Dynamic culture of a thermosensitive collagen hydrogel as an extracellular matrix improves the construction of tissue-engineered peripheral nerve
Lanfeng Huang, Rui Li, Wanguo Liu, Jin Dai, Zhenwu Du, Xiaonan Wang, Jianchao Ma, Jinsong Zhao
1 Department of Joint Surgery, Orthopedics Hospital of the Second Hospital of Jilin University, Changchun, Jilin Province, China
2 Centre of Hand & Foot Surgery and Reparative & Reconstructive Surgery, Orthopedics Hospital of the Second Hospital of Jilin University, Changchun, Jilin Province, China
3 Department of Orthopedics Surgery, the Third Hospital of Jilin University, Changchun, Jilin Province, China
4 Institute of Orthopedics, the Second Hospital of Jilin University, Changchun, Jilin Province, China
5 Department of Ophthalmology, the Second Hospital of Jilin University, Changchun, Jilin Province, China
Dynamic culture of a thermosensitive collagen hydrogel as an extracellular matrix improves the construction of tissue-engineered peripheral nerve
Lanfeng Huang1, Rui Li2, Wanguo Liu3, Jin Dai1, Zhenwu Du4, Xiaonan Wang1, Jianchao Ma1, Jinsong Zhao5
1 Department of Joint Surgery, Orthopedics Hospital of the Second Hospital of Jilin University, Changchun, Jilin Province, China
2 Centre of Hand & Foot Surgery and Reparative & Reconstructive Surgery, Orthopedics Hospital of the Second Hospital of Jilin University, Changchun, Jilin Province, China
3 Department of Orthopedics Surgery, the Third Hospital of Jilin University, Changchun, Jilin Province, China
4 Institute of Orthopedics, the Second Hospital of Jilin University, Changchun, Jilin Province, China
5 Department of Ophthalmology, the Second Hospital of Jilin University, Changchun, Jilin Province, China
Lanfeng Huang and Rui Li contributed equally to this paper.
Tissue engineering technologies offer new treatment strategies for the repair of peripheral nerve injury, but cell loss between seeding and adhesion to the scaffold remains inevitable. A thermosensitive collagen hydrogel was used as an extracellular matrix in this study and combined with bone marrow mesenchymal stem cells to construct tissue-engineered peripheral nerve composites in vitro. Dynamic culture was performed at an oscillating frequency of 0.5 Hz and 35° swing angle above and below the horizontal plane. The results demonstrated that bone marrow mesenchymal stem cells formed membrane-like structures around the poly-L-lactic acid scaffolds and exhibited regular alignment on the composite surface. Collagen was used to fi ll in the pores, and seeded cells adhered onto the poly-L-lactic acid fi bers. The DNA content of the bone marrow mesenchymal stem cells was higher in the composites constructed with a thermosensitive collagen hydrogel compared with that in collagen I scaffold controls. The cellular DNA content was also higher in the thermosensitive collagen hydrogel composites constructed with the thermosensitive collagen hydrogel in dynamic culture than that in static culture. These results indicate that tissue-engineered composites formed with thermosensitive collagen hydrogel in dynamic culture can maintain larger numbers of seeded cells by avoiding cell loss during the initial adhesion stage. Moreover, seeded cells were distributed throughout the material.
nerve regeneration; peripheral nerve; biomaterials; extracellular matrix; tissue engineering; nerve scaffold; bone marrow mesenchymal stem cells; thermosensitive collagen hydrogel; poly-L-lactic acid; dynamic culture; NSFC grant; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 31071222; Jilin Province Science and Technology Development Project in China, No. 20080738; and the Frontier Interdiscipline Program of Norman Bethune Health Science Center of Jilin University in China, No. 2013106023.
Huang LF, Li R, Liu WG, Dai J, Du ZW, Wang XN, Ma JC, Zhao JS. Dynamic culture of a thermosensitive collagen hydrogel as an extracellular matrix improves the construction of tissue-engineered peripheral nerve. Neural Regen Res. 2014;9(14):1371-1378.
Introduction
Peripheral nerve injuries and defects are common following trauma or surgical procedures. The surgical repair of severe peripheral nerve injuries represents not only a pressing medical need, but also a dif fi cult clinical challenge. Autologous nerve grafting is the current gold standard for bridging extended gaps in transected nerves (Gu et al., 2011). However, this technique has several limitations. For example, the repair is effective only if it is performed within 2 months after trauma and only in young patients. Moreover, many patients still suffered from permanent neurological de fi cits or recovered only limited neurological function, and these patients frequently experienced irreversible neuropathic pain (Teles et al., 2010).
Tissue-engineered nerve grafts are typically constructed by combining a neural scaffold and a variety of cellular and molecular components. Schwann cells, or cells with similar functions, are typically seeded in such scaffolds. The factor used most frequently is neurotrophic factor. Because bone marrow mesenchymal stem cells display the characteristics of stem cells, including multi-differentiation potential and strong proliferation, as well as being convenient to source, they are often used tissue engineering approaches (Lin et al., 2012; Tan et al., 2013). Bone marrow mesenchymal stem cells can also express phenotypic characteristics of neurons after specific induction, as demonstrated in several studieson nerve regeneration (Jia et al., 2012; Zheng and Cui, 2012; Hsu et al., 2013).
