Restricting carbohydrates to fi ght head and neck cancer—is this realistic?
Rainer J. Klement
Department of Radiotherapy and Radiation Oncology, Leopoldina Hospital, Schweinfurt 97421, Germany
Restricting carbohydrates to fi ght head and neck cancer—is this realistic?
Rainer J. Klement
Department of Radiotherapy and Radiation Oncology, Leopoldina Hospital, Schweinfurt 97421, Germany
Head and neck cancers (HNCs) are aggressive tumors that typically demonstrate a high glycolytic rate, which results in resistance to cytotoxic therapy and poor prognosis. Due to their location these tumors speci fi cally impair food intake and quality of life, so that prevention of weight loss through nutrition support becomes an important treatment goal. Dietary restriction of carbohydrates (CHOs) and their replacement with fat, mostly in form of a ketogenic diet (KD), have been suggested to accommodate for both the altered tumor cell metabolism and cancer-associated weight loss. In this review, I present three specific rationales for CHO restriction and nutritional ketosis as supportive treatment options for the HNC patient. These are (1) targeting the origin and specific aspects of tumor glycolysis; (2) protecting normal tissue from but sensitizing tumor tissue to radiation- and chemotherapy induced cell kill; (3) supporting body and muscle mass maintenance. While most of these bene fi ts of CHO restriction apply to cancer in general, speci fi c aspects of implementation are discussed in relation to HNC patients. While CHO restriction seems feasible in HNC patients the available evidence indicates that its role may extend beyond fi ghting malnutrition to fi ghting HNC itself.
Ketogenic diet (KD); head and neck neoplasms; diet; carbohydrate restricted (CHO restricted); nutritional support
Introduction
Head and neck cancer (HNC) is a collective term for cancers originating from the lip, oral and nasal cavity, paranasal sinuses, pharynx, larynx and trachea. Approximately 90% of HNCs are head and neck squamous cell carcinoma (HNSCC) originating from the mucosal lining (epithelium) of these regions.
Frequent comorbidities of HNCs include various feeding di ffi culties and malnutrition that are oen aggravated by tobacco and alcohol abuse and a general unhealthy lifestyle1. At time of diagnosis up to 60% of all HNC patients present with improper nutritional status2,3, so that nutritional support becomes an important aspect in the treatment of these patients. A general recommendation is that even HNC patients who appear healthy should be counseled and advised to eat a high-calorie andhigh-protein diet4. In practice, however, the variety of available supplementary nutrition formulas and general inconsistent dietary advices for cancer patients5pose a di ffi culty for deciding on the optimal diet for preventing muscle loss, improving the quality of life, reducing in fl ammation and withstanding therapyinduced side-e ff ects. Many physicians seem unaware of the fact that besides the amount of caloric intake, the composition of the diet may have profound influences on these dietary goals. This is exempli fi ed by a recent investigation of enteral and parenteral feeding practices in a Chinese university teaching hospital6where only 2.1% of cancer patients received Supportan, a diseasespecific high-fat nutrition formula that has been shown to improve nutritional and functional parameters in HNC patients compared to a standard formula7.
Like most aggressive tumors, HNCs exhibit a high rate of and dependence on glycolysis to meet their metabolic demands8,9. It has therefore been reasoned that diets restricted in carbohydrates (CHOs) could target the altered metabolism of such glycolytic tumors10,11. Indeed, there is some evidence that a ketogenic diet (KD), a high-fat low-CHO diet that leads to the elevation of circulating ketone bodies into the mM range, may not onlyimpair tumor cell metabolism and growth, but also fi ght cachexia and therapy-induced side e ff ects12-14.
In this review I am going to present three main rationales for the implementation of CHO restricted and KDs in HNC patients. Briefly, these are (1) targeting the altered tumor cell metabolism; (2) increasing the radio- and chemosensitivity of malignant cells while protecting normal cells; (3) accounting for the altered metabolism of the tumor-bearing host. Due to the various problems regarding food intake, the question remains whether CHO restriction is feasible in HNC patients. In the final part of this paper, I therefore address specific aspects and practical issues of such a dietary intervention.
A hallmark of HNC, like most cancers in general, is their high avidity for glucose uptake. Oo Warburg and his co-workers at the former Kaiser Wilhelm-Institute for Biology in Berlin were the first to quantify glucose uptake and energy generation in a large variety of animal and human tumors15-19. Using both in vivo and in vitro measurements, Warburg showed that compared to normal tissues, tumors would take up several times more glucose from the surroundings and ferment the majority of it to lactate even in the presence of sufficient oxygen that would normally suppress lactate production.is metabolic phenotype of increased glucose uptake and lactate production is therefore known as the Warburg effect or aerobic glycolysis since it also happens in normoxic conditions.
The Warburg effect is the basic principle behind molecular imaging using positron emission tomography (PET) with the glucose analog 2-(18F)fluoro-2-deoxy-D-glucose (FDG). FDG is structurally similar to glucose except for the substitution of an OH group by the positron emitter18F. Similar to glucose it therefore enters the cells through glucose transporters and gets phosphorylated by the enzyme hexokinase. Unlike glucose, however, FDG cannot be further metabolized after phosphorylation to FDG-6-phosphate and stays trapped inside the cell until it decays. The amount of assimilated FDG is quantified by the standardized uptake value (SUV) which expresses the ratio between the measured tissue activity and the injected activity standardized to body weight. In HNC FDGPET in combination with computer tomography (PET/CT) has shown great benefit for tumor and lymph node staging, detection of an unknown primary tumor, radiation treatment planning, evaluation of therapy response and long-term surveillance20,21(Figure 1). Furthermore, several studies have found that pretreatment tumor SUV—either as maximal SUV22-25or combined with tumor volume into a total lesion glycolysis parameter26—is an independent significant predictor of local control, disease free and overall survival rates, while high lymph node SUVs were predictive for distant recurrence at 1 year27. Plasma glucose levels are able to falsify SUVs in highly glycolytic tumors28which might account for negative results reported in some studies29.
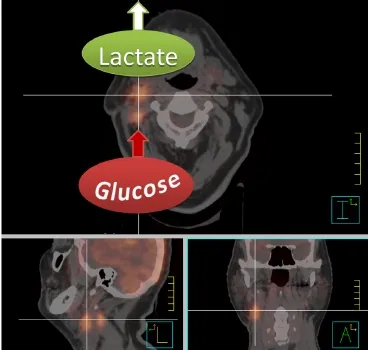
Figure 1 Fusion image of a radiotherapy planning CT and FDGPET scan of a patient with a primary right-sided cT1 cN2b tonsillar squamous cell carcinoma after tonsillectomy. The high FDG uptake of the right lymph node conglomerates is indicative of highly glycolytic metastasis. Note, however, that FDG-PET only measures glucose uptake and conversion into glucose-6-phosphate, and can therefore not discriminate between lactate production or feeding of glycolysis intermediates and end products into the pentose phosphate pathway or citric acid cycle. The high lactate release which can be measured with other techniques such as magnetic resonance spectroscopy is, however, indicated for illustrative purposes since it is characteristic for aggressive metastasis.
The correlation between glycolytic rate and tumor aggressiveness is not only re fl ected on the side of glucose in fl ux and hexokinase activity, but also at the final step of glucose fermentation in which pyruvate gets reduced to lactate. A large proportion of HNSCC overexpress lactate dehydrogenase 5, the enzyme that catalyzes this conversion, and this was correlated with poor prognosis30. Mechanistically, lactate is a key metabolite linking the Warburg effect to the other hallmarks of cancer such as sustained proliferative signaling, resisting cell death and activating invasion and metastasis31. Lactate production asmeasured by magnetic resonance spectroscopy may be a more sensitive parameter for the Warburg effect and thus tumor aggressiveness than FDG-PET32. There are several reasons why tumor lactate correlates with tumor aggressiveness. First of all, lactate acts as an antioxidant, protecting tumor cells not only from intrinsic reactive oxygen species (ROS) production, but also cytotoxic therapies. Indeed, high pretreatment lactate concentrations in head and neck tumors have been shown to increase the probability of metastatic spread in patients undergoing either postoperative or primary radiotherapy33,34. This is consistent with 1-(11C)-acetate PET measurements of tumor perfusion and oxidative metabolism in a small cohort of HNSCC patients that indicated that tumors utilizing predominantly glycolysis are more resistant to ionizing radiation than those with high cellular respiration35. Second, lactate (as well as pyruvate) seems able to stabilize hypoxia-inducible factor-1α (HIF-1α), a transcription factor that is also stabilized by hypoxia or oncogenic signaling and increases the expression of most glycolytic genes36.is provides a feed-forward loop in which tumor glycolysis sustains itself. Third, there is evidence showing that lactate promotes angiogenesis and metastatic spread37. Forth, lactate impairs the anti-tumor immune response by increasing the frequency of myeloid-derived suppressor cells and decreasing the cytolytic activity of NK cells38. Finally, lactate can be used as a fuel by some aerobic cancer cells39,40, and this may also apply to HNSCC8,41. Investigating cancerous oral mucosa slices from 40 patients, Curry et al.41described a tumor compartment consisting of mitochondrial-rich proliferating cells staining positive for monocarboxylate transporter 1 (MCT1), the MCT isoform that normally mediates lactate uptake into respiring cells. This compartment was adjacent to non-proliferating, mitochondrial-poor tumor compartments staining positive for MCT4, the MCT isoform that passively transports lactate and protons out of glycolytic cells. Based on these findings the authors proposed a model of “metabolic compartmentalization” in which non-proliferating, glycolytic cells within the tumor stroma and epithelium would shuffle lactate and ketone bodies to mitochondrial-rich and highly proliferative epithelial stem-cell like cancer cells, thus fueling tumor growth and metastasis. However, besides the fact that no mechanism how stromal cells in the head and neck region would produce ketone bodies has ever been described, this study has not ruled out the possibility that MCT1-positive cells export lactate rather than importing it. Indeed, it was shown that in cells de fi cient in the p53 protein, MCT1 is up-regulated in response to hypoxia and able to work in the reverse mode, releasing lactate out of the cell, if glucose is abundant42.erefore, as also argued recently by Doherty and Cleveland43, this model is probably no generalizable explanation for the observed relation between lactate and cancer aggressiveness.
