbon content was similar to levels recorded at the time of initial plantation. Similar trends were recorded for available N and P and K. This might have been due to production of certain allelo-chemicals/mixture of acids released from tree biomass as well as from root and shoot biomass of grasses. Gill et al. (1987) and Lal (1998) reported threefold and twofold increases in organic carbon in surface (0?15 cm) soil over a span of five years under A. nilotica and E. teriticornis, respectively. Garg (1999) reported greater accumulation of organic carbon by P. juliflora as compared to other tree species due to differences in root distribution in the soil profile. Tidemann and Klemmedson (1986), Kaur et al. (2000) and Kaur et al. (2001) also reported that soils under Prosopis species contained 2?3 times as much organic matter as soils without any plantation. The increment in available K+content might have been due to release of K+from the K+bearing minerals following reclamation and recycling of K+from litter decomposition (Mishra et al. 2002; Mongia et al. 1998).
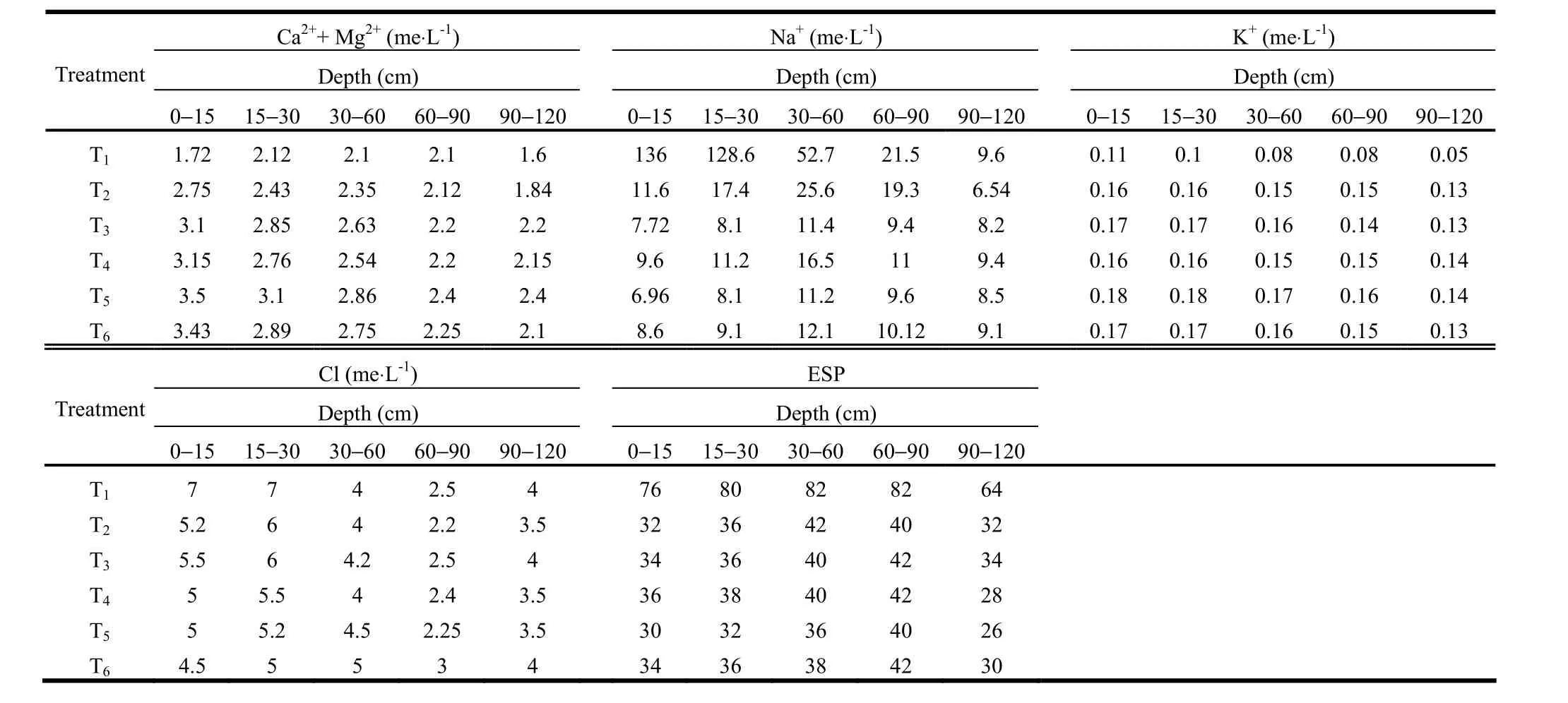
Table 7: Changes in nutrient levels up to 120cm soil depth under different agroforestry systems ten years after plantation
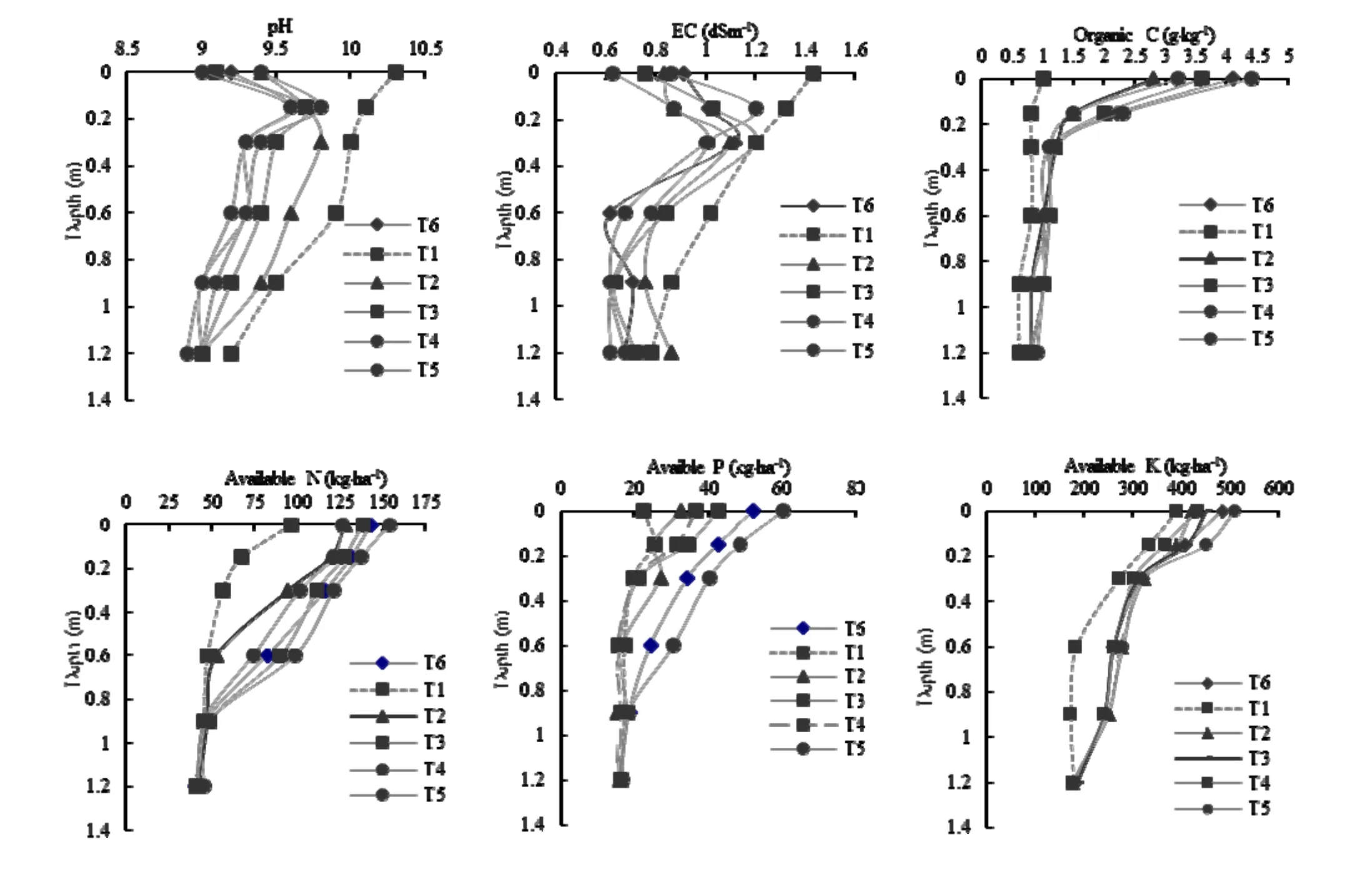
Fig. 3: Changes in pH, EC, organic carbon, available N, available P and available K under different agroforestry systems compared with control