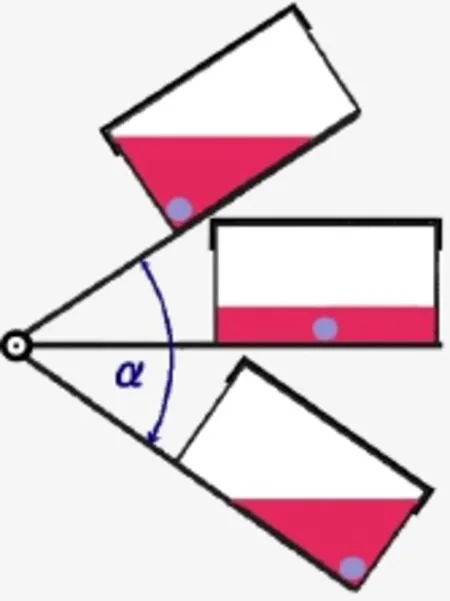
Figure 1 Schematic showing the path of the dynamic cultivation device.
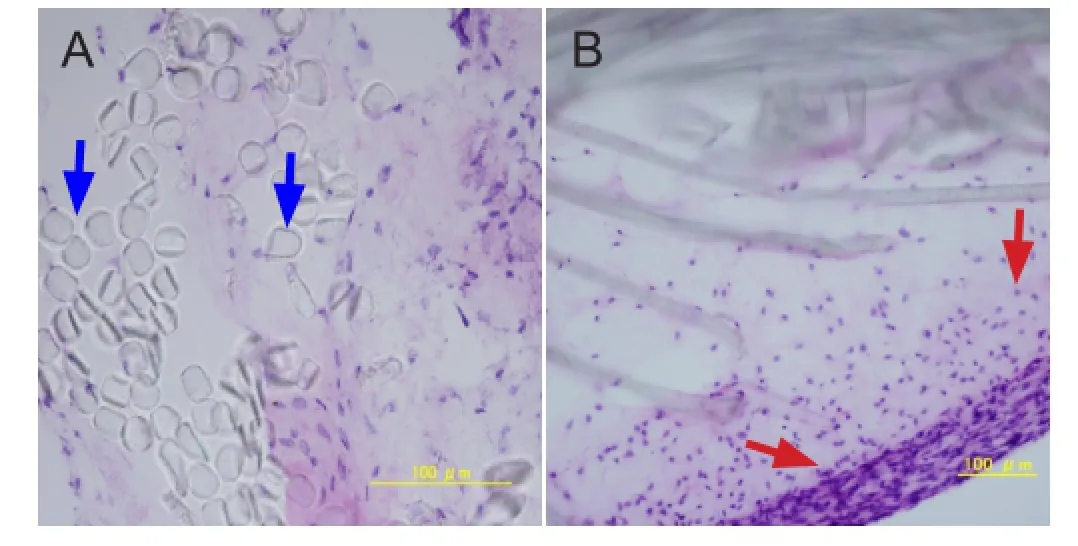
Figure 4 Histology of the composites after dynamic culture (hematoxylin-eosin staining).
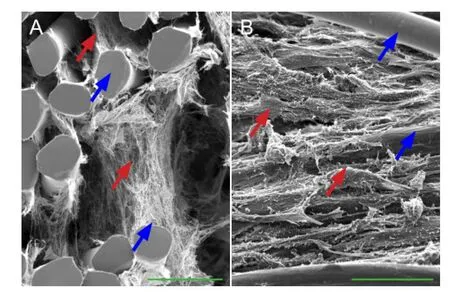
Figure 7 Relationship between bone marrow mesenchymal stem cells and the thermosensitive collagen hydrogel composite (scanning electron microscopy).
Neural tissue engineering constructs currently play a role in bridging, support, and nutrition after nerve injury (Kojima et al., 2014). In past studies, due to its cytocompatibility (Feinberg and Parker, 2010), collagen has served as a template in the design of “l(fā)iving” nerve conduits, which may promote nerve regeneration by extending across nerve gaps. Porous collagen scaffolds also enhance the regeneration of injured peripheral nerves (Soller et al., 2012). The use of tissue engineering technologies to repair peripheral nerve injury is a new treatment strategy. One important dif fi culty in creating such scaffolds for tissue engineering is the ability to cultivate sufficient numbers of cells to seed for growth in the biomaterial in vitro. Standard collagen formulations that are currently used as extracellular matrix can improve cell compatibility with different materials, but they do not prevent cell loss between seeding and cell adhesion to the scaffold. Thermosensitive collagen hydrogels are made from natural collagen and inorganic salts. They display many characteristics of normal collagen and can be crosslinked to form gels with increasing temperature, which may increase their cytocompatibility. Thus, the aim of this study was to determine whether thermosensitive collagen hydrogels can improve tissue-engineered peripheral nerve composites.
