Lentivirus-mediated Persephin over-expression in Parkinson’s disease rats
Xiao-feng Yin, Hua-min Xu, Yun-xia Jiang, Yun-lai Zhi, Yu-xiu Liu, Heng-wei Xiang, Kai Liu, Xiao-dong Ding,, Peng Sun,
1 Department of Neurosurgery, the Second Af liated Hospital of Shanxi Medical University, Taiyuan, Shanxi Province, China
2 Department of Physiology, Qingdao University, Qingdao, Shandong Province, China
3 Nursing College of Qingdao University, Qingdao, Shandong Province, China
4 Department of Pediatric Surgery, Af liated Hospital of Qingdao University, Qingdao, Shandong Province, China
5 Department of Nursing, Af liated Hospital of Qingdao University, Qingdao, Shandong Province, China
6 Department of Neurosurgery, Af liated Hospital of Medical College, Qingdao University, Qingdao, Shandong Province, China
Lentivirus-mediated Persephin over-expression in Parkinson’s disease rats
Xiao-feng Yin1,#, Hua-min Xu2,#, Yun-xia Jiang3, Yun-lai Zhi4, Yu-xiu Liu5, Heng-wei Xiang6, Kai Liu6, Xiao-dong Ding6,*, Peng Sun6,*
1 Department of Neurosurgery, the Second Af liated Hospital of Shanxi Medical University, Taiyuan, Shanxi Province, China
2 Department of Physiology, Qingdao University, Qingdao, Shandong Province, China
3 Nursing College of Qingdao University, Qingdao, Shandong Province, China
4 Department of Pediatric Surgery, Af liated Hospital of Qingdao University, Qingdao, Shandong Province, China
5 Department of Nursing, Af liated Hospital of Qingdao University, Qingdao, Shandong Province, China
6 Department of Neurosurgery, Af liated Hospital of Medical College, Qingdao University, Qingdao, Shandong Province, China
Persephin, together with glial cell line-derived neurotrophic factor and neurturin, has a neurotrophic ef ect and promotes the survival of motor neurons cultured in vitro. In this study, dopaminergic neurons in the substantia nigra of rats were transfected with the Persephin gene. One week later 6-hydroxydopamine was injected into the anterior medial bundle to establish a Parkinson’s disease model in the rats. Results found that the number of dopaminergic neurons in the substantia nigra increased, tyrosine hydroxylase expression was upregulated and concentrations of dopamine and its metabolites in corpus striatum were increased after pretreatment with Persephin gene. In addition, the rotating ef ect of the induced Parkinson’s disease rats was much less in the group pretreated with the Persephin gene. Persephin has a neuroprotective ef ect on the 6-hydroxydopamine-induced Parkinson’s disease through protecting dopaminergic neurons.
nerve regeneration; Persephin; lentivirus; Parkinson’s disease; dopaminergic neurons; gene therapy; over-expression; transfection; striatum; neural regeneration
Funding: This work was supported by the National Natural Science Foundation of China, No. 81171208 and the Natural Science Foundation of Shandong Province of China, No. Z2008C06.
Yin XF, Xu HM, Jiang YX, Zhi YL, Liu YX, Xiang HW, Liu K, Ding XD, Sun P (2015) Lentivirus-mediated Persephin over-expression in Parkinson’s disease rats. Neural Regen Res 10(11):1814-1818.
Introduction
Parkinson’s disease (PD) is a multi-system degenerative disorder, involving not only the nigral dopaminergic cells but also other predisposed nerve cells, i.e., non-dopaminergic structures of the lower brainstem or in the olfactory bulb (Braak et al., 2002; Burn and Tr?ster, 2004; Katzenschlager, 2014). Progressive degeneration of substantia nigra dopaminergic neurons leads to a subsequent loss of dopaminergic terminals in the caudate-putamen and the clinical symptoms, such as akinesia, resting tremor, muscle rigidity, and postural imbalance (Lotharius and Brundin, 2002; de la Fuente-Fernández et al., 2004).
Neurotrophic factors are important for the development and maintenance of the nervous system (Airaksinen and Saarma, 2002; Zihlmann et al., 2005; Wanigasekara and Keast, 2006). Neurturin supports the survival of ventral midbrain dopaminergic and motor neurons and induces neurite outgrowth in the spinal cord (Bespalov et al., 2011; Wang et al., 2014). Glial cell line-derived neurotrophic factor (GDNF) maintains the survival and function of midbrain dopaminergic neurons in vitro and attenuates neuronal damage induced by 6-hydroxydopamine (6-OHDA) (Zhao et al., 2014).