Genetic and epigenetic alterations promote aerobic glycolysis
On a molecular level HNC is a heterogeneous disease, complicating prognostication and the search for causative factors44,45. Yet the glycolytic phenotype appears to be a universal feature with good prognostic potential. Intriguingly, as Table 1 shows, many of the most frequently mutated genes in HNSCC play a role in activating the metabolic switch towards aerobic glycolysis46,48,49,51-53. This metabolic shift also influences the epigenome by altering the availability of certain metabolic cofactors that are needed for epigenetic enzymes such as acetylCoA for histone acetylation or NAD+ for the class III histone deacetylase (HDAC) family of the sirtuins. In turn, epigenetic regulation of the expression of genes involved in metabolism may contribute to the altered tumor cell metabolism54. Epigenetic alterations are frequently observed in HNSCC and involve the methylation status of DNA (genome-wide hypomethylation and promoter region hypermethylation), post-translational histone modifications and post-transcriptional modifications through microRNAs55. Both DNA methylation and histone modi fi cations such as methylation, acetylation or phosphorylation regulate gene expression by altering the chromatin structure between an open and closed form, thus allowing or blocking, respectively, the access of transcription factors to gene promoter regions. The activity of the enzymes catalyzing histone modifications is thereby not limited to the epigenetic regulation of gene expression, but also able to modify other non-histone proteins involved in cellular metabolism54.
The tumor suppressor p53 is one example since its activity and stability can be regulated through lysine methylation and acetylation by various histone-modifying proteins. Although epigenetic silencing of p53 seems rare in HNSCC56, the TP53 gene is found to be mutated in approximately 60% of all HNSCC, and its protein product p53 inactive in another 20% due to degradation by the human papilloma virus oncoprotein E644. A loss of p53 promotes aerobic glycolysis, increases the fl ux through the pentose phosphate pathway (PPP) and downregulates mitochondrial oxidative phosphorylation (OXPHOS) through various pathways (reviewed in46,47). Furthermore, p53 deficiency increases the levels of ROS which promotes further DNA mutations and an up-regulation of glycolysis via hypoxia-independent stabilization of HIF-1α57. In this way HIF-1α protects tumor cells against steady-state oxidative stress through the production of lactate and pyruvate by glycolysisand regeneration of reduced glutathione (GSH) via NADPH production in the oxidative PPP37,58. This protection probably extends to the oxidative stress induced by chemotherapy and ionizing radiation as shown in HNSCC xenogras59,60. It breaks down upon glucose deprivation, leading to ROS-induced cell death61-64. Interestingly, a p53-independent overexpression of the p53 target gene TP53-induced glycolysis and apoptosis regulator (TIGAR) is often observed in tumor cells, causing an up-regulation of the oxidative and non-oxidative PPP which are important for the production of NADPH and ribose-5-phosphate (an anabolic intermediate needed for nucleotide production), respectively47. Another gene related to the PPP is transketolase-like-1 (TKTL1). TKTL1 seems frequently overexpressed in HNSCC due to promoter hypomethylation and increases aerobic glycolysis and HIF-1α accumulation65. Consistent with this, a high degree of staining for TKTL1 has been linked to a signi fi cantly shorter disease-speci fi c survival in laryngeal66and oral67SCC patients.
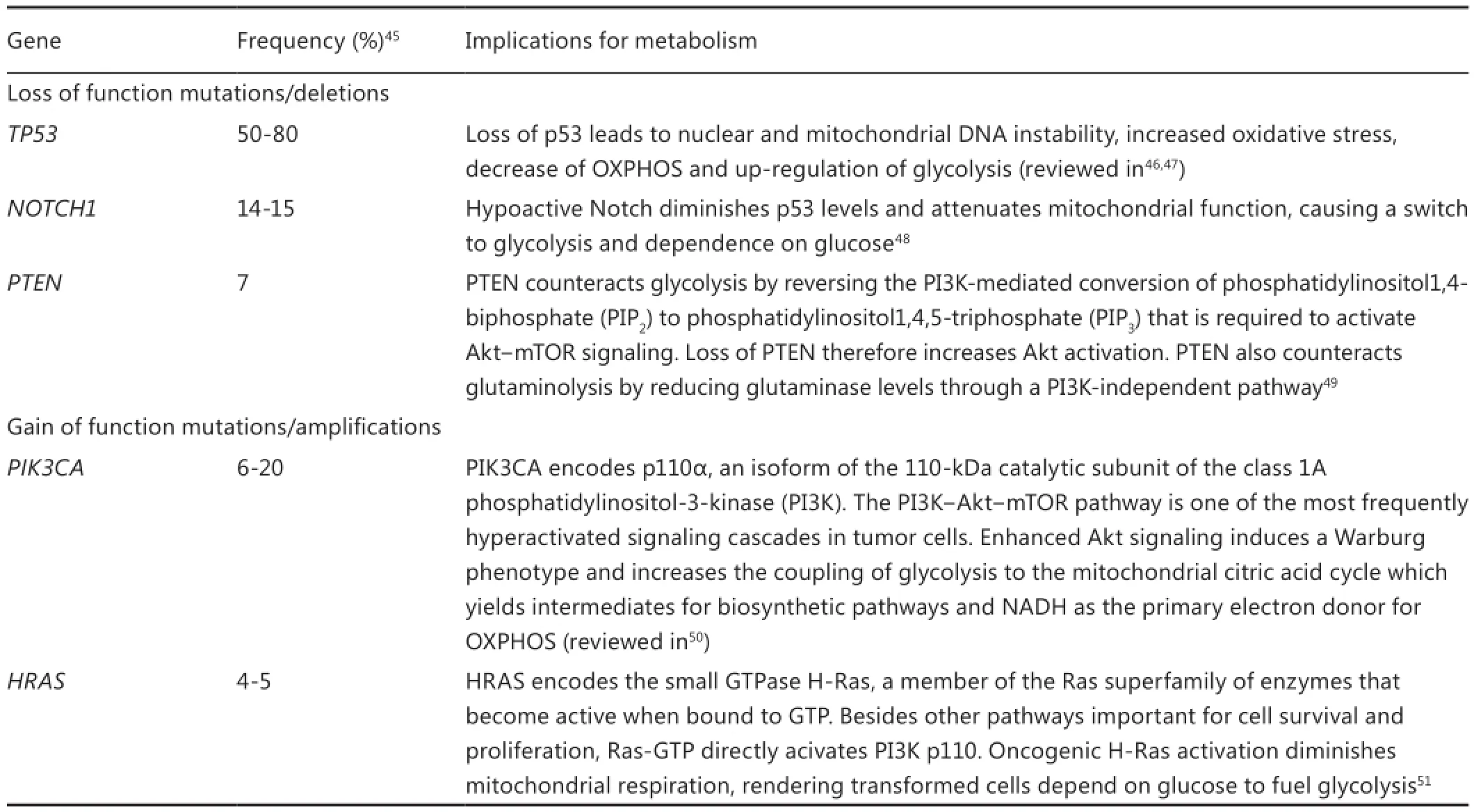
Table 1 Common genetic mutations in HNSCC and their implication for tumor cell metabolism
Finally, p53 plays an important role in mitochondrial DNA (mtDNA) repair and stability, and some studies have shown associations between mutant p53 and mtDNA mutations68,69. MtDNA mutations may cause mitochondrial dysfunction since the mtDNA encodes 20% of the OXPHOS genes70.is may also impair nuclear DNA stability by leading to an increased release of ROS into the cytosol70and a retrograde response involving downregulation of the nuclear DNA repair protein APE171. About 24%-47% of HNSCC are estimated to harbor pathogenic mtDNA mutations that may alter mitochondrial function through aberrant transcription, translation or replication68,69. High steady state levels of ROS caused by mitochondrial dysfunction would have to be neutralized by a high rate of glycolysis else they induce tumor cell death61-64,72. Zhou and colleagues68,73have provided evidence that mtDNA mutations in HNSCC contribute to the Warburg effect via ROS-induced stabilization of HIF-1α, although Challen et al.74found no association between mtDNA mutations and the expression of four HIF-1α target genes. The complex interaction between the nucleus and the hundreds to thousands of mitochondria in the cell may explain part of the controversy about the role of mtDNA mutations as drivers or bystanders of tumor progression74. However, there is evidence that mtDNA mutations and a dysfunctional TCA cycle induce a compensatory up-regulation of glycolysis10,11. This relates to the original hypothesis by Oo Warburg that cancer and aerobic glycolysis are caused by respiratory insufficiency75. Some rare forms of familial and sporadic head and neck paragangliomas indeed arise due to a dysfunctional TCA cycle. These tumors are caused by germline loss-of-function mutations in the SDHC gene encoding the C subunit of the TCA cycle enzyme succinatedehydrogenase. As a consequence succinate accumulates in the mitochondria and leaks out into the cytosol where it inhibits certain prolyl hydroxylase enzymes leading to stabilization of HIF-1α and aerobic glycolysis76. Furthermore Yang and colleagues provided evidence that oncogenic HRAS transformation impairs mitochondrial respiration which subsequently increases glycolysis without compromising mitochondrial mass or content51.