Maximum Ca2++ Mg2+and K+contents were recorded in T5and T6. Ca2++ Mg2+contents in the surface soils of silvipastoral systems (T5and T6) increased to double over the control (T1) and initial values over a period of ten years. The increases in these cations resulted from the combined root action of trees and grasses that reclaimed the soil. Khanduja et al. (1986) reported a reduction in Na+and improvement in Ca2+content of soil under P. juliflora and other fruit tree cover. A similar trend was observed in Na+content. The Na+content was lowest in the surface layer and increased with increasing depth in all treatments. Exchangeable Na+was replaced by Ca2+as a result of growing C. dactylon for two years followed by barley and alfalfa. The reduction in Clcontent was more pronounced in the surface layer in all agroforestry systems (Table 6).
Reduction in bulk density was greatest in T5and least in T2but enhancement in porosity was greatest in T5and T6and least in T1(Fig. 4). Soil moisture retention in the surface layer in T5and T6was significantly greater than in T3, T4and T2. Moisture retention under silvipastoral systems was about five times that in the control where the soil was completely barren. Similarly, infiltration rate in T5and T6was higher than in T3, T4and T2. This was due to better tree growth, improved soil physico-chemical properties, enhanced biological activities of the soil due to greater root growth of trees and decomposition of grass residues. Singh et al. (1989) compared the effects of several tree species on sodic soils and found the largest improvement in infiltration rate under P. juliflora.
Conclusion