The physiological functions of Persephin protein (PSPN) remain unclear at present. PSPN shares some neurotrophic ef ects previously described for GDNF and neurturin (Sidorova et al., 2010). It supports the survival of motor neurons cultured in vivo after sciatic nerve axotomy (Yang et al., 2007). However, because of the extremely low expression levels of PSPN, tissue distribution has been studied by reverse transcription-PCR only. Low PSPN mRNA expression level is found throughout the embryonic and adult central nervous system and in all peripheral tissues examined (including heart, kidney, liver, skin, and muscle) (Akerud et al., 2002).
Previous studies showed that neural stem cells modif ed using GDNF could survive longer than unmodified neural stem cells after intracerebral transplantation in a rat model of middle cerebral artery occlusion (Kameda et al., 2007). In the present study, we investigated whether lentivirus (LV)-mediated PSPN over-expression could enhance the survival of dopaminergic neurons in the 6-OHDA-induced PD rat model.
Materials and Methods
6-OHDA injury and LV-PSPN intervention
Forty healthy, clean, 8-month-old male Wistar rats, weighing 200–250 g, were provided by the Animal Center of Qingdao Food and Drug Administration (license No. D20131004; Qingdao, Shandong Province, China). All rats were housed in a temperature-controlled room, under a 12-hour day/night cycle, allowing free access to water and food. All experimental procedures were in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Qingdao University in China.
Rats adapted to the experiment conditions for 1 week before the experiment and were randomly divided into four groups. Normal group: rats were free of any treatment; 6-OHDA group: rats were injected with 6-OHDA (Sigma, St. Louis, MO, USA; dissolved to normal saline containing 0.2% ascorbic acid, 4 mg/mL) into the anterior medial bundle of the right forebrain. The coordinates of the two injection sites were: (1) 4.4 mm posterior to the bregma, 1.2 mm lateral to the median line and 7.8 mm below the dura mater; (2) 4.0 mm posterior to the bregma, 0.8 mm lateral to the median line and 8.0 mm below the dura mater. The injection volume was 2.25 μL and 2.7 μL, respectively (He et al., 2014). 6-OHDA + LV-null group: rats were f rst injected with 1 μL of LV only (titer 1 × 108, Shanghai Genechem, Shanghai, China) on the right substantia nigra (coordinates: 5.3 mm posterior to the bregma, 1.9 mm lateral to the median line and 7.5 mm below the skull), and then injected with 6-OHDA as above 1 week later; 6-OHDA + LV-PSPN group: rats were f rst injected with LV-PSPN lentivirus (titer 1 × 108, Shanghai Genechem) on the right substantia nigra, and then injected with 6-OHDA as above 1 week later (He et al., 2014).
Behavioral testing
After 21 days of 6-OHDA injection, subcutaneous injections with 0.05 mg/kg apomorphine (Sigma; dissolved to normal saline containing 0.2% ascorbic acid) were applied to the napes of the rats to induce the rotating behaviors of rats. The number of rotations within 30 minutes was recorded.
Immunof uorescence test
At 21 days after the 6-OHDA injection, rats were anesthetized with 8% chloral hydrate via intraperitoneal injection. A needle was inserted into the aortic ventricle of rats, and 400 mL of 37°C normal saline was infused rapidly until the liver turned white, then 4% pre-cooled paraformaldehyde was infused until the whole body of the rat became stif . The brains were harvested and f xed in 4% paraformaldehyde for 4 hours and then hydrated in a 4°C 20% sucrose phosphate solution until the brains sank to the bottom of the centrifugation tube. Then the brains were hydrated in a 4°C 30% sucrose phosphate solution until the brains sank to the bottom of the centrifugation tube. Tissues were cut into coronal frozen sections at 20 μm thickness for immunof uorescence staining. The sections were placed in 24-well culture plates and incubated with 400 μL of mouse anti-rat TH monoclonal antibody (1:2,000; Sigma). The culture plates were then transferred to 4°C refrigerators overnight, and rinsed with PBS three times, for 10 minutes each. Then the sections were incubated with DyLight 488-labeled donkey anti-mouse IgG (1:500; Sigma) for 1 hour at room temperature, and rinsed with PBS three times, for 10 minutes each. Finally, the sections were mounted with 70% glycerol and observed under the microscope (ZEISS, Oberkochen, Germany).