Vulnerability of HNC cells to CHO restriction
The shift in HNC metabolism towards aerobic glycolysis concurrently provides high energy production, cytotoxic stress resistance, building blocks for rapid proliferation and the ability to migrate out of the cell compartment. However, this comes at the expense of metabolic flexibility. The genetic and epigenetic alterations found in HNC cells in conjunction with mitochondrial defects and hypoxia leave these cells extremely dependent on a steady supply of nutrients, especially glucose. Numerous in vitro experiments have shown that cancer cells are particularly vulnerable to glucose (and glutamine) restriction63,64,77-81. Although glutamine also plays a role as an energetic and anaplerotic substrate in cancer cells it seems to be less important than glucose in HNSCC9. The biophysical theory of quantum metabolism predicts that cancer cells relying on glycolysis outperform normal metabolically flexible cells only as long as glucose is abundant and dominates other metabolic fuels82,83.is implies to impose limitations on glucose abundance and increase the diversity of alternative substrates as a therapeutic strategy83. Dietary CHO restriction is a non-toxic approach of reducing the supply of blood glucose to cancer cells and increasing the utilization of fatty acids and ketone bodies in normal cells10,12,84,85. In particular, KDs may have beneficial effects when used as a supportive dietary manipulation in cancer patients. A KD mimics the metabolism of fasting without restricting energy intake, mainly by replacing CHOs with fat. The restriction of CHOs seems to be responsible for most of the bene fi cial e ff ects of calorie restriction. A moderate to severe restriction of CHOs without limiting energy intake is therefore a good alternative to a calorie restricted diet when weight loss must be prevented14,86. One main rationale for dietary CHO restriction in cancer patients is its ability to simultaneously exploit several of the following factors underlying tumor glycolysis (Table 2):
CHO restriction down-regulates glycolysis
CHO restriction and its replacement with fat elevate free fatty acids and decreases glucose concentrations in serum. Fay acids—in particular the saturated ones—have been shown to inhibit the key glycolytic enzymes hexokinase, phosphofructokinase, pyruvate kinase and lactate dehydrogenase87. While even low physiological blood glucose levels should not be rate-limiting to glucose influx into tumor cells since their glucose transporters have low Km values and therefore a high a ffi nity for glucose, this situation may be different in poorly vascularized tumor areas as glucose concentrations decrease along their di ff usion paths. FDGPET studies con fi rm that a KD is able to inhibit tumor glycolysis in some cancer patients88-90. Two recent studies in mice showed that a KD lowered lactate production and resulted in less tumor growth38,40. Importantly, Schroeder et al.91recently described a KD-induced reduction of lactate levels in a small group of HNSCC patients using implanted microdialysis catheters. For 4 days the patients received a diet consisting of solely meat, fi sh, eggs, salad, cheese and sausages; these foods were grinded for patients with dysphagia and tolerated very well (Ursula Schroeder, private communication). This short-term KD decreased blood glucose levels and induced a decline of intra-tumoral lactate levels that was far greater than in normal mucosa. Although the reduction of blood glucose concentrations may be facilitated and increased with concurrent calorie restriction11, this is no option for HNC patients with a high risk for weight loss.
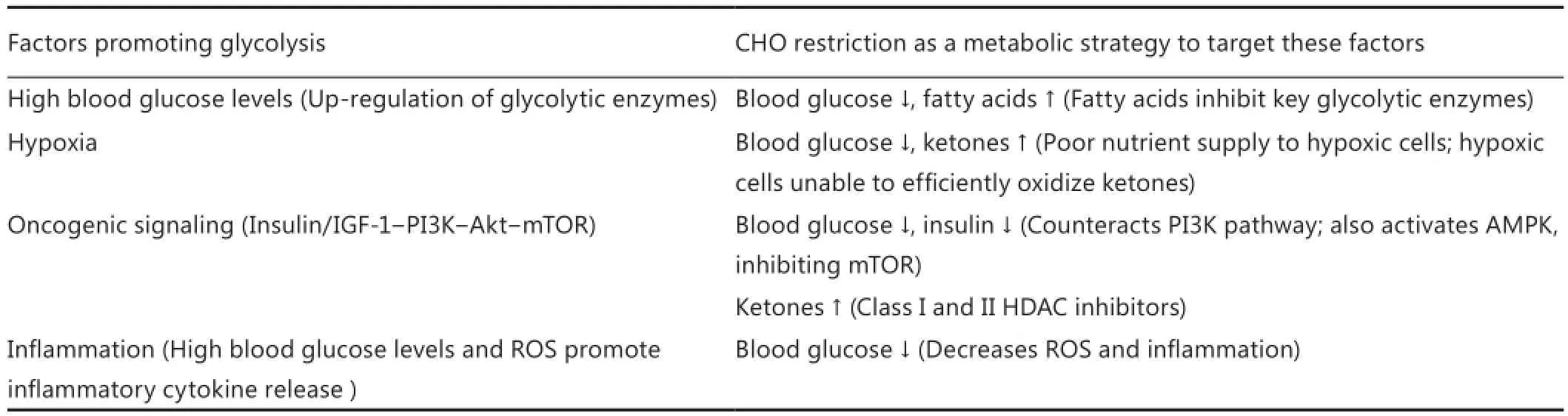
Table 2 Targeting tumor glycolysis through carbohydrate restriction
CHO restriction is especially problematic for hypoxic cells
The role of hypoxia in up-regulating glycolytic enzymes and glucose entry into the cells is well established. Already Warburg was aware of the poor capillary network of tumor tissue and hypothesized that tumors are more vulnerable to simultaneous glucose and oxygen deprivation due their worse “channels of supply”19. In principle, a lowering of blood glucose levels might cut some of the chronically hypoxic cells lying far from blood vessels completely o ff their supply. If CHOs are restricted severely enough, the liver also starts to produce larger amounts of ketone bodies that serve as a high quality fuel for normal tissues, in particular the brain and muscles92. Although measurements in HNSCC patients have shown that their tumors take up ketone bodies, the absolute amounts were small and their metabolic fate was not determined8. Even if HNSCC would have the necessary enzymes to utilize ketone bodies—which seems not the case for many other tumor cells93-96—utilization of ketones requires oxygen and is therefore impaired in large parts of the tumor40. Thus, a lowering of blood glucose levels will have a much harder impact on hypoxic tumor cells than on normal cells that are metabolically fl exible and possess an intact nutrient supply network.
CHO restriction inhibits oncogenic signaling
CHO restriction has the ability to counteract signaling through the phosphatidylinositol-3 kinase?Akt?mammalian target of rapamycin (PI3K?Akt?mTOR) pathway. This pathway is activated by insulin and growth factors such as insulin-like growth factor-1 (IGF-1) and its e ff ect is, among others, an upregulation of glycolysis50,97.e complexity of the IGF signaling network, tyrosine kinase receptor crosstalk as well as autocrine activation of non-targeted receptors all provide resistance mechanisms against overly speci fi c tyrosine kinase inhibitors that additionally often induce systemic side effects98,99. In contrast, CHO restriction is a non-toxic strategy to simultaneously target the same molecular pathways that are individually targeted with pharmaceutical drugs.
Conflicting and often negative results concerning an association between IGF-1 and cancer have been reported for a variety of cancers including HNC98. This leaves insulin, hyperglycemia and in fl ammation as more plausible mediators of the well-established metabolic syndrome-cancer connection100. A recent study has extended this link to HNC by showing that obesity is an independent risk factor for disease-specific mortality from early stage oral SCC when the influence of cancer-associated weight loss is accounted for101.us, in early stage HNSCC insulin inhibition through CHO restriction may be beneficial against tumor glycolysis and progression. CHO restriction also increases AMP kinase (AMPK) activity, an intracellular energy sensor. Although AMPK activation can acutely up-regulate glycolysis in some cells, in the longer term it acts as an “anti-Warburg” tumor suppressor and inhibits mTOR signaling102. AMPK has therefore emerged as an aractive anticancer target that is tried to be activated using anti-diabetic drugs such as metformin99,103.
Insulin inhibition may be less e ff ective in later stages of tumor progression as genetic and epigenetic alterations accumulate and chronically activate the PI3K?Akt?mTOR pathway104. Work from Adrienne Scheck and co-workers implies, however, that KDs exert effects extending beyond those of insulin inhibition by inducing global changes in tumor gene expression that counteract glycolysis and tumor growth105,106. Interestingly, β-hydroxybutyrate and, to a lesser extent, acetoacetate act as class I and II HDAC inhibitors107,108. As such, ketone bodies may alter signaling pathways in HNC through epigenetic and nonepigenetic mechanisms, providing novel anti-cancer functions. For instance, chemically induced chromatin acetylation through the HDAC inhibitor Trichostatin A has been shown to signi fi cantly impair the proliferation of HNSCC spheres and to reduce the fraction of cancer stem cells109. Furthermore, fi ndings relating the overexpression of HDAC 2 to post-translational HIF-1α stabilization in oral SCC imply a role for HDAC inhibitors as “anti-Warburg” agents110. It must be noted, however, that ketone bodies are less potent than other clinically employed HDAC inhibitors suggested for the treatment of HNSCC, so that their anti-cancer e ff ects relating to their role as HDAC inhibitors remain to be elucidated.
In summary CHO restriction and KDs in particular exert systemic e ff ects on oncogenic signaling pathways that counteract tumor glycolysis but—owing to the complexity of the signaling networks involved and the large genetic heterogeneity of HNC tumors—need to be further investigated.