Materials and Methods
Bone marrow mesenchymal stem cell culture and identi fi cation
Bone marrow mesenchymal stem cells were obtained from eight Fischer 344 rats (male, 7 weeks of age, speci fi c pathogen free, acquired from the Laboratory Animal Centre of Jilin University, China). Following anesthesia with a lethal dose of diethyl ether and under sterile conditions, the bilateral femurs and tibias were separated, the bone ends were cut, and the bone marrow cavities were rinsed with a 5-mL syringe filled with Dulbecco’s modified Eagle’s medium (DMEM; Gibco, New York, NY, USA). The rinse solution was centrifuged at 1,500 r/min at 4°C for 10 minutes, and the supernatant was discarded. The pelleted cells were resuspended in DMEM containing 10% fetal bovine serum (Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin. The cells were then incubated in a T-75 fl ask in an incubator containing 5% CO2at 37°C with 100% humidity. After 4 days, the fi rst medium change was performed. Non-adherent cells were gently removed by the medium change. From then on, the medium was replaced once every 2 days until cells reached 80% confluency. The cultures were treated with trypsin-ethylenediamine tetraacetic acid solution (0.25% trypsin, 0.02% ethylenediamine tetraacetic acid; Sigma, St. Louis, MO, USA), and were then harvested and diluted to 1:2 per passage for further expansion (Zhang and Lv, 2013). To identify bone marrow mesenchymal stem cells, 80% confluent cells were induced towards osteogenesis for 3 weeks with osteogenic medium, which was supplemented with 0.1 μmol/L dexamethasone, 50 mg/L ascorbic acid, and 10 mmol/L beta glycerin sodium phosphate.Adipogenic induction was also performed for 3 weeks using an adipogenic medium that included 0.25 μmol/L dexamethasone, 50 μmol/L indomethacin, 0.5 mmol/L 3-isobutyl-methyl xanthine, and 10 mg/L insulin. The effects of the osteogenic and adipogenic induction were observed with separate alizarin red and oil red O staining (Gambardella et al., 2011; James, 2013; Kollmer et al., 2013; El-Mahgoub et al., 2014).
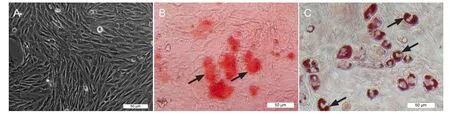
Figure 2 Morphological characteristics and differentiation of cultured bone marrow mesenchymal stem cells (inverted phase contrast and bright- fi eld microscopy).
Figure 3 Appearance of the thermosensitive collagen hydrogel composites.
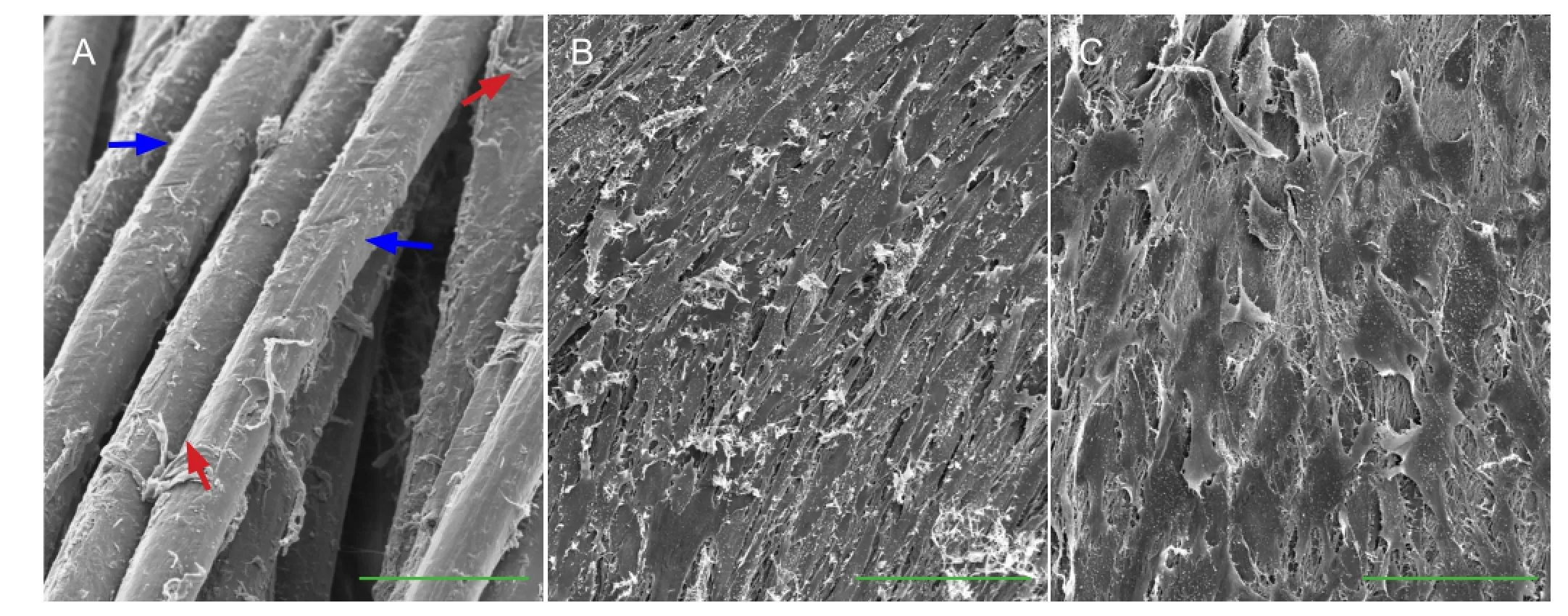
Figure 5 Ultrastructure of the thermosensitive collagen hydrogel composite after 7 days of culture (scanning electron microscopy).