Western blot assay
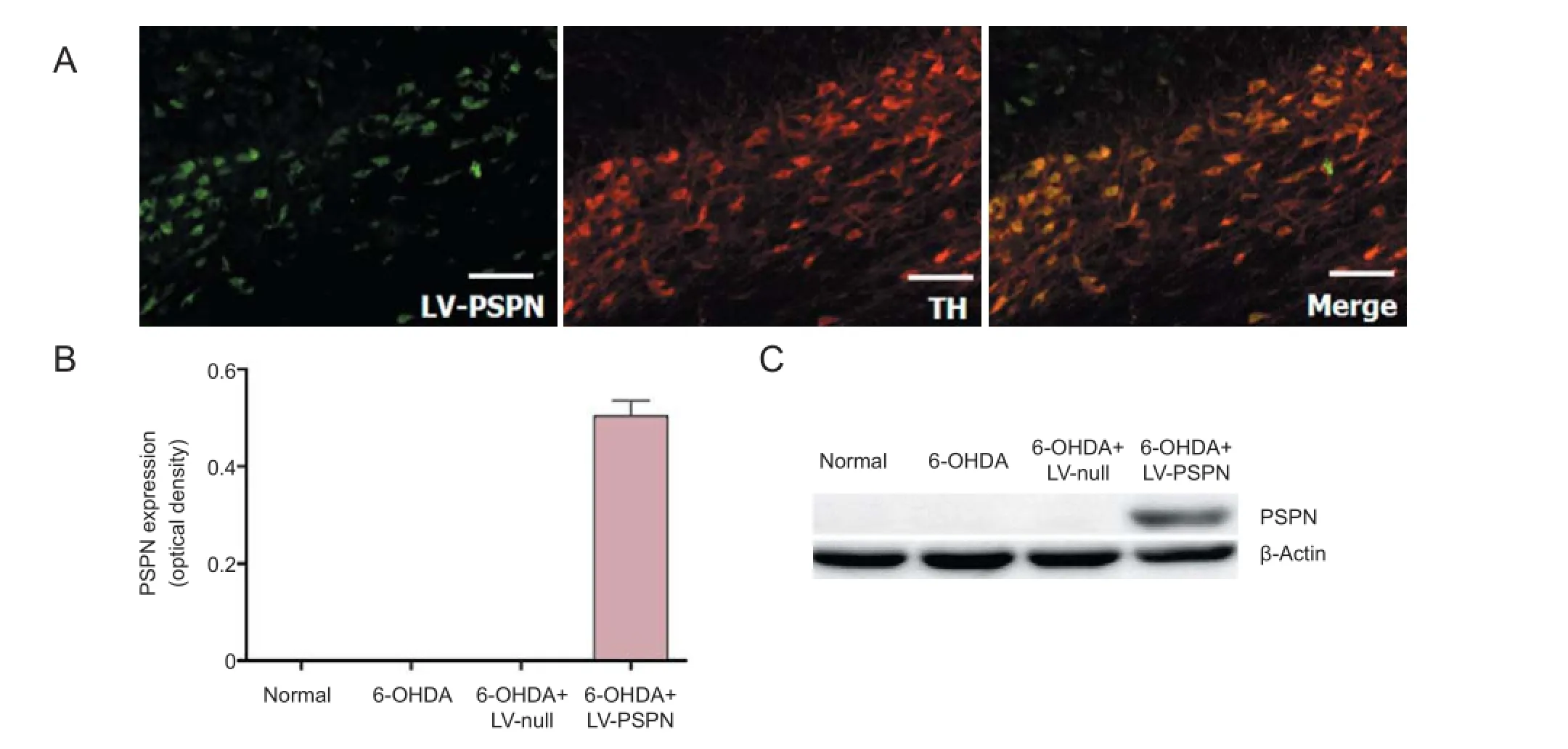
Figure 1 Lentivirus-mediated Persephin (LV-PSPN) over-expressed PSPN in dopaminergic neurons of rat substantia nigra.