CHO restriction targets in fl ammation
The relationship between inflammation and HNSCC becomes apparent from a Hungarian study showing an increased prevalence of oral inflammatory, premalignant and cancerous lesions among diabetics compared to healthy controls111.ese authors also found that with 14.6% and 9.7%, respectively, the prevalence of diabetes and elevated blood glucose levels (>6.1 mmol/L) was significantly higher in 610 oral carcinoma patients than in a tumor-free control group. High blood glucose levels promote the release of inflammatory cytokines andROS from monocytes and macrophages in a dose-dependent manner112,113; both inflammatory cytokines and ROS are activators of HIF-1α and therefore glycolysis. The connection between inflammation and high blood glucose levels is also seen in cancer cachexia syndrome12. In fact, already in 1885 Ernst Freund described signs of hyperglycemia in 70 out of 70 cancer patients, which led him to conclude that the abnormally high blood sugar content would be necessary for the existence of a carcinoma114. Along these lines, hyperglycemia is now an established predictor of poor survival in a variety of cancers115-122.
It therefore seems prudent to limit high blood glucose spikes that may particularly occur with nutritional support containing simple sugars. In a retrospective analysis of data from the RTOG 90-03 trial involving 1,073 HNSCC patients, Rabinovitch et al.123clearly showed that baseline nutrition support before de fi nitive radiotherapy significantly decreased locoregional control and 5-year overall survival by an absolute amount of 28% and 33%, respectively, despite diminishing treatment-related weight loss. After adjusting for a range of other prognostic factors through recursive partitioning analysis, baseline nutrition support remained as a highly significant (P<0.0001) risk factor for locoregional failure and death. Although detailed information on the composition of the nutritional support was lacking, it is clear that the standard nutrition support formulas used at that time contained a high percentage of high glycemic CHOs which would lead to blood sugar and insulin spikes and fuel in fl ammation. It is also clear from these results that the treating physician should not only focus on the quantity of calories, but that their quality might be more important for patient survival.
CHO restriction during radiation treatment
The optimal treatment of HNC requires a multidisciplinary approach in which radiation therapy constitutes the major modality besides surgery124. Unfortunately HNCs oen exhibit increased radioresistance that is linked to their glycolytic phenotype58. These resistance mechanisms might be targeted by dietary modulation. Preclinical data indicate that calorie and CHO restriction during cancer therapy differently alter the stress resistance of tumor and normal tissue such that the former experiences sensitization to and the latter protection against ionizing radiation and chemotherapy72,106,125-128. Central to this di ff erential stress resistance is the energy sensing network consisting of AMPK, the NAD+-dependent class III HDAC silent mating type information regulation 2 homologue 1 (SIRT1), peroxisome proliferator-activated receptor α (PPARα) and the transcription factor peroxisome proliferator-activated receptor γ coactivator α (PGC-1α)14,129. In normal human tissue, this network is generally activated through any stress that decreases blood glucose levels and activates lipid mobilization and oxidation: calorie restriction, fasting, exercise or—as emerging evidence indicates—CHO restriction. AMPK/SIRT1/ PPARα/PGC-1α signalling not only serves to up-regulate mitochondrial biogenesis and respiration, but also functions to “clean up” cells via autophagy and to protect them against in fl ammation and DNA damage.us, proper activation of this network would o ff er protection to normal cells during radio- and chemotherapy (Figure 2).
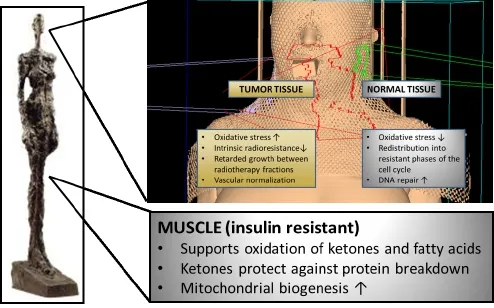
Figure 2 Putative effects of CHO restriction on normal and tumor tissue. During radiotherapy CHO restriction may induce a differential stress response between normal and tumor cells such that the former experience protection from and the latter sensitization to ionizing radiation. Additionally, through the elevation of ketone bodies and fatty acids, CHO restriction helps to conserve muscle tissue.
With few exceptions, normal tissues readily oxidize ketonebodies thereby decreasing the mitochondrial NADP+/NADPH ratio. This in turn increases the amount of reduced GSH available for scavenging ROS130. This antioxidative property of ketone bodies would not bene fi t tumor cells which are unable to metabolize them due to a lack of the necessary enzymes93-96or hypoxia40. In contrast, the HDAC inhibiting activity of ketones could be useful against HNSCC stem cells which typically exhibit the highest radioresistance109.
CHO restriction also up-regulates lipid oxidation which increases the intracellular NAD+/NADH ratio and thus amplifies the NAD+-dependent activity of SIRT1129. SIRT1 enhances the repair of single and double strand breaks that are induced by ionizing radiation, in this respect acting as a tumor suppressor131. SIRT1 also interacts with p53 and forkhead box O (FOXO) transcription factor proteins to induce cell cycle arrest in order to keep cells from transitioning into replicative phases of the cell cycle in which they are most vulnerable to cytotoxic insults. Thus, CHO restriction before a radiotherapy session could be employed to redistribute normal cells into a non-dividing resistant state. These SIRT1-mediated protection mechanisms are probably less pronounced or defective in HNC cells. For instance nuclear SIRT1 expression generally seems to be lower in HNSCC than in normal mucosa132, suggesting an impaired global genome nucleotide excision repair through repression of xeroderma pigmentosum C expression133. Furthermore phosphorylation of FOXO transcription factors through Akt leads to their exclusion from the nucleus and cytosolic degradation.us constitutive activation of Akt as well as loss of p53 in HNSCC tumors would disrupt FOXO-mediated transcription of DNA repair genes and induction of cell cycle arrest upon CHO restriction.
CHO restriction may also impair tumor re-growth during radiotherapy fractions and delay the accelerated proliferation that is known to start in HNSCC at some point during radiation treatment. We have previously reviewed the wealth of preclinical data showing that CHO restriction alone delays tumor growth in a variety of tumor models12. Most of these studies tested KDs, and in vitro data indicate that ketone bodies themselves can have anti-proliferative e ff ects on some tumor cells134,135. Unfortunately, most human studies assessing growth inhibition through KDs have speci fi cally focused on advanced stage astrocytoma patients that have a particularly bad prognosis88,89,136. In addition, subject numbers in the studies to date are small, reducing the statistical reliability of the results. Nevertheless some hints for a reduction of tumor cell proliferation with CHO restriction in extra-cranial tumors have been found in small pilot trials90,137.
Some preclinical studies have further shown that CHO restriction either in the form of overall calorie restriction138-140or an unrestricted KD141may target the vascular endothelial growth factor pathway that is also targeted by the drug bevacizumab (Avastin) for which clinical benefits have been shown when combined with radio- and/or chemo-therapy in HNSCC142. VEGF inhibition is employed in order to normalize the tumor vasculature and radiosensitize tumor tissue by facilitating the delivery of oxygen and chemotherapeutic drugs. Along these lines, hyperbaric oxygen therapy (HBOT) has been employed to enhance the e ffi cacy of radiotherapy. A Cochrane review has concluded that HBOT during radiotherapy signi fi cantly lowers the risk of tumor recurrence at one and five years in HNSCC, but at the expense of increased normal tissue injury and central nervous system oxygen toxicity. In this context it is interesting to note that two preclinical studies provide evidence that ketone bodies might have synergistic e ff ects with HBOT143and signi fi cantly delay the onset of HBOT-induced seizures144.
In summary, there is evidence that CHO restriction acts as a cytotoxic sensitizer in tumor tissue while simultaneously protecting normal tissue which supports its implementation during standard treatment for HNC. The main effects are summarized in Figure 2, and a more thorough review of the underlying mechanisms has recently been published14.
CHO restriction to positively in fl uence body composition
Unintentional weight loss is a common problem in HNC patients. It is oen present already at the time of diagnosis and indicates malnutrition or an early cachectic state. Weight loss is usually in fl uenced by disease- and treatment-associated problems with food intake such as dysphagia, xerostomia, mucositis and anorexia. Cachexia differs from malnutrition or physiological states of low calorie intake in that it is characterized by a progressively increasing change in whole-body metabolism that induces a continuous loss of skeletal, but not visceral, tissue. It is driven by a complex interaction between the tumor and its host involving a multitude of heterogeneous factors that are, however, mostly connected to chronic systemic inflammation145. Muscle wasting is mainly responsible for the negative e ff ects of cancerrelated weight loss.ese include declines in strength, quality of life and tolerability of cancer treatment. A simple and easy-toobtain indicator of muscle mass and strength exists in the form of the phase angle (PA) measured by tetrapolar bioelectrical impedance analysis146.e PA is determined by tissue cellularity, hydration and membrane potential and therefore is useful to assess malnutrition at the cellular level. Consistently, low PAs have been shown to strongly predict outcome in cancer patients147-149. Bioelectrical impedance measurements haveshown that already at early stages of disease, i.e., with normal BMI and minimal previous weight loss, HNC patients exhibit a signi fi cantly lower PA than age-matched healthy controls that is not explainable by an altered hydration status150. This sign of “sub-clinical malnutrition” therefore seems to occur early in the progression of disease and may be connected to the early systemic insulin resistance that has been described in a variety of cancer patients151-154. Pro-in fl ammatory cytokines are thought to play a causal role in this insulin resistance, similar to adipokines in obesity. Insulin resistance has important consequences for whole-body metabolism: In the liver, the rate of gluconeogenesis increases, utilizing lactate from the tumor, alanine from muscle and glycerin from lipolysis as substrates. As an energy-consuming process gluconeogenesis might therefore contribute to weightloss. In liver and skeletal muscle, glucose uptake and storage are inhibited155-157, and some156,158, but not all159, studies have found a decreased glucose oxidation rate. In contrast, gradually increased lipid oxidation rates have been measured in weightstable and weight-losing patients158,160, and even exogenous glucose ingestion was not able to reverse lipid oxidation to normal levels159. Finally, with disease progression the prolonged in fl ammatory state further takes its toll on muscle as amino acids are increasingly broken down and used for the synthesis of acutephase proteins in the liver.