Poly-L-lactic acid scaffolds
The diameter of the poly-L-lactic acid scaffold materials (Ai-medic Co. Ltd., Tokyo, Japan) was 3.0 mm, woven from poly-L-lactic acid fi bers with 10 μm diameter. The rupture strength was 366 N under a pulling speed of 100 mm/min. The surface of the scaffolds was first treated with 70% alcohol, which was then replaced with DMEM to make them hydrophilic.
Thermosensitive collagen hydrogel preparation
The thermosensitive collagen gel culture kit (Nitta-gelatin Co. Ltd., Osaka, Japan) consisted of Cellmatrix Type I-A that was prepared from pig collagen type I, NaHCO3-free 10 × concentrated DMEM, and restructured buffer that consisted of 260 mmol/L NaHCO3, 50 mmol/L NaOH, and 200 mmol/L hydroxyethyl piperazine ethanesulfonic acid. The thermosensitive collagen hydrogel was mixed together on ice at a constituent ratio of 8 parts Cellmatrix Type IA to 1 part NaHCO3-free concentrated DMEM to 1 part restructured buffer. The thermosensitive collagen hydrogel remained as a liquid when kept at temperatures below 10°C and gelled when the temperature was increased to 37°C in an incubator for 30 minutes. In the control group, the thermosensitive collagen hydrogel was replaced with 10 × concentrated DMEM diluted 10-fold with collagen type I.
Construction of compositein vitro
Mesenchymal stem cells were suspended in thermosensitive collagen hydrogel solution at a cell density of 1 × 106/mL, while keeping the solution on ice. A total of 0.2 mL of the bone marrow mesenchymal stem cell suspension was injected slowly into the poly-L-lactic acid scaffolds placed in 96-well plates with a 24G syringe. The plate was placed in an incubator for gelation at 37°C for 30 minutes. The composites were removed and placed in T-25 fl asks for dynamic or static culture. Scaffolds made from normal type I collagen instead of thermosensitive collagen hydrogel served as controls.
Dynamic culture
An oscillating device (Strex, Osaka, Japan) was used to generate swing through a specific angle (α), which was set as 70°, and half (35°) occurred above and half below the horizontal plane (Figure 1) at an oscillating frequency of 0.5 Hz. The culture fl ask was fi xed on the plate, and the composite was allowed to roll within the fl ask from end to end with the gravitational fl ow of the medium. The medium was changed every day. For static culture, the fl ask was placed horizontally without any movement.
Morphology of the tissue-engineered peripheral nerve observed by hematoxylin-eosin staining
The composites were fi xed in 1% glutaraldehyde in 0.1 mol/L PBS for 12 hours, dehydrated in a graded ethanol series, and embedded in paraf fi n. For light microscopy (Olympus, Tokyo, Japan), serial sections 1 μm thick were cut and stained with hematoxylin-eosin.
Ultrastructure of the tissue-engineered peripheral nerve observed by scanning electron microscopy
The morphology of the composites was evaluated using scanning electron microscopy on the surface and longitudinal and transverse cross-sections. The composites were cut along the longitudinal or transverse axis with a scalpel, hydrated in sterile PBS, dried in a critical point dryer, sputter-coated with gold, and then observed with a scanning electron microscope (Hitachi S-800, Tokyo, Japan) (Scott et al., 2011).
Total DNA quanti fi cation to assess cell proliferation
To quantify total DNA content, the composites were cut into 1 mm × 1 mm pieces, and these fragments were treated in an Ultrasonic Cell Crusher (Sonics VCX800, Newtown, CT, USA). Cell proliferation was quantified by measuring the DNA concentration with a DNA/RNA extraction kit (Wako, Osaka, Japan) according to the manufacturer’s protocol. The lysis solution was centrifuged at 1,000 r/min for 5 minutes, and the supernatant was collected for DNA quantification by measuring the absorbance at 260 nm with an ultraviolet spectrophotometer (Shimadzu, Kyoto, Japan). The DNA concentration was normalized to the initial number of bone marrow mesenchymal stem cells (Lundborg; Gu et al., 2014).
Statistical analysis
All data were expressed as mean ± SD. Data were analyzed using normal distribution tests and tests of homogeneity of variance between groups with SPSS 17.0 software (SPSS, Chicago, IL, USA). Comparisons between two groups were performed using one-way analysis of variance followed by Student-Newman-Keuls test. Values of P < 0.05 were considered statistically signi fi cant.
Results
Culture and identi fi cation of bone marrow mesenchymal stem cells
After seeding bone marrow cells into the fl asks, many round cells were found adhered to the bottom after 24 hours. The adherent, fusiform-shaped bone marrow mesenchymal stem cells were isolated from the non-adherent hematopoietic cells, which were removed with the medium changes. Small cell clusters were found starting at 3 days after culture, and the number of clusters increased, they fused, and become gradually confluent with cell proliferation over 7-10 days (Figure 2A). To demonstrate the differentiation potential of the bone marrow mesenchymal stem cells, osteogenic and adipogenic inductions were performed over 3 weeks. The results of osteogenic induction via alizarin red staining showedred calcium deposits in the cytoplasm (Figure 2B), and lipid-laden adipocyte phenotype were found after adipogenic induction via oil red O staining (Figure 2C).