After the rats were anesthetized with 8% chloral hydrate via intraperitoneal injection and killed, their brains were harvested. The substantia nigra was separated and weighed in the Eppendorf tube, then the tissue was ground using 100 μL of 4 mg lysis buf er and centrifuged for 30 minutes at 14,000 × g at 4°C. The supernatant was collected and denatured with 4× loading buf er for 5 minutes at 95 °C. Forty μg totalprotein was used for protein electrophoresis and transferred onto membranes. The membranes were blocked for 2 hours and incubated with mouse anti-rat TH monoclonal antibody (1:2,000; Sigma) and mouse anti-rat PSPN monoclonal antibody (1:200; Invitrogen, Karlsruhe, Germany) at 4°C overnight. After three washes in TBST, samples were incubated with donkey anti-mouse IgG (1:2,000; Abcam, Cambridge, MA, USA) for 1 hour. Immunoreactivity was visualized using ECL western blot assay and optical density was analyzed using a UVP Image System (Upland, CA, USA).
High-performance liquid chromatography (HPLC)
At 8 weeks post-transplantation, striatal concentrations of DA, Dihydroxy phenyl acetic acid (DOPAC) and homovanillic acid (HVA) were measured by HPLC with an electrochemical detector (ZEISS). Rats were decapitated at 4°C under sterile conditions. The brain tissue was rinsed with icecold PBS, precisely weighed, and homogenized in an ice-cold perchloric acid and EDTA solution (0.4 M HClO4, 0.5 mM Na2-EDTA, 0.01% L-cysteine) to yield a 10% (w/v) homogenate at 0 °C. The homogenates were centrifuged at 14,000 × g at 4°C for 15 minutes. After centrifugation, the supernatants were removed, and 0.5 volume of potassium salt solution (20 mmol/L potassium citrate, 300 mmol/L KH2PO4, 2 mmol/L Na2-EDTA) was added at 0°C for 15 minutes. The precipitates were then centrifuged at 14,000 × g at 0–4°C for 15 minutes. After centrifugation, the supernatants were frozen at –80°C or immediately applied to the HPLC system (ZEISS). Dialysis samples were assayed for DA, DOPAC and HVA by HPLC with electrochemical detection. The samples were placed in the 717 Plus Autosampler (Waters, Milford, MA, USA) connected to the 2465ECD (Waters) equipped with a C18 reverse-phase column (4.6 × 75 mm, 3.5 μm; Waters). The samples were eluted by mobile phase (100 mM Na-citrate, 0.1 mM EDTA, 75 mM Na2HPO4, 2 mM NaCl, 1 mM C-7 at pH 3.9, 10% methanol) at a f ow rate of 0.6 mL/min. DA, DOPAC and HVA levels were calculated by extrapolating the peak area from a standard curve (ranging from 1 nM to 100 nM of mixed DA, DOPAC and HVA). The identification of peaks was carried out by comparison with standards. The amounts of DA in each sample were quantif ed by comparing the peak area of the samples with those of the standards.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism 6.0 software (GraphPad Software Inc., LaJolla, CA, USA). The data are expressed as the mean ± SD and analyzed by one-way or two-way analysis of variance. Statistical signif cance was determined as P < 0.05.
Results
LV-PSPN over-expressed PSPN in dopaminergic neurons
Seventy-two hours after dopaminergic neurons were transfected with LV-PSPN, 95% of green fluorescent protein (GFP)-positive dopaminergic neurons were observed. Western blot assay results showed that dopaminergic neurons expressed endogenous PSPN and that LV-null infection did not af ect the expression of PSPN, whereas LV-PSPN signif -cantly induced high expression of PSPN (Figure 1A). Immunof uorescence staining showed that dopaminergic neurons mostly expressed the TH and PSPN (Figure 1B). Therefore, LV-PSPN could ef ectively infect dopaminergic neurons and increase the protein expression of PSPN.
Neuroprotection by PSPN on 6-OHDA-lesioned dopaminergic neurons
Rats in the 6-OHDA group and 6-OHDA + LV-null group had about 15% residual substantia nigra TH cells, which was signif cantly lower than the normal group (P < 0.05); rats in the 6-OHDA + LV-PSPN group had approximately 50% residual substantia nigra TH cells, which was higher than the 6-OHDA group and the 6-OHDA + LV-null group (P < 0.05; Figure 2).
Transplantation of LV-PSPN-transfected neural stem cells increased the striatal dopamine expression in 6-OHDA-induced injury rats
Dopamine levels in the striatum were determined by HPLC. Mean dopamine level in the striatum of rats in the 6-OHDA group and 6-OHDA + LV-null group was lower than that of the normal group (P < 0.05). Dopamine level in the striatum of rats showed no signif cant dif erence between the 6-OHDA group and 6-OHDA + LV-null group (P > 0.05). After pretreatment with LV-PSPN, the dopamine level was increased by 179% compared with the two other groups (P < 0.05; Figure 3).