These metabolic alterations indicate a general dysfunction in the utilization of glucose and an increased demand on fat as an energy source. This is quite contrary to the metabolism of tumor tissue that mainly utilizes glucose. It has therefore been recommended to account for these metabolic differences between the tumor and its host through either a high-fat low-CHO diet or a KD12,13(Figure 2). Unfortunately, clinical studies on these diets are rare and concentrate on advancedstage patients. In a randomized controlled trial on patients with advanced gastrointestinal cancer undergoing chemotherapy, Breitkreuz et al.161supplemented the conventional diet of 12 patients with a drink containing 66% energy from fat, while 11 patients remained on their conventional diet. Although there were no signi fi cant di ff erences between the groups with respect to non-nitrogenous energy intake, the treatment group had gained weight at 4 and 8 weeks and retained their body cell mass, while the control group continued to lose weight. Fearon et al.162administered a KD containing 70% energy from medium chain triglycerides and supplemented with β-hydroxybutyrate to five extremely cachectic patients (mean body weight 38.6 kg). Aer 1 week, the patients had regained approximately 2 kg body weight and improved their physical performance status. However, in this study there was no change in nitrogen balance that would have explained the significant weight gain. Other findings suggest that the physiological role of ketone bodies in the conservation of muscle tissue during prolonged starvation is retained even in cachectic patients163. Rat studies provide evidence that physiological levels of ketone bodies diminish muscle catabolism by inhibiting the oxidation of branched chain amino acids164and reducing the release of the gluconeogenic amino acid alanine165. We166and others167,168have shown that several weeks of a KD combined with ample protein intake increased the muscle mass in recreational and top-level athletes, respectively, despite a small overall weight loss.
In HNC patients, even “su ffi cient” energy and protein intakes have been shown to be insufficient for preventing significant weight and lean tissue loss during treatment169. Accordingly a recent position paper from a European School of Oncology Task Force states that “every e ff ort should be made to prevent muscle loss rather than relying on attempts to regain what has been lost”170. Following this statement the usage of ketone esters or KDs could be tried as part of such an e ff ort.
Discussion: is CHO restriction in HNC patients realistic?
Despite the evidence outlined above showing how CHO restriction counteracts tumor glycolysis, accounts for the altered metabolism of the tumor-bearing patient and may even improve the tolerability of radiation treatment, some authors still question the scienti fi c rationale for the KD and deny any possible benefits171. This probably reflects a general skepticism towards the implementation of low CHO or KDs that are “extreme” in the sense that they go against o ffi cial standard recommendations of the food agencies. In this context it should be noted, however, that the 2006 guidelines of the European Society for Enteral and Parenteral Nutrition (ESPEN) stated that the observations on altered patient metabolism “may be taken to support recommendations to increase the fat/CHO ratio in feeding cancer patients”172. Arguments against a KD include the fear of “ketoacidosis”, excessive weight loss, high cholesterol levels, kidney problems or the belief that the brain depends on a certain CHO intake171. However, none of these fears and assumptions are justified in light of the evidence from the literature13,173-175. Quite to the contrary, the literature on cancer patients suggests that even in very advanced stages low CHO diets combined with moderate to high protein intake may be anti-catabolic162, without serious side e ff ects90,136,176and able to improve blood parameters and some aspects of quality of life176. It is clear, however, that once a cachectic state has been reached any nutritional support will be insu ffi cient in stopping the weight loss170,177. Furthermore the ability to develop ketosis might be impaired in a largefraction of cachectic patients163. A low-CHO diet as a supportive treatment for HNC should therefore be offered as soon as the patient has been assessed for malnutrition, and then be implemented before weight loss becomes irreversible170.
Studies so far have shown that individual dietary counselling during radiotherapy of HNC is more effective than standard or no dietary advice for preventing long-term weight loss and improving quality of life, although the effects of these interventions on mortality have not been assessed178.is would be important, however, since nutrition support using standard high-CHO formulas has been shown to increase mortality rates despite better preservation of body weight during therapy123. Already in 1979 Donaldson and Lennon warned against this danger of nutrition support in the HNC patient2. From the mechanistic insights outlined here, this is now understandable. Figure 3 therefore presents a possible fl ow chart for individual counselling of HNC patients when the goal is to implement a low-CHO diet in order to minimize the risk of inducing high blood glucose levels and spurring tumor growth. First of all any individual problems with food intake and the nutritional status have to be assessed. Common problems such as dysphagia, xerostomia or odynophagia can oen be addressed by blending foods and using liquid supplements1. The high-fat nature of low CHO diets allows one to offer a variety of foods with a creamy texture that facilitate swallowing. If a patient is not yet malnourished, but unable to tolerate or accept a diet high in fat, then simple CHO restriction, the avoidance of high glycemic CHOs or intermittend fasting are options to try14. If, however, the patient has been classified as malnourished or is unable to meet his or her caloric needs through oral intake, supplemental tube feeding should be considered according to recommendations from the literature4. This can be seen as an opportunity for implementing low-CHO high-fat feeding since suitable feeding formulas are available for prescription.ereby it seems preferable to try a KD based on the bene fi cial properties of ketone bodies outlined in this paper. In addition or as an alternative, ketone esters may be used to induce “therapeutic ketosis” with ketone levels in the 2-7 mM range lasting for several hours upon ingestion179. To further optimize ketosis, it may be considered that the ability of di ff erent protein sources to elevate blood glucose levels via gluconeogenesis negatively correlates with the fraction of their amino acids utilized in anabolic pathways, also known as net nitrogen utilization180. Accordingly, protein sources with high net nitrogen utilization such as eggs and meat or the Master Amino Acid Pattern supplement180, a blend of the essential amino acids with a net nitrogen utilization of 99%, might be preferred, although the e ff ects of such strategies on ketosis have not yet been systematically evaluated. Finally, to fully exploit the potential of CHO restriction in HNC it should be used within a multimodal approach combined with antiinflammatory170and anti-cancer metabolic11therapy. This may further include nutraceuticals such as ω-3 fatty acids as well asresistance training that has shown great potential in the recovery of muscle mass and strength aer HNC therapy181.
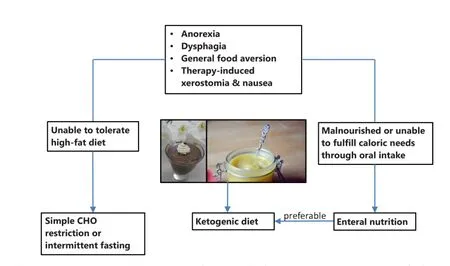
Figure 3 Flow chart showing the proposed implementation of a low CHO diet for the HNC patient. The pictures show foods compatible with a ketogenic diet that have a creamy texture and thus are easy to swallow.
In conclusion, CHO restriction in the HNC patient seems feasible and therefore realistic, but requires additional time and e ff ort as it has to be tailored towards the individual patient.is, however, is a general problem in HNC patients, and e ff orts on nutritional counselling generally seem to pay off. Clearly, the tolerability of and response to CHO restricted diets is also individual and some patients reach ketosis more easily than others. Hopefully future studies will show which patients bene fi t most from CHO restriction. Currently a phase I clinical trial (NCT01975766) at the university of Iowa recruits HNSCC patients to investigate the safety of a KD plus concurrent chemoradiation with a secondary outcome being progressionfree survival. Until the results are published, this paper hopefully encourages physicians to make their own experience with offering CHO restriction to their patients as a non-toxic approach to fi ght HNC.
Acknowledgements
Con fl ict of interest statement
No potential con fl icts of interest are disclosed.
1. Wood RM, Lander VL, Mosby EL, HiaWR. Nutrition and the head and neck cancer patient. Oral Surg Oral Med Oral Pathol 1989;68:391-395.
4. Alshadwi A, Nadershah M, Carlson ER, Young LS, Burke PA, Daley BJ. Nutritional Considerations for Head and Neck Cancer Patients: A Review of the Literature. J Oral Maxillofac Surg 2013;71:1853-1860.
5. Champ CE, Mishra M V, Showalter TN, Ohri N, Dicker AP, Simone NL. Dietary recommendations during and after cancer treatment: consistently inconsistent? Nutr Cancer 2013;65:430-439.
6. Zhu XP, Zhu LL, Zhou Q. Prescribing practice and evaluation of appropriateness of enteral nutrition in a university teaching hospital.er Clin Risk Manag 2013;9:37-43.
7. Fietkau R, Lewitzki V, Kuhnt T, H?lscher T, Hess CF, Berger B, et al. A Disease-Speci fi c Enteral Nutrition Formula Improves Nutritional Status and Functional Performance in Patients With Head and Neck and Esophageal Cancer Undergoing Chemoradiotherapy: Results of a Randomized, Controlled, Multicenter Trial. Cancer 2013;119:3343-3353.