Morphological changes of the composite material
After pre-culture (30 minutes), the thermosensitive collagen hydrogel cell suspension solidified into a gel (Figure 3A). During culture, the thermosensitive collagen hydrogel gradually contracted, surrounding the poly-L-lactic acid scaffold (Figure 3B-D).
Hematoxylin-eosin staining results of the composite showed that the poly-L-lactic acid scaffolds were surrounded by a membrane formed by the seeded cells and thermosensitive collagen hydrogel, and the seeded cells were also seen in the space between poly-L-lactic acid fi bers on the interior of the composite at 7 days after culture (Figure 4).
Ultrastructure of tissue-engineered peripheral nerve
Scanning electron microscopy revealed that in the composite constructed with collagen type I, few cells attached to the poly-L-lactic acid and no membrane structure formed on the surface (Figure 5A). In the composite constructed with the thermosensitive collagen hydrogel, the cells on the surface were spindle-shaped. They were arranged among the longitudinal axis of the composite after dynamic cultivation (Figure 5B), and the cells were extended into a fl at shape after static culture (Figure 5C).
After dynamic culture, a membrane structure formed from the thermosensitive collagen hydrogel and seeded cells surrounded the composite (Figure 6A). The cells were arranged as spindles close together on the surface (Figure 6B), and their activity was seen by the large number of villis formed on their surfaces (Figure 6C).
Seeded cells had adhered to the collagen and poly-L-lactic acid fi bers (Figure 7A), and the collagen fi bers fi lled in the space around the poly-L-lactic acid fi bers (Figure 7B).
Change of cell proliferation in tissue-engineered peripheral nerve composite
The total DNA content of the composites formed with the thermosensitive collagen hydrogel was significantly higher than that in normal collagen I composites at both 30 minutes and 24 hours after seeding (P < 0.01;Figure 8A). The total DNA content of the composites under dynamic cultivation was higher than that in the composites under static culture (P < 0.05). The total DNA content at 7 days after culture was higher than the control (0 day) in both of the groups (P < 0.05;Figure 8B).
Discussion
Tissue engineering techniques have been recently used to construct composite conduits in vitro for promoting the functional recovery of peripheral nerve defects (Konofaos and Ver Halen, 2013). Biodegradable materials seeded with cells that have neural differentiation potential and neurotrophic factors are considered to be essential components for tissue-engineered nerve regeneration (Marquardt and Sakiyama-Elbert, 2013; Quigley et al., 2013; Salgado et al., 2013; Saracino et al., 2013). A classical method of tissue engineering was used in the present study, and peripheral nerve-like composites were constructed in vitro using a thermosensitive collagen hydrogel combined with poly-L-lactic acid scaffolds as the extracellular matrix and bone marrow mesenchymal stem cells as the cell source. The main objective in this study was to determine the effect of the thermosensitive collagen hydrogel on the seeding efficiency by morphological observation and analysis of total DNA content. The seeding efficiency was improved from 35% to 85% with the thermosensitive collagen hydrogel, avoiding most cell loss. The number of adhered cells may play a very important role in the construction of tissue-engineered peripheral nerve (Liu et al., 2013; Qi et al., 2013; Zhang and Lv, 2013).
Collagen, as a natural material, has many advantages in tissue engineering applications. Combining collagen and poly-L-lactic acid (or other biodegradable materials), we can obtain a composite with good biocompatibility and mechanical strength (Feinberg and Parker, 2010; Soller et al., 2012; Hwang et al., 2013). Many natural macromolecules and synthetic polymers/monomers can be made into thermoresponsive hydrogels (Hsu et al., 2013). Such materials have been extensively used for drug delivery studies (Hoshi et al., 2000; Feinberg and Parker, 2010; Gong et al., 2013). Collagen has also been used to prepare thermoresponsive hydrogels for use in three-dimensional cell culture (embedded cell culture) and tissue engineering studies (Ding et al., 2012; Qi et al., 2013). The thermosensitive collagen hydrogel used in this study was derived from collagen type I, which is the most widely distributed and important protein in the body. Its most representative characteristic is that it undergoes a phase separation in response to temperature change, gelling above its lower critical solution temperature (Oh et al., 2008; Ji et al., 2010; Xu et al., 2011). Thermosensitive collagen hydrogel solutions can be the diluted to obtain an injectable cell suspension, and the porous network and high water content formed upon gelation promotes cell metabolism in composites (Johnson et al., 2005; Chung et al., 2011).
The bene fi ts of using the thermosensitive collagen hydrogel in this study were that it retained the most seeded cells, limited migration of the cells, maintained the seeded cells in the scaffolds, reduced cell loss during culture, and avoided the need for cells to grow into the scaffolds. Using the thermosensitive collagen hydrogel, we created a composite with high cell density at the initial stage of culture, and the composite contracted during culture. The contractile properties of the composite may be associated with the concentration of collagen and the cell number, as well as with any associated fibroblasts (Ding et al., 2012; Hwang et al., 2013). To achieve composite contraction, a certain number of fibroblasts may be needed among the seeded cells, and such cells may be useful in constructing tissue-engineered peripheral nerves in vitro.