The amelioration of alkali soils is feasible using silvipastoral systems. Establishment of a P. juliflora silvipastoral system with planting of L. fusca for four years followed by T. alexandrium for six years might be a more remunerative land use system than pure pastoral or silvicultural systems because of its capacity to produce more biomass and improve physico-chemical properties of alkali soil. It is thus inferred that the alkali soils rehabilitated with silvipastoral systems for ten years can be put under cultivation of highly remunerative crops to harness the productivity potential of these soils. Our results can be applied to the utilization of salt-affected soils for biomass and bio-energy production and their rehabilitation for productive uses.
Acknowledgement
The authors are thankful to the then Director of the Institute (Dr. N. K. Tyagi) for providing all financial assistance to conduct such a long-term study. We thank Dr. B. Singh, Head, Restora-tion Ecology, National Botanical Research Institute, Lucknow for his guidance and technical help in preparing the manuscript.
Abrol IP, Prasad R. 1985. Karnal grass (Leptochloa fusca) for reclaiming alkali soil. In: Better farming in salt affected soils. Karnal, Haryana: No. 5: Central Soil Salinity Research Institute.
Bogdan AV. 1977. Tropical Pasture and Fodder Plants. New York: Longman Inc., pp. 77?86.
Bowen GD, Rovira AD. 1991. The rhizosphere, the hidden half of the hidden half. In: Waisel Y, Eshel A, Kafkafi U (eds), The Plant Roots: The hidden half, New York: Marcel Dekker, pp. 641?669.
Brady NC. 1990. The nature and Properties of Soils. New York: Macmillan Publishers.
Brechtel HM. 1976. Application of an inexpensive double ring infiltration: hydrological techniques for upstream conservation. Conservation Guide 2. Rome: FAO.