8. Richtsmeier WJ, Dauchy R, Sauer LA. In Vivo Nutrient Uptake by Head and Neck Cancers. Cancer Res 1987;47:5230-5233.
9. Sandulache VC, Ow TJ, Pickering CR, Frederick MJ, Zhou G, Fokt I, et al. Glucose, Not Glutamine, Is the Dominant Energy Source Required for Proliferation and Survival of Head and Neck Squamous Carcinoma Cells. Cancer 2011;117:2926-2938.
10. Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (Lond) 2010;7:7.
11. Seyfried TN, Flores RE, Po ff AM, D’Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 2014;35:515-527.
12. Klement RJ, K?mmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond) 2011;8:75.
13. Holm E, K?mmerer U. Lipids and Carbohydrates in Nutritional Concepts for Tumor Patients. Aktuel Ern?hrungsmed 2011;36:286-298.
14. Klement RJ, Champ CE. Calories, carbohydrates, and cancer therapy with radiation: exploiting the fi ve R’s through dietary manipulation. Cancer Metastasis Rev 2014;33:217-229.
15. Minami S. Versuche an überlebendem Carcinomgewebe. Biochem Zeitschr 1923;142:334-350.
16. Warburg O, Posener K, Negelein E. über den Sto ff wechsel der Carcinomzelle. Biochem Zeitschr 1924;152:309-343.
17. Warburg O. über den Sto ff wechsel der Carcinomzelle. Klin Wochenschr 1925;4:534-536.
18. Warburg O, Wind F, Negelein E. über den Sto ff wechsel von Tumoren im K?rper. Klin Wochenschr 1926;5:829-832.
19. Warburg O, Wind F, Negelein E.e Metabolism of Tumors in the Body. J Gen Physiol 1927;8:519-530.
21. Subramaniam RM, Truong M, Peller P, Sakai O, Mercier G. Fluorodeoxyglucose-positron-emission tomography imaging of head and neck squamous cell cancer. AJNR Am J Neuroradiol 2010;31:598-604.
22. Allal AS, Dulguerov P, Allaoua M, Haenggeli CA, El-Ghazi el A, Lehmann W, et al. Standardized Uptake Value of 2-[(18)F] Fluoro-2-Deoxy-D-Glucose in Predicting Outcome in Head and Neck Carcinomas Treated by Radiotherapy With or Without Chemotherapy. J Clin Oncol 2002;20:1398-1404.
23. Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of Outcome in Head-And-Neck Cancer Patients Using the Standardized Uptake Value of 2-[(18)F] Fluoro-2-Deoxy-D-Glucose. Int J Radiat Oncol Biol Phys 2004;59:1295-1300.
24. Torizuka T, Tanizaki Y, Kanno T, Futatsubashi M, Naitou K, Ueda Y, et al. Prognostic Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR Am J Roentgenol 2009;192:W156-W160.
25. Suzuki H, Kato K, Fujimoto Y, Itoh Y, Hiramatsu M, Naganawa S, et al. Prognostic value of (18)F- fl uorodeoxyglucose uptake before treatment for pharyngeal cancer. Ann Nucl Med 2014;28:356-362.
26. Chan SC, Chang JT, Lin CY, Ng SH, Wang HM, Liao CT, et al. Clinical utility of 18 F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: predictive role for di ff erent survival endpoints and impact on prognostic strati fi cation. Nucl Med Commun 2011;32:989-996.
27. Kubicek GJ, Champ C, Fogh S, Wang F, Reddy E, Intenzo C, et al. FDG-PET staging and importance of lymph node SUV in head and neck cancer. Head Neck Oncol 2010;2:19.
28. Langen KJ, Braun U, Rota Kops E, Herzog H, Kuwert T, Nebeling B, et al.e In fl uence of Plasma Glucose Levels on Uptake in Bronchial Carcinomas. J Nucl Med 1993;34:355-359.
29. Greven KM, Williams DW 3rd, McGuirt WF Sr, Harkness BA, D’Agostino RB Jr, Keyes JW Jr, et al. Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck 2001;23:942-946.
30. Koukourakis MI, Giatromanolaki A, Winter S, Leek R, Sivridis E, Harris AL. Lactate dehydrogenase 5 expression in squamous cell head and neck cancer relates to prognosis following radical or postoperative radiotherapy. Oncology 2009;77:285-292.
31. Walenta S, Mueller-Klieser WF. Lactate: Mirror and Motor of Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol 2004;14:267-274.
32. Serganova I, Rizwan A, Ni X,akur SB, Vider J, Russell J, et al. Metabolic imaging: a link between lactate dehydrogenase A, lactate, and tumor phenotype. Clin Cancer Res 2011;17:6250-6261.
33. Walenta S, Salameh A, Lyng H, Evensen JF, Mitze M, Rofstad EK, et al. Correlation of High Lactate Levels in Head and Neck Tumors with Incidence of Metastasis. Am J Pathol 1997;150:409-415.
34. Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, et al. Elevated Tumor Lactate Concentrations Predict for an Increased Risk of Metastases in Head-And-Neck Cancer. Int J Radiat Oncol Biol Phys 2001;51:349-353.
35. Sun A, Johansson S, Turesson I, Dasu A, S?rensen J. Imaging tumor perfusion and oxidative metabolism in patients with headand-neck-cancer using 1- [11C]-acetate PET during radiotherapy: preliminary results. Int J Radiat Oncol Biol Phys 2012;82:554-560.
36. Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 2010;20:51-56.
38. Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modi fi es antitumor immune response: e ff ect on myeloid-derived suppressor cells and NK cells. J Immunol 2013;191:1486-1495.
39. Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 2008;118:3930-3942.
41. Curry JM, Tuluc M, Whitaker-Menezes D, Ames JA, Anantharaman A, Butera A, et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle 2013;12:1371-1384.
42. Boidot R, Végran F, Meulle A, Le Breton A, Dessy C, Sonveaux P, et al. Regulation of Monocarboxylate Transporter MCT1 Expression by p53 Mediates Inward and Outward Lactate Fluxes in Tumors. Cancer Res 2012;72:939-948.
43. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest 2013;123:3685-3692.
44. Leemans CR, Braakhuis BJM, Brakenho ff RH.e molecular biology of head and neck cancer. Nat Rev Cancer 2011;11:9-22.
45. Suh Y, Amelio I, Guerrero Urbano T, Tavassoli M. Clinical update on cancer: molecular oncology of head and neck cancer. Cell Death Dis 2014;5:e1018.
47. Lee P, Vousden KH, Cheung EC. TIGAR, TIGAR, burning bright. Cancer Metab 2014;2:1.
48. Landor SK, Mutvei AP, Mamaeva V, Jin S, Busk M, Borra R, et al. Hypo- and hyperactivated Notch signaling induce a glycolytic switch through distinct mechanisms. Proc Natl Acad Sci 2011;108:18814-18819.
49. Garcia-Cao I, Song MS, Hobbs RM, Laurent G, Giorgi C, de Boer VC, et al. Systemic elevation of PTEN induces a tumor suppressive metabolic state. Cell 2012;149:49-62.
50. Robey RB, Hay N. Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol2009;19:25-31.
51. Yang D, Wang M-T, Tang Y, Chen Y, Jiang H, Jones, et al. Impairment of mitochondrial respiration in mouse fi broblasts by oncogenic H-S(Q61L). Cancer Bioler 2010;9:122-133.
52. DeBerardinis RJ, Lum JJ, Hatzivassiliou G,ompson CB.e Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab 2008;7:11-20.
53. Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem 2001;276:9519-9525.
54. Gerh?user C. Cancer cell metabolism, epigenetics and the potential in fl uence of dietary components – A perspective. Biomed Res 2012;23.
55. Gasche JA, Goel A. Epigenetic mechanisms in oral carcinogenesis. Future Oncol 2012;8:1407-1425.
56. Yeh KT, Chang JG, Lin TH, Wang YF, Tien N, Chang JY, et al. Epigenetic changes of tumor suppressor genes, P15, P16, VHL and P53 in oral cancer. Oncol Rep 2003;10:659-663.
57. Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, et al. JunD Reduces Tumor Angiogenesis by Protecting Cells from Oxidative Stress. Cell 2004;118:781-794.
58. Meijer TWH, Kaanders JHAM, Span PN, Bussink J. Targeting Hypoxia, HIF-1, and Tumor Glucose Metabolism to Improve Radiotherapy E ffi cacy. Clin Cancer Res 2012;18:5585-5594.
59. Quennet V, Yaromina A, Zips D, Rosner A, Walenta S, Baumann M, et al. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother Oncol 2006;81:130-135.
61. Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose Deprivation-Induced Oxidative Stress in Human Tumor Cells. A Fundamental Defect in Metabolism? Ann N Y Acad Sci 2000;899:349-362.
62. Ahmad IM, Aykin-Burns N, Sim JE, Walsh SA, Higashikubo R, Buener GR, et al. Mitochondrial O2- and H2O2 Mediate Glucose Deprivation-induced Stress in Human Cancer Cells. J Biol Chem 2005;280:4254-4263.
63. Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the di ff erential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J 2009;418:29-37.
64. Graham NA, Tahmasian M, Kohli B, Komisopoulou E, Zhu M, Vivanco I. Glucose deprivation activates a metabolic and signaling ampli fi cation loop leading to cell death. Mol Syst Biol 2012;8:589.
65. Sun W, Liu Y, Glazer CA, Shao C, Bhan S, Demokan S, et al. TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1alpha stabilization. Clin Cancer Res 2010;16:857-866.
66. V?lker H-U, Scheich M, Schmausser B, K?mmerer U, Eck M. Overexpression of transketolase TKTL1 is associated with shorter survival in laryngeal squamous cell carcinomas. Eur Arch Otorhinolaryngol 2007;264:1431-1436.