The use of a bioreactor can promote the growth of cells in three-dimensional materials and can increase the in vitro biological activity of tissue-engineered composite (Sun et al.,2008; Valmikinathan et al., 2011).
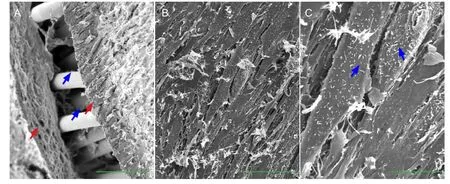
Figure 6 Surface characteristics of the thermosensitive collagen hydrogel composite after 7 days of dynamic culture (scanning electron microscopy).
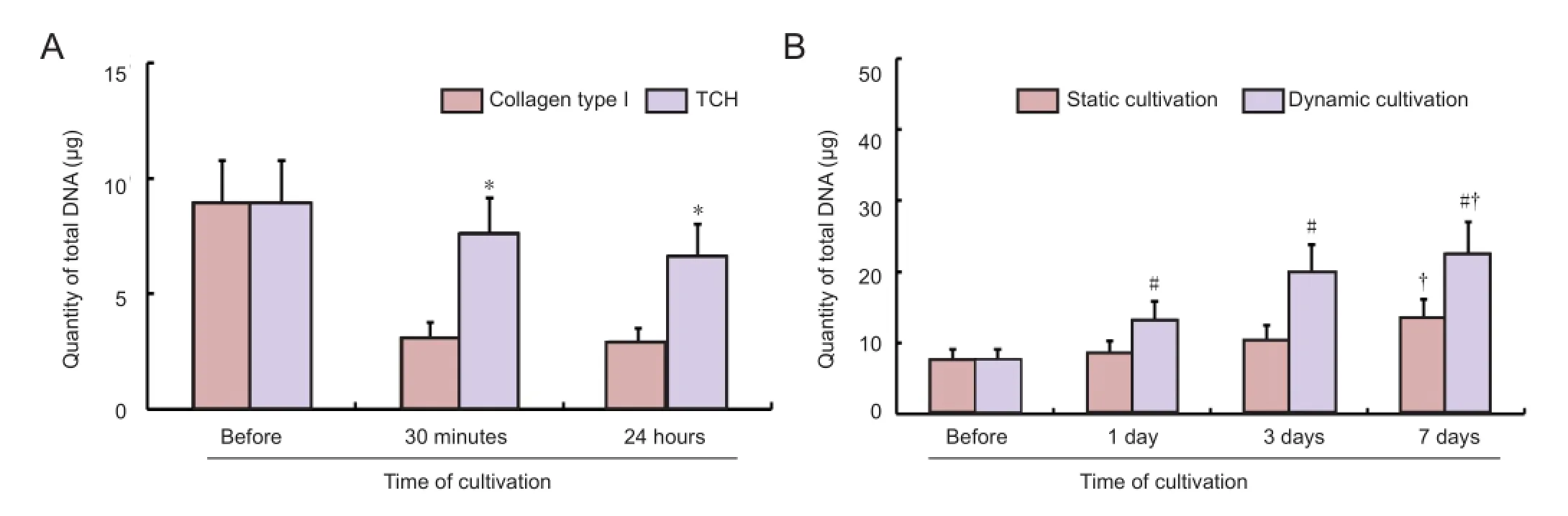
Figure 8 Cell proliferation in the tissue-engineered peripheral nerve composites.
Hydromechanical stimulation, generated using an oscillating device, can provide a better environment for cell survival, promote adhesion of seeded cells to biological materials, and increase the cellular biological activities in the three-dimensional composite structure (Sun et al., 2008). The results from this study indicate that compared with static plate culture, dynamic plate culture can improve the environment for cell adhesion and growth, as shown by the total DNA content increase. Cell alignment is thought to play a critical role in various cell behaviors and in tissue regeneration (Li et al., 2013), and also exists in native nerve tissues. During axonal regeneration, neural cells spontaneously orient parallel to aligned Schwann cells in injured peripheral (Guenard et al., 1992) and central (Brook et al., 1998) nerves in vivo. The transplantation of nerve conduits seeded with aligned Schwann cells has also been used to promote nerve regeneration (Lietz et al., 2006; Laulicht et al., 2011), which is largely caused by the improved rate and extent of neurite elongation when cultured on aligned Schwann cells (Miller et al., 2001; Lietz et al., 2006). The morphological observations in the present study showed that mesenchymal stem cells were arranged in parallel and had formed a membrane structure on the surface of the composite by 7 days after culture in thedynamic cultivation system. This organization may be useful for peripheral nerve tissue engineering.
Because the main objective in the fi rst stage of this study was the construction and morphological examination of a tissue-engineered composite in vitro, no neural inducing factors such as neurotrophic factors were used on the bone marrow mesenchymal stem cells to promote neural cell differentiation. No neurologic functional assessments of the composites were performed either. These analyses will be conducted in the next step of this project. By using a thermosensitive collagen hydrogel as the extracellular matrix, the seeded cells remained in the tissue-engineered composite with sufficient cell numbers and a uniform distribution, avoiding the typical cell loss after seeding. This thermosensitive collagen hydrogel may be widely applicable as an extracellular matrix for the construction of peripheral nerves or any other in vitro tissue-engineered composite. The oscillating culture device provided a dynamic culture environment that promoted cell proliferation, alignment, and increased cell biological activity.