Transplantation of LV-PSPN-transfected neural stem cells improved the behavior of 6-OHDA-induced injury rats
The apomorphine-induced rotational behaviors of rats in all groups are shown in Figure 4. The average rotational rate of the normal group hardly changed from the baseline (0 rotation/30 minutes). In contrast, the rotational rates of rats in the 6-OHDA + LV-null group and the 6-OHDA group averaged more than 300 rotations per 30 minutes, signif -cantly greater than before treatment (P < 0.05). The average rotational rates of rats implanted with 6-OHDA + LV-PSPN were half those in the other two 6-OHDA groups (P < 0.05).
Discussion
Neurotrophic factors have been demonstrated to exert potent ef ects on neurons, such as the promotion of survival, neurite branching, synaptogenesis, modulation of electrophysiological properties and synaptic plasticity (Kuhlmann et al., 2006; Wang et al., 2013; Zhang et al., 2014). GDNF, a distantly related member of the transforming growth factor-beta superfamily and a potent neurotrophic factor, can af ect neuronal dif erentiation, development, growth and survival in the central nervous system and has neuroprotective ef ects against a variety of neuronal insults (Conover et al., 1993; Tang et al., 2014). However, the ef ects of GDNF are transient, and need repeated administration into brain parenchyma or intraventricular space (Parker et al., 2014). In addition, as a large protein, GDNF has dif culty in crossing the bloodbrain barrier (Barichello et al., 2014). This limits the clinical application of GDNF. Direct intravenous administration ofa clinical applicable gene in rats subjected to middle cerebral artery occlusion increased clinical applicability levels, reduced infarct volume at the affected hemisphere, and improved behavioral performance (Bensadoun et al., 2000). Moreover, transplantation of GDNF gene-modif ed stem cells promotes dif erentiation into neurof lament-positive cells and has better therapeutic ef ects in intracerebral hemorrhage models in rats than the transplantation of empty virus-transfected stem cells (DeWitt et al., 2014; Du et al., 2014). Therefore, GDNF may have some therapeutic potential for central nervous system diseases.
In the past decade, gene therapy has painted the prospect of better PD treatments (Arimura et al., 2014). If the genes of neuroprotective factors are delivered to the striatum, the progressive loss of dopaminergic neurons should be interrupted. PSPN, a recently cloned member of the transforming growth factor-β superfamily and GDNF subfamily, is distributed throughout the nervous system at extremely low levels. Therefore, we used PSPN as the therapeutic gene to provide a protective ef ect against progressive degeneration of dopaminergic neurons.
The f ndings of this study show a promising application of PSPN in gene therapy to treat PD. In this study, we observed that PSPN could prevent the severe reduction in dopaminergic cell count in substantia nigra and striatal dopamine content in the striatum caused by 6-OHDA treatment. This study also indicates that intracellular transduction of the PSPN gene can reduce the rotational behavior in a rat model of PD to some extent. This indicates that PSPN can directly protect nigra dopaminergic neurons against the toxicity of 6-OHDA by countering its ef ects.
Author contributions: PS and XDD conceived and designed this study. XFY and YXJ performed the experiments. YXL and HWX analyzed the data. KL and YLZ provided reagents/ materials/analysis tools. HMX wrote the paper. All authors approved the f nal version of this paper.
Conf icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383-394.
Akerud P, Holm PC, Castelo-Branco G, Sousa K, Rodriguez FJ, Arenas E (2002) Persephin-overexpressing neural stem cells regulate the function of nigral dopaminergic neurons and prevent their degeneration in a model of Parkinson’s disease. Mol Cell Neurosci 21:205-222.
Arimura S, Okada T, Tezuka T, Chiyo T, Kasahara Y, Yoshimura T, Motomura M, Yoshida N, Beeson D, Takeda S, Yamanashi Y (2014) Neuromuscular disease. DOK7 gene therapy benef ts mouse models of diseases characterized by defects in the neuromuscular junction. Science 345:1505-1508.