68. Zhou S, Kachhap S, Sun W, Wu G, Chuang A, Poeta L, et al. Frequency and phenotypic implications of mitochondrial DNA mutations in human squamous cell cancers of the head and neck. Proc Natl Acad Sci 2007;104:7540-7545.
69. Lai CH, Huang SF, Liao CT, Chen IH, Wang HM, Hsieh LL. Clinical Signi fi cance in Oral Cavity Squamous Cell Carcinoma of Pathogenic Somatic Mitochondrial Mutations. PLoS One 2013;8:e65578.
70. Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol 2010;5:297-348.
71. Singh KK, Kulawiec M, Still I, Desouki MM, Geradts J, Matsui S-I. Inter-genomic cross talk between mitochondria and the nucleus plays an important role in tumorigenesis. Gene 2005;354:140-146.
72. Allen BG, Bhatia SK, Buai JM, Brandt KE, Lindholm KE, Buon AM, et al. Ketogenic Diets Enhance Oxidative Stress and Radio-Chemo-erapy Responses in Lung Cancer Xenogras. Clin Cancer Res 2013;19:3905-3913.
73. Sun W, Zhou S, Chang SS, McFate T, Verma A, Califano JA. Mitochondrial mutations contribute to HIF1alpha accumulation via increased reactive oxygen species and up-regulated pyruvate dehydrogenease kinase 2 in head and neck squamous cell carcinoma. Clin Cancer Res 2009;15:476-484.
74. Challen C, Brown H, Cai C, Bes G, Paterson I, Sloan P, et al. Mitochondrial DNA mutations in head and neck cancer are infrequent and lack prognostic utility. Brit J Cancer 2011;104:1319-1324.
75. Warburg O. On the Origin of Cancer Cells. Science 1956;123:309-314.
77. Demetrakopoulos GE, Linn B, Amos H. Rapid loss of ATP by tumor cells deprived of glucose: contrast to normal cells. Biochem Biophys Res Commun 1978;82:787-794.
78. Shim H, Chun YS, Lewis BC, Dang CV. A unique glucosedependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci 1998;95:1511-1516.
79. Li Y, Liu L, Tollefsbol TO. Glucose restriction can extend normal cell lifespan and impair precancerous cell growth through epigenetic control of hTERT and p16 expression. FASEB J 2010;24:1442-1453.
80. Priebe A, Tan L, Wahl H, Kueck A, He G, Kwok R, et al. Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells. Gynecol Oncol 2011;122:389-395.
81. Mathews EH, Stander BA, Joubert AM, Liebenberg L. Tumor cell culture survival following glucose and glutamine deprivation at typical physiological concentrations. Nutrition 2014;30:218-227.
82. Demetrius LA, Coy JF, Tuszynski JA. Cancer proliferation and therapy: the Warburg e ff ect and quantum metabolism.eor Biol Med Model 2010;7:2.
83. Davies P, Demetrius LA, Tuszynski JA. Implications of quantum metabolism and natural selection for the origin of cancer cells and tumor progression Implications of quantum metabolism and natural selection. AIP Adv 2012;2:11101.
84. Champ CE, Baserga R, Mishra MV, Jin L, Sotgia F, Lisanti MP, et al. Nutrient restriction and radiation therapy for cancer treatment: when less is more. Oncologist. 2013;18:97-103.
85. Simone BA, Champ CE, Rosenberg AL, Berger AC, Monti DA, Dicker AP, et al. Selectively starving cancer cells through dietary manipulation: methods and clinical implications. Future Oncol 2013;9:959-976.
86. Klement RJ. Calorie or Carbohydrate Restriction?e Ketogenic Diet as Another Option for Supportive Cancer Treatment. Oncologist 2013;18:1056.
87. Marchut E, Gumińska M, Kedryna T.e inhibitory e ff ect of various fay acids on aerobic glycolysis in Ehrlich ascites tumour cells. Acta Biochim Pol 1986;33:7-16.
88. Nebeling LC, Miraldi F, Shurin SB, Lerner E. E ff ects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr 1995;14:202-208.
89. Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr Metab (Lond) 2010;7:33.
90. Fine EJ, Segal-isaacson CJ, Feinman RD, Herszkopf S, Romano MC, Tomuta N, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: A pilot safety and feasibility dietary trial in 10 patients. Nutrition 2012;28:1028-1035.
91. Schroeder U, Himpe B, Pries R, Vonthein R, Nitsch S, Wollenberg B. Decline of lactate in tumor tissue aer ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr Cancer 2013;65:843-849.
92. Cahill GF Jr, Veech RL. Ketoacids? Good medicine? Trans Am Clin Climatol Assoc 2003;114:149-61; discussion 162-3.
94. Skinner R, Trujillo A, Ma X, Beierle EA. Ketone bodies inhibit the viability of human neuroblastoma cells. J Pediatr Surg 2009;44:212-216.
95. Maurer GD, Brucker DP, B?hr O, Harter PN, Haingen E, Walenta S, et al. Di ff erential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 2011;11:315.
96. Chang HT, Olson LK, Schwartz. Ketolytic and glycolytic enzymatic expression pro fi les in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond) 2013;10:47.
97. Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell 2007;129:1261-1274.
98. Limesand KH, Chibly AM, Fribley A. Impact of targeting insulinlike growth factor signaling in head and neck cancers. Growth Horm IGF Res 2013;23:135-140.
100. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915-928.
101. Iyengar NM, Kochhar A, Morris PG, Morris LG, Zhou XK, Ghossein, et al. Impact of Obesity on the Survival of Patients With Early-Stage Squamous Cell Carcinoma of the Oral Tongue. Cancer 2014;120:983-991.
102. Hardie DG, Alessi DR. LKB1 and AMPK and the cancermetabolism link – ten years aer. BMC Biol 2013;11:36.
103. Porporato PE, Dhup S, Dadhich RK, Copei T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors : a compehensive review. Front Pharmacol 2011;2:49.
104. Curry NL, Mino-Kenudson M, Oliver TG, Yilmaz OH, Yilmaz VO, Moon JY, et al. Pten-Null Tumors Cohabiting the Same Lung Display Di ff erential AKT Activation and Sensitivity to Dietary Restriction. Cancer Discov 2013;3:908-921.
105. Sta ff ord P, Abdelwahab MG, Kim do Y, Preul MC, Rho JM, Scheck AC.e ketogenic diet reverses gene expression paerns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond) 2010;7:74.
106. Scheck AC, Abdelwahab MG, Fenton KE, Sta ff ord P.e ketogenic diet for the treatment of glioma: insights from genetic pro fi ling. Epilepsy Res 2012;100:327-337.
107. Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013;339:211-214.
108. Newman JC, Verdin E. Ketone bodies as signaling metabolites.Trends Endocrinol Metab 2014;25:42-52.
109. Giudice FS, Pinto DSJ, N?r JE, Squarize CH, Castilho RM. Inhibition of Histone Deacetylase Impacts Cancer Stem Cells and Induces Epithelial-Mesenchyme Transition of Head and Neck Cancer. PLoS One 2013;8:e58672.
110. Chang CC, Lin BR, Chen ST, Hsieh TH, Li YJ, Kuo MY. HDAC2 promotes cell migration/invasion abilities through HIF-1a stabilization in human oral squamous cell carcinoma. J Oral Pathol Med 2011;40:567-575.
111. Ujpál M, Matos O, Bíbok G, Somogyi A, Szabó G, Suba Z. Diabetes and Oral Tumors in Hungary. Diabetes Care 2004;27:770-774.
112. Shanmugam N, Reddy MA, Guha M, Natarajan R. High Glucose–Induced Expression of Proin fl ammatory Cytokine and Chemokine Genes in Monocytic Cells. Diabetes 2003;52:1256-1264.
113. Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL. Elevated Glucose and Diabetes Promote Interleukin-12 Cytokine Gene Expression in Mouse Macrophages. Endocrinology 2006;147:2518-2525.
114. Freund E. Zur Diagnose des Carcinoms. Wiener Medizinische Bl?er 1885;9.
115. Maestu I, Pastor M, Aparicio J, Oltra A, Herranz C, Montalar J, et al. Pretreatment prognostic factors for survival in small-cell lung cancer: A new prognostic index and validation of three known prognostic indices on 341 patients. Ann Oncol 1997;8:547-553.
116. Weiser MA, Cabanillas ME, Konopleva M,omas DA, Pierce SA, Escalante CP, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/ methotrexate-cytarabine regimen. Cancer 2004;100:1179-1185.
117. McGirt MJ, Chaichana KL, Gathinji M, Aenello F,an K, Ruiz AJ, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival aer primary resection of malignant brain astrocytomas. Neurosurgery 2008;63:286-291.
118. Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol 2009;27:1082-1086.
119. Lamkin DM, Spitz DR, Shahzad MM, Zimmerman B, Lenihan DJ, Degeest K, et al. Glucose as a Prognostic Factor in Ovarian Carcinoma. Cancer 2009;115:1021-1027.
120. Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD, et al. Clinically Defined Type 2 Diabetes Mellitus and Prognosis in Early-Stage Breast Cancer. J Clin Oncol 2011;29:54-60.
121. Villarreal-Garza C, Shaw-Dulin R, Lara-Medina F, Bacon L, Rivera D, Urzua L, et al. Impact of diabetes and hyperglycemia on survival in advanced breast cancer patients. Exp Diabetes Res 2012;2012:732027.
122. Minicozzi P, Berrino F, Sebastiani F, Falcini F, Vaiato R, Cioccoloni F, et al. High fasting blood glucose and obesity signi fi cantly and independently increase risk of breast cancer death in hormone receptor-positive disease. Eur J Cancer 2013;49:3881-3888.