Author contributions:Zhao JS obtained the funding and supervised the study. Huang LF and Li R obtained funding, were responsible for research concept and design and manuscript authorization. Liu WG wrote the manuscript, executed the experiments, provided and processed the experimental and statistical data. Du ZW provided technical and material supports. Dai J, Wang XN, and Ma JC participated in the experiments and provided experimental data. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Brook GA, Plate D, Franzen R, Martin D, Moonen G, Schoenen J, Schmitt AB, Noth J, Nacimiento W (1998) Spontaneous longitudinally orientated axonal regeneration is associated with the Schwann cell framework within the lesion site following spinal cord compression injury of the rat. J Neurosci Res 53:51-65.
Chung TW, Yang MC, Tseng CC, Sheu SH, Wang SS, Huang YY, Chen SD (2011) Promoting regeneration of peripheral nerves in-vivo using new PCL-NGF/Tirofiban nerve conduits. Biomaterials 32:734-743.
Ding K, Yang Z, Dai F, Jin HY, Chang ZQ, Xu JZ (2012) Effects of injectable chitosan/β-glycerol phosphate disodium/collagen scaffold on the growth and differentiation of myoblasts. Zhonghua Yi Xue Za Zhi 92:1357-1360.
El-Mahgoub ER, Ahmed E, Afifi RA, Kamal MA, Mousa SM (2014) Mesenchymal stem cells from pediatric patients with aplastic anemia: isolation, characterization, adipogenic, and osteogenic differentiation. Fetal Pediatr Pathol 33:9-15.
Feinberg AW, Parker KK (2010) Surface-initiated assembly of protein nanofabrics. Nano Lett 10:2184-2191.
Gambardella A, Nagaraju CK, O’Shea PJ, Mohanty ST, Kottam L, Pilling J, Sullivan M, Djerbi M, Koopmann W, Croucher PI, Bellantuono I (2011) Glycogen synthase kinase-3α/β inhibition promotes in vivo ampli fi cation of endogenous mesenchymal progenitors with osteogenic and adipogenic potential and their differentiation to the osteogenic lineage. J Bone Miner Res 26:811-821.
Gong C, Wu Q, Wang Y, Zhang D, Luo F, Zhao X, Wei Y, Qian Z (2013) A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 34:6377-6387.
Gu X, Ding F, Yang Y, Liu J (2011) Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol 93:204-230.
Gu Y, Zhu J, Xue C, Li Z, Ding F, Yang Y, Gu X (2014) Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials 35:2253-2263.
Guénard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P (1992) Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci 12:3310-3320.
Hoshi M, Harada A, Kawase T, Uyemura K, Yazaki T (2000) Antitumoral effects of defective herpes simplex virus-mediated transfer of tissue inhibitor of metalloproteinases-2 gene in malignant glioma U87 in vitro: consequences for anti-cancer gene therapy. Cancer Gene Ther 7:799-805.
Hsu SH, Kuo WC, Chen YT, Yen CT, Chen YF, Chen KS, Huang WC, Cheng H (2013) New nerve regeneration strategy combining laminin-coated chitosan conduits and stem cell therapy. Acta Biomater 9:6606-6615.
Hwang YS, Chiang PR, Hong WH, Chiao CC, Chu IM, Hsiue GH, Shen CR (2013) Study in vivo intraocular biocompatibility of in situ gelation hydrogels: poly(2-ethyl oxazoline)-block-poly(ε-caprolactone)-block-poly(2-ethyl oxazoline) copolymer, matrigel and pluronic F127. PLoS One 8:e67495.
James AW (2013) Review of signaling pathways governing MSC wsteogenic and adipogenic differentiation. Scientifica (Cairo) 2013: 684736.
Ji QX, Zhao QS, Deng J, Lü R (2010) A novel injectable chlorhexidine thermosensitive hydrogel for periodontal application: preparation, antibacterial activity and toxicity evaluation. J Mater Sci Mater Med 21:2435-2442.
Jia H, Wang Y, Tong XJ, Liu GB, Li Q, Zhang LX, Sun XH (2012) Sciatic nerve repair by acellular nerve xenografts implanted with BMSCs in rats xenograft combined with BMSCs. Synapse 66:256-269.
Johnson EO, Zoubos AB, Soucacos PN (2005) Regeneration and repair of peripheral nerves. Injury 36 Suppl 4:S24-29.
Kojima T, Matsumoto Y, Ibrahim OM, Wakamatsu TH, Dogru M, Tsubota K (2014) Evaluation of a thermosensitive atelocollagen punctal plug treatment for dry eye disease. Am J Ophthalmol 157:311-317. e1.
K?llmer M, Buhrman JS, Zhang Y, Gemeinhart RA (2013) Markers are shared between adipogenic and osteogenic differentiated mesenchymal stem cells. J Dev Biol Tissue Eng 5:18-25.
Konofaos P, Ver Halen JP (2013) Nerve repair by means of tubulization: past, present, future. J Reconstr Microsurg 29:149-164.
Laulicht B, Gidmark NJ, Tripathi A, Mathiowitz E (2011) Localization of magnetic pills. Proc Natl Acad Sci U S A 108:2252-2257.