Barichello T, N Gon?alves JC, Generoso JS, Simoes LR, Tashiro MH, Goularte JA, Vuolo F, Rodrigues DH, Vilela MC, Petronilho F, Teixeira AL, Quevedo J (2014) Protection of blood brain barrier integrity and modulation of inflammatory mediators during treatment of pneumococcal meningitis with daptomycin or ceftriaxone. Curr Neurovasc Res.
Bensadoun JC, Deglon N, Tseng JL, Ridet JL, Zurn AD, Aebischer P (2000) Lentiviral vectors as a gene delivery system in the mouse midbrain: cellular and behavioral improvements in a 6-OHDA model of Parkinson’s disease using GDNF. Exp Neurol 164:15-24.
Bespalov MM, Sidorova YA, Tumova S, Ahonen-Bishopp A, Magalh?es AC, Kulesskiy E, Paveliev M, Rivera C, Rauvala H, Saarma M (2011) Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J Cell Biol 192:153-169.
Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U (2002) Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol 249 Suppl 3:III/1-5.
Burn DJ, Tr?ster AI (2004) Neuropsychiatric complications of medical and surgical therapies for Parkinson’s disease. J Geriatr Psychiatry Neurol 17:172-180.
Conover JC, Ip NY, Poueymirou WT, Bates B, Goldfarb MP, DeChiara TM, Yancopoulos GD (1993) Ciliary neurotrophic factor maintains the pluripotentiality of embryonic stem cells. Development 119:559-565.
de la Fuente-Fernández R, Sossi V, Huang Z, Furtado S, Lu JQ, Calne DB, Ruth TJ, Stoessl AJ (2004) Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain 127:2747-2754.
DeWitt J, Pappas A, Nishi R (2014) Ciliary neurotrophic factor reduces the proliferation and promotes the differentiation of TH- MYCN transformed sympathoadrenal progenitors. Dev Neurosci 36:422-431.
Du J, Gao XQ, Deng L, Chang NB, Xiong HL, Zheng Y (2014) Transfection of the glial cell line-derived neurotrophic factor gene promotes neuronal dif erentiation. Neural Regen Res 9:33-40.
He Z, Jiang Y, Xu H, Jiang H, Jia W, Sun P, Xie J (2014) High frequency stimulation of subthalamic nucleus results in behavioral recovery by increasing striatal dopamine release in 6-hydroxydopamine lesioned rat. Behav Brain Res 263:108-114.
Kameda M, Shingo T, Takahashi K, Muraoka K, Kurozumi K, Yasuhara T, Maruo T, Tsuboi T, Uozumi T, Matsui T, Miyoshi Y, Hamada H, Date I (2007) Adult neural stem and progenitor cells modif ed to secrete GDNF can protect, migrate and integrate after intracerebral transplantation in rats with transient forebrain ischemia. Eur J Neurosci 26:1462-1478.
Katzenschlager R (2014) Parkinson’s disease: recent advances. J Neurol 261:1031-1036.
Kuhlmann T, Remington L, Cognet I, Bourbonniere L, Zehntner S, Guilhot F, Herman A, Guay-Giroux A, Antel JP, Owens T, Gauchat JF (2006) Continued administration of ciliary neurotrophic factor protects mice from inf ammatory pathology in experimental autoimmune encephalomyelitis. Am J Pathol 169:584-598.
Lotharius J, Brundin P (2002) Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 3:932-942.
Parker N, Falk H, Singh D, Fidaleo A, Smith B, Lopez MS, Shokat KM, Wright WW (2014) Responses to glial cell line-derived neurotrophic factor change in mice as spermatogonial stem cells form progenitor spermatogonia which replicate and give rise to more dif erentiated progeny. Biol Reprod 91:92.
Sidorova YA, M?tlik K, Paveliev M, Lindahl M, Piranen E, Milbrandt J, Arum?e U, Saarma M, Bespalov MM (2010) Persephin signaling through GFRα1: the potential for the treatment of Parkinson’s disease. Mol Cell Neurosci 44:223-232.
Tang S, Liao X, Shi B, Qu Y, Huang Z, Lin Q, Guo X, Pei F (2014) The ef ects of controlled release of neurotrophin-3 from PCLA scaf olds on the survival and neuronal dif erentiation of transplanted neural stem cells in a rat spinal cord injury model. PLoS One 9:e107517.
Wang K, Demir IE, D’Haese JG, Tieftrunk E, Kujundzic K, Schorn S, Xing B, Kehl T, Friess H, Ceyhan GO (2014) The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis 35:103-113.