Chaturvedi AN, Behl HM. 1996. Biomass production trials on sodic site. Indian Forester, 122: 439–455.
Draper NR, Smith H. 1998. Applied Regression Analysis. New York: John Wiley and Sons.
Garg VK, Jain RK. 1992. Influence of fuel wood trees on sodic soils. Canadian Journal of Forest Research, 22: 729–735.
Garg VK, Jain RK. 1996. Effect of fuel wood plantation on some properties of sodic wastelands. Journal of Ttropical Forest Science, 9: 194?205.
Garg VK. 1998. Interaction of tree crops with a sodic soil environment: potential for rehabilitation of degraded environments. Land Degradation and Development, 9: 81?93.
Garg VK. 1999. Leguminous trees for the rehabilitation of sodic wastelands in Northern India. Restoration Ecology, 7: 281?287.
Gill HS, Abrol IP, Samara JS. 1987. Nutrient recycling through litter production in young plantations of A. nilotica and E. tereticornis in a highly alkali soil. Forest Ecology and Management, 22(1-2): 57?69.
Gill HS, Abrol IP. 1991. Salt affected soils, their afforestation and its ameliorating influence. International Tree Crops Journal, 6: 239?260.
Hellden U. 1992. Desertification time for an assessment. Ambio, 20: 372?383.
Jain RK, Garg VK. 1996. Effect of a decade old tree stands on some properties of soils while revegetating sodic wastelands. The Indian Forester, 122: 467?475.
Jain RK, Singh B. 1998. Biomass production and soil amelioration in a high density Terminalia arjuna plantation on sodic soils. Biomass and Bioenergy, 15: 187?192.
Kaur B, Gupta SR, Singh G. 2000. Soil carbon, microbial activity and nitrogen availability in agroforestry systems on moderately alkali soils in northern India. Applied Soil Ecology, 15: 283?294.
Kaur B, Gupta SR, Singh G. 2001. Bioamelioration of sodic soils by silvipastoral systems in north-western India. Agroforestry Systems, 54: 13?20.
Khanduja SD, Chandra V, Srivastava GS, Jain RK, Mishra PN, Garg VK. 1986. Utilization of alkali soils in the plains of northern India- a case study. In: Prinsley RT, Swift MJ (eds), Amelioration of Soil by Trees. London: Commonwealth Science Council, pp. 54?61.
Kumar A, Abrol IP. 1983a. Effect of gypsum on five tropical grasses grown in normal and extremely sodic soil. Experimental Agriculture, 19: 169?177.
Kumar A, Abrol IP. 1986. Grasses in Alkali soils. Bulletin No. 11, 95. Karnal: Central Soil Salinity Research Institute, ICAR.
Kumar A, Singh NT, Batra L. 1990?1991. Tree canopy inter-crop relationships in an agro forestry system. Report 1990?91. Karnal, Haryana: Central Soil Salinity Research Institute.
Kumar A. 1988a. Performance of forage grasses in saline soils. Indian Journal of Agronomy, 33: 26?-30.
Kumar A. 1988b. Long term forage yields of five tropical grasses on an extremely sodic soil and resultant soil amelioration. Experimental Agriculture, 24: 89?96.
Kumar A. 1996. Use of Leptochloa fusca for the improvement of salt affected soils. Experimental Agriculture, 32: 243?149.
Lal B. 1998. Comparative performance of forage grasses with amendments and row spacing on saline sodic soils. In: 1stAgronomy Congress: Agronomy, Environment and food security for 21stCentury. November, 23?27, 1998, New Delhi.
Lazarides M. 1970. The grasses of central Australia. Canberra: Australian national University Press.
Luken JO, Fonda RW. 1983. Nitrogen accumulation in a chronosequence of red alder communities along the Hon River., Olympic National Park, Washington. Canadian Journal of Forest Research, 13(6): 1228?1237.