Li Y, Huang G, Zhang X, Wang L, Du Y, Lu TJ, Xu F (2014) Engineering cell alignment in vitro. Biotechnol Adv 32:347-365.
Lietz M, Dreesmann L, Hoss M, Oberhoffner S, Schlosshauer B (2006) Neuro tissue engineering of glial nerve guides and the impact of different cell types. Biomaterials 27:1425-1436.
Lin H, Yang G, Tan J, Tuan RS (2012) In fl uence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials 33:4480-4489.
Liu HW, Wen WS, Hu M, Bi WT, Chen LJ, Liu SX, Chen P, Tan XY (2013) Chitosan conduits combined with nerve growth factor microspheres repair facial nerve defects. Neural Regen Res 8:3139-3147.
Lundborg G (2000) A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am 25:391-414.
Marquardt LM, Sakiyama-Elbert SE (2013) Engineering peripheral nerve repair. Curr Opin Biotechnol 24:887-892.
Miller C, Jeftinija S, Mallapragada S (2001) Micropatterned Schwann cell-seeded biodegradable polymer substrates signi fi cantly enhance neurite alignment and outgrowth. Tissue Eng 7:705-715.
Oh SH, Kim JH, Song KS, Jeon BH, Yoon JH, Seo TB, Namgung U, Lee IW, Lee JH (2008) Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials 29:1601-1609.
Qi BW, Yu AX, Zhu SB, Zhou M, Wu G (2013) Chitosan/poly(vinyl alcohol) hydrogel combined with Ad-hTGF-β1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp Biol Med (Maywood) 238:23-30.
Quigley AF, Bulluss KJ, Kyratzis IL, Gilmore K, Mysore T, Schirmer KS, Kennedy EL, O’Shea M, Truong YB, Edwards SL, Peeters G, Herwig P, Razal JM, Campbell TE, Lowes KN, Higgins MJ, Moulton SE, Murphy MA, Cook MJ, Clark GM, et al (2013) Engineering a multimodal nerve conduit for repair of injured peripheral nerve. J Neural Eng 10:016008.
Salgado AJ, Oliveira JM, Martins A, Teixeira FG, Silva NA, Neves NM, Sousa N, Reis RL (2013) Tissue engineering and regenerative medicine: past, present, and future. Int Rev Neurobiol 108:1-33.
Saracino GA, Cigognini D, Silva D, Caprini A, Gelain F (2013) Nanomaterials design and tests for neural tissue engineering. Chem Soc Rev 42:225-262.
Scott JB, Afshari M, Kotek R, Saul JM (2011) The promotion of axon extension in vitro using polymer-templated fi brin scaffolds. Biomaterials 32:4830-4839.
Soller EC, Tzeranis DS, Miu K, So PT, Yannas IV (2012) Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials 33:4783-4791.
Sun T, Norton D, Vickers N, L McArthur S, Neil SM, Ryan AJ, Haycock JW (2008) Development of a bioreactor for evaluating novel nerve conduits. Biotechnol Bioeng 99:1250-1260.
Tan SL, Ahmad TS, Selvaratnam L, Kamarul T (2013) Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J Anat 222:437-450.
Teles H, Vermonden T, Eggink G, Hennink WE, de Wolf FA (2010) Hydrogels of collagen-inspired telechelic triblock copolymers for the sustained release of proteins. J Control Release 147:298-303.
Valmikinathan CM, Hoffman J, Yu X (2011) Impact of scaffold micro and macro architecture on Schwann cell proliferation under dynamic conditions in a rotating wall vessel bioreactor. Mater Sci Eng C Mater Biol Appl 31:22-29.
Xu H, Yan Y, Li S (2011) PDLLA/chondroitin sulfate/chitosan/NGF conduits for peripheral nerve regeneration. Biomaterials 32:4506-4516.
Zhang CS, Lv G (2013) Repair of sciatic nerve defects using tissue engineered nerves. Neural Regen Res 8:1985-1994.
Zheng L, Cui HF (2012) Enhancement of nerve regeneration along a chitosan conduit combined with bone marrow mesenchymal stem cells. J Mater Sci Mater Med 23:2291-2302.
Copyedited by McCarty W, Pack M, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.137590
Jinsong Zhao, M.D., Ph.D., Department of Ophthalmology, the Second Hospital of Jilin University, Changchun 130041, Jilin Province, China, jinsong@jlu.edu.cn.
http://www.nrronline.org/
Accepted: 2014-04-25
- 中國神經(jīng)再生研究(英文版)的其它文章
- Cholecystokinin octapeptide antagonizes apoptosis in human retinal pigment epithelial cells
- Heat shock protein 72 confers protection in retinal ganglion cells and lateral geniculate nucleus neurons via blockade of the SAPK/JNK pathway in a chronic ocular-hypertensive rat model
- Amplitude of sensory nerve action potential in early stage diabetic peripheral neuropathy: an analysis of 500 cases
- Ultrasound imaging of chitosan nerve conduits that bridge sciatic nerve defects in rats
- Green tea polyphenols protect spinal cord neurons against hydrogen peroxide-induced oxidative stress
- Electroacupuncture attenuates neuropathic pain after brachial plexus injury