Wang S, Fang J, Ma J, Wang Y, Liang S, Zhou D, Sun G (2013) Electroacupuncture-regulated neurotrophic factor mRNA expression in the substantia nigra of Parkinson’s disease rats. Neural Regen Res 8:540-549.
Wanigasekara Y, Keast JR (2006) Nerve growth factor, glial cell line-derived neurotrophic factor and neurturin prevent semaphorin 3A-mediated growth cone collapse in adult sensory neurons. Neuroscience 142:369-379.
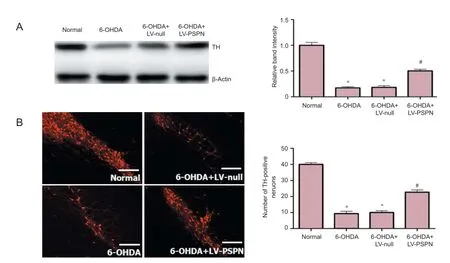
Figure 2 PSPN increased TH expression in dopaminergic neurons of rat substantia nigra.
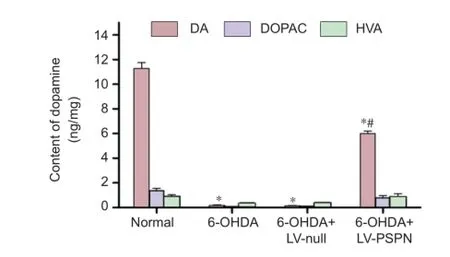
Figure 3 The levels of dopamine (DA), 3-4-dihydroxy-phenylacetic acid (DOPAC) and homovanillic acid (HVA) in Parkinson’s disease rats after transfection.
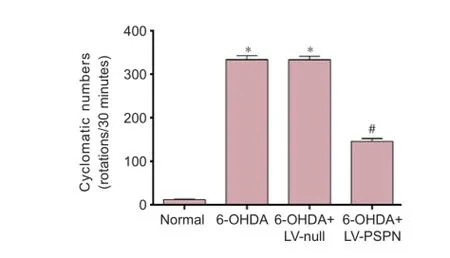
Figure 4 The apomorphine-induced rotational behaviors of 6-OHDA-induced Parkinson’s disease rats after transplantation of LV-PSPN-transfected neural stem cells.
Yang J, Runeberg-Roos P, Leppanen VM, Saarma M (2007) The mouse soluble GFR[alpha]4 receptor activates RET independently of its ligand persephin. Oncogene 26:3892-3898.
Zhang HY, Song N, Jiang H, Bi MX, Xie JX (2014) Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor inhibit ferrous iron inf ux via divalent metal transporter 1 and iron regulatory protein 1 regulation in ventral mesencephalic neurons. Biochim Biophys Acta 1843:2967-2975.
Zhao Y, Haney MJ, Gupta R, Bohnsack JP, He Z, Kabanov AV, Batrakova EV (2014) GDNF-transfected macrophages produce potent neuroprotective ef ects in Parkinson’s disease mouse model. PLoS One 9:e106867.
Zihlmann KB, Ducray AD, Schaller B, Huber AW, Krebs SH, Andres RH, Seiler RW, Meyer M, Widmer HR (2005) The GDNF family members neurturin, artemin and persephin promote the morphological dif erentiation of cultured ventral mesencephalic dopaminergic neurons. Brain Res Bull 68:42-53.
Copyedited by Norman C, Dawes EA, Yang Y, Li CH, Song LP, Zhao M
*Correspondence to: Peng Sun, M.D. or Xiao-dong Ding, M.D., sunpengqd@163.com or 15154258721@163.com.
# These authors contributed equally to this work.
orcid: 0000-0001-5202-3819 (Peng Sun) 0000-0002-7872-0919 (Xiao-dong Ding)
10.4103/1673-5374.170309 http://www.nrronline.org/
Accepted: 2015-09-14
- 中國神經(jīng)再生研究(英文版)的其它文章
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- Targeting brain microvascular endothelial cells: a therapeutic approach to neuroprotection against stroke
- Severe bilateral anterior cingulum injury in patients with mild traumatic brain injury
- Injury of corticoreticular pathway and corticospinal tract caused by ventriculoperitoneal shunting
- Susceptibility weighted imaging in the evaluation of hemorrhagic dif use axonal injury
- Mechanical properties of nerve roots and rami radiculares isolated from fresh pig spinal cords