Malik KA, Aslam Z, Naqvi M. 1986. Kallar grass-plant for saline lands. Faislabad, Pakistan: Nuclear Institute of Agriculture and Biology (NAIB).
Mannetje T, Kersten SMM. 1992. Chloris gayana Kunth. In: ’t Mannetje L, Jones RM (Eds), Plant Resources of South-East Asia No 4: Forages. Wageningen, Netherlands: Pudoc-DLO, pp. 90?92.
Mishra A, Sharma SD, Khan GH. 2002. Rehabilitation of degraded sodic lands during a decade of Dalbergia sissoo plantation in Sultanpur district of Uttar Pradesh, India. Land Degradation and Development, 13: 375?386.
Mongia AD, Dey P, Singh G. 1998. Ameliorating effect of forest trees on a highly sodic soil in Haryana. Journal of Indian Society of Soil Science, 46: 664?668.
National remote Sensing Application and Associates (NRSA and Associates). 1996. Mapping of salt affected soils of India, 1: 250,000 map sheets, Legend. Hyderabad, INDIA: NRSA.
Oster JD, Shainberg I, Abrol IP. 1996. Reclamation of salt affected soils. In: Agassi M (ed), Soil Erosion, Conservation and Rehabilitation. New York: Marcel Dekker, pp. 315?352.
Oster JD, Shainberg I, Abrol IP. 1999. Reclamation of salt affected soils. In: Skaggs RW, van Schilfgaarde J (eds), Agricultural Drainage. Madison, Wisconsin: ASA-CSSASSSA, pp. 659?691,
Qadir M, Qureshi RH, Ahmad N. 1996. Reclamation of a saline sodic soil by gypsum and Leptochloa fusca. Geoderma, 74: 207?217.
Qadir M, Qureshi RH, Ahmad N. 1997. Nutrient availability in a calcarious saline sodic soil during vegetative biorememediation. Arid Soil Research and Rehabilitation, 111: 343?352.
Qadir M, Schubert S, Ghafoor A, Murtaza G. 2001. Amelioration strategies for sodic soils: a review. Land Degradation and Development, 12: 357?386.
Qureshi RH, Salim M, Abdullah M, Pitman MG. 1982. Diplachne fusca: An Australian salt tolerant grass used in Pakistan Agriculture. The Journal of the Australian Institute of Agricultural Science, 48: 195?199.
Richards LA. 1954. Diagnosis and improvement of saline and alkali soils. USDA (Washington) Handbook No. 60, p.112.
Rundel PW, Nelson ET, Sharifi RA, Virgginia WM, Jarreland DH, Shearer GB. 1982. Seasonal dynamics of nitrogen cycling for Prosopis woodland in the sonorant desert. Plant and Soil, 67: 343?353.
Sandhu GR, Aslam Z, Salim M, Sattar A, Qureshi RH, Ahmad N, Wyn Jones RG. 1981. The effect of salinity on the yield and composition of Diplachne fusca (kallar grass). Plant, Cell and Environment, 4: 177?181.
Sharma RC, Singh R, Singh YP, Singh G. 2006. Sodic soils of Shivri Experimental Farm; Site characteristics, reclamability and use potential for different land uses. Karnal: Central Soil Salinity Research Institute, Publ. no.1/2006, p. 36.
Singh B. 1975. Role of forestry in mitigating the energy crisis in India. Indian Forester, 101: 589?596.
Singh D, Chhonkar PK, Pandey RN. 1999. Soil Plant Water Analysis: A Methods Manual. New Delhi: IARI, pp. 11?14.
Singh G, Abrol IP, Cheema SS. 1989a. Effect of spacing and lopping on Prosopis + Kallar grass (L. fusca) Agroforesty systems in a sodic soil. Experimental Agriculture, 25: 401?408.
Singh G, Abrol IP, Cheema SS. 1989b. Effect of management practices on mesquite (Prosopis chilensis) in highly alkaline soils. Indian Journal of Agricultural Science, 59: 1?7.
Singh G, Dagar JC. 2005. Greening Sodic Lands: Bichhian Model. Tech. Bull. No.2/2005. Karnal: Central Soil Salinity Research Institute, p. 51.
Singh G, Singh NT. 1993. Mesquite for the Vegetation of Salt Lands. Bull. No. 18. Karnal, Haryana: Central Soil Salinity Research Institute, p. 24.
Singh G. 1995. An Agroforestry practice for the development of salt lands using Prosopis juliflora and Laptocloa fusca. Agroforestry Systems, 29: 61?75.
Singh G. 2009. Salinity-related desertification and management strategies: Indian experience. Land Degradation and Development, 20: 367?385.
Singh G., Abrol I.P. and Cheema S.S. 1987. Effect of some management practices on biomass production and nutrient cycling through mesquite-Diplachne silvipastoral system in an extremely alkali soil. National symposium on Macronutrients in soils and crops. Punjab Agricultural University, Ludhiana, 21-23 December, 1987.
Singh YP, Sharma DK, Singh G, Nayak AK, Mishra VK, Singh R. 2008. Alternate Land Use Management for Sodic Soils. Bull. No. 2. Lucknow, Uttar Pradesh: Central Soil Salinity Research Institute, Regional Research Station, p. 7.
Singh YP, Singh G, Sharma DK. 2010. Biomass and bio-energy production of ten multipurpose tree species planted in sodic soils of Indo-gangetic plains. Journal of Forestry Research, 21(1): 63?70.
Subbiah BV, Asija GL. 1956. A rapid procedure for estimation of available nitrogen in soils. Current Science, 25: 259?263.
Swaminathan MS. 1980. Indian forestry at the crossroads. International Tree Crop Journal, 1: 61?67.
Thorrold BS, Knowles RL, Nicholas ID, Power IL, Carter JL. 1997. Evaluation of agroforestry options for three tree species. Proceedings of the Newzeland Grassland Association, 59: 187?190.
Tidemann AR, Klemmedson JO. 1986. Long term effect of Mesquite removal on soil characteristics. 1. Nutrients and bulk density. Soil Science Society of America Journal, 50: 472?475.
Toth B. 1981. Afforestation on salt affected soils in Hungary. Agrokemia Telajtan, 30: 205–212.
Vance ED, Brookes PC, Jenkinson DS. 1987. An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry, 19: 703?707.
Verboon WG, Brunt MA. 1970. An ecological survey of western province, Zambia, with special reference to the fodder resources. Vol. 2. The Grasslands and their development. Land Research Study No.8. Tolworth, UK: Ministry of Overseas Development.
Walkley A. 1947. An examination of methods for determining organic carbon and nitrogen in soils. Journal of Agricultural Sciences, 25: 598?609.
Wilde SA, Voigt GK, Ayer JG. 1964. Soil and Plant Analysis for Tree Culture. Calcutta: Oxford Publishing House.
Yadav YP, Vasistha HB. 1989. Infiltration capacity of forest soils under Cryptomeria japonica. The Indian Forester, 115: 435?441.
DOI 10.1007/s11676-014-0535-1
The online version is available at http://www.springerlink.com
Central Soil Salinity Research Institute, Regional Research Station, Lucknow (U.P.) 226005, India. E-mail: ypsingh_5@yahoo.co.in
Gurbachan Singh
Indian Council of Agricultural Research, New Delhi 110001, India.
D.K. Sharma
Central Soil Salinity Research Institute, Karnal, Haryana 132001, India. Corresponding editor: Yu Lei
 Journal of Forestry Research2014年4期
Journal of Forestry Research2014年4期
- Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Full length cDNA cloning and expression analysis of annexinA2 gene from deer antler tissue
- Bamboo resources of Sikkim Himalaya: diversity, distribution and utilization
- Implications of crude oil pollution on natural regeneration of plant species in an oil-producing community in the Niger Delta Region of Nigeria
- Enhancement of seed germination in Macaranga peltata for use in tropical forest restoration
- Spatial variability of soil organic carbon in the forestlands of northeast China