123. Rabinovitch R, Grant B, Berkey BA, Raben D, Ang KK, Fu KK, et al. Impact of nutrition support on treatment outcome in patients with locally advanced head and neck squamous cell cancer treated with de fi nitive radiotherapy: a secondary analysis of RTOG trial 90-03. Head Neck 2006;28:287-296.
124. Mu?oz IP, Plaza FC. Head and Neck Cancer: Multidisciplinary Approach is a must, Including Radiation Oncologist and Anaesthesiologist. Surgery Curr Res 2014;4:157.
125. Ra ff aghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, et al. Starvation-dependent di ff erential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci 2008;105:8215-8220.
126. Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, et al. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One 2012;7:e44603.
127. Lee C, Ra ff aghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med 2012;4:124ra27.
128. Saleh AD, Simone BA, Savage J, Sano Y, Jin L, Champ C, et al. Caloric restriction augments radiation e ffi cacy in breast cancer. Cell Cycle 2013;12:1955-1963.
129. Cantó C, Auwerx J. Targeting Sirtuin 1 to Improve Metabolism: All You Need Is NAD+? Pharmacol Rev 2012;64:166-187.
132. Yu XM, Liu Y, Jin T, Liu J, Wang J, Ma C, et al.e Expression of SIRT1 and DBC1 in Laryngeal and Hypopharyngeal Carcinomas. PLoS One 2013;8:e66975.
133. Ming M, Shea CR, Guo X, Li X, Soltani K, Han W, et al. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc Natl Acad Sci U S A 2010;107:22623-8.
134. Magee BA, Potezny N, Rofe AM, Conyers.e inhibition of malignant cell growth by ketone bodies. Aust J Exp Biol Med Sci 1979;57:529-539.
135. Fine EJ, Miller A, Quadros E V, Sequeira JM, Feinman RD. Acetoacetate reduces growth and ATP concentration in cancercell lines which over-express uncoupling protein 2. Cancer Cell Int 2009;9:14.
136. Rieger J, B?hr O, Maurer GD, Haingen E, Franz K, Brucker D, et al. ERGO: A pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol 2014;44:1843-1852.
137. Rossi-Fanelli F, Franchi F, Mulieri M, Cangiano C, Cascino A, Ceci F, et al. E ff ect of energy substrate manipulation on tumor cell proliferation in parenterally fed cancer patients. Clin Nutr 1991;10:228-232.
138. Mukherjee P, Sotnikov AV, Mangian HJ, Zhou J, Visek WJ, Clinton SK. Energy Intake and Prostate Tumor Growth, Angiogenesis, and Vascular Endothelial Growth Factor Expression. J Natl Cancer Inst 1999;91:512-523.
139. Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and Proapoptotic E ff ects of Dietary Restriction on Experimental Mouse and Human Brain Tumors Antiangiogenic and Proapoptotic E ff ects of Dietary Restriction on Experimental Mouse and Human Brain Tumors. Clin Cancer Res 2004;10:5622-5629.
140. Urits I, Mukherjee P, Meidenbauer J, Seyfried TN. Dietary restriction promotes vessel maturation in a mouse astrocytoma. J Oncol 2012;2012:264039.
142. Christopoulos A, Ahn SM, Klein JD, Kim S. Biology of Vascular Endothelial Grwoth Factor and its Receptors in Head And Neck Cancer: Beyond Angiogenesis. Head Neck 2011;33:1220-1229.
143. Po ff AM, Ari C, Seyfried TN, Agostino DPD.e Ketogenic Diet and Hyperbaric Oxygenerapy Prolong Survival in Mice with Systemic Metastatic Cancer. PLoS One 2013;8:e65522.
144. D’Agostino DP, Pilla R, Held HE, Landon CS, Puchowicz M, Brunengraber H, et al.erapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol Regul Integr Comp Physiol 2013;304:R829-R836.
145. Johns N, Stephens NA, Fearon KCH. Muscle wasting in cancer. Int J Biochem Cell Biol 2013;45:2215-2229.
146. Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol 2002;86:509-516.
147. Toso S, Piccoli A, Gusella M, Menon D, Bononi A, Crepaldi G, et al. Altered Tissue Electric Properties in Lung Cancer Patients as Detected by Bioelectric Impedance Vector Analysis. Nutrition 2000;16:120-124.
148. Gupta D, Lammersfeld CA, Burrows JL, Dahlk SL, Vashi PG, Grutsch JF, et al. Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. Am J Clin Nutr 2004;80:1634-1638.
149. Gupta D, Lammersfeld CA, Vashi PG, King J, Dahlk SL, Grutsch JF, et al. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer 2008;8:249.
150. De Luis DA, Aller R, Izaola O, Terroba M, Cabezas G, Cuellar L. Tissue electric properties in head and neck cancer patients. Ann Nutr Metab 2006;50:7-10.
151. McCall JL, Tuckey JA, Parry BR. Serum tumour necrosis factor alpha and insulin resistance in gastrointestinal cancer. Br J Surg 1992;79:1361-1363.
152. Noguchi Y, Yoshikawa T, Marat D, Doi C, Makino T, Fukuzawa K, et al. Insulin Resistance in Cancer Patients Is Associated with Enhanced Tumor Necrosis Factor-alpha Expression in Skeletal Muscle. Biochem Biophys Res Commun 1998;253:887-892.
153. Makino T, Noguchi Y, Yoshikawa T, Doi C, Nomura K. Circulating interleukin 6 concentrations and insulin resistance in patients with cancer. Br J Surg 1998;85:1658-1662.
154. Yoshikawa T, Noguchi Y, Doi C, Makino T, Nomura K. Insulin resistance in patients with cancer: relationships with tumor site, tumor stage, body-weight loss, acute-phase response, and energy expenditure. Nutrition 2001;17:590-593.
157. Norton JA, Maher M, Wesley R, White D, Brennan MF. Glucose lntolerance in Sarcoma Patients. Cancer 1984;54:3022-3027.
158. Hansell DT, Davies JWL, Burns HJG, Shenkin A.e Oxidation of Body Fuel in Cancer Patients. Ann Surg 1986;204:637-642.
159. Gambardella A, Paolisso G, D’Amore A, Granato M, Verza M, Varricchio M. Di ff erent Contribution of Substrates Oxidation to Insulin Resistance in Malnourished Elderly Patients with Cancer. Cancer 1993;72:3106-3113.
160. K?rber J, Pricelius S, Heidrich M, Müller MJ. Increased lipid utilization in weight losing and weight stable cancer patients with normal body weight. Eur J Clin Nutr 1999;53:740-745.
161. Breitkreutz R, Tesdal K, Jentschura D, Haas O, Leweling H, Holm E. E ff ects of a high-fat diet on body composition in cancer patients receiving chemotherapy: a randomized controlled study. Wien Klin Wochenschr 2005;117:685-692.
162. Fearon KC, Borland W, Preston T, Tisdale MJ, Shenkin A, Calman KC. Cancer cachexia: in fl uence of systemic ketosis on substrate levels and nitrogen metabolism. Am J Clin Nutr 1988;47:42-48.
163. Rich AJ, Wright PD. Ketosis and nitrogen excretion in undernourished surgical patients. JPEN J Parenter Enteral Nutr 1979;3:350-354.
164. Buse MG, Biggers JF, Friderici KH, Buse JF. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat.e e ff ect of fay acids, glucose, and pyruvate respiration. J Biol Chem1972;247:8085-8096.
165. Palaiologos G, Felig P. E ff ects of ketone bodies on amino acid metabolism in isolated rat diaphragm. Biochem J 1976;154:709-716.
166. Klement RJ, Frobel T, Albers T, Fikenzer S, Prinzhausen J, K?mmerer U. A pilot case study on the impact of a selfprescribed ketogenic diet on biochemical parameters and running performance in healthy and physically active individuals. Nutr Med 2013;1:10.
167. Volek JS, Sharman MJ, Love DM, Avery NG, Gómez AL, ScheeTP, et al. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism 2002;51:864-870.
168. Paoli A, Grimaldi K, D’Agostino D, Cenci L, Moro T, Bianco A, et al. Ketogenic diet does not a ff ect strength performance in elite artistic gymnasts. J Int Soc Sports Nutr 2012;9:34.
170. Aapro M, Arends J, Bozzei F, Fearon K, Grunberg SM, Herrstedt J, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol 2014;25:1492-1499.
171. Huebner J, Marienfeld S, Abbenhardt C, Ulrich C, Muenstedt K, Micke O, et al. Counseling Patients on Cancer Diets: A Review of the Literature and Recommendations for Clinical Practice. Anticancer Res 2014;34:39-48.
172. Arends J, Bodoky G, Bozzei F, Fearon K, Muscaritoli M, Selga G, et al. ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin Nutr 2006;25:245-259.
173. Manninen AH. High-Protein Weight Loss Diets and Purported Adverse E ff ects: Where is the Evidence? J Int Soc Sport Nutr 2004;1:45.
174. Manninen AH. Metabolic e ff ects of the very-low-carbohydrate diets: misunderstood “villains” of human metabolism. J Int Soc Sports Nutr 2004;1:7-11.
175. Manninen A. High-Protein Diets: Puing Rumors to Rest. J Am Coll Cardiol 2004;44:1526.
176. Schmidt M, Pfetzer N, Schwab M, Strauss I, K ?mmerer U. E ff ects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr Metab (Lond) 2011;8:54.
177. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. De fi nition and classi fi cation of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-495.
178. Langius JA, Zandbergen MC, Eerenstein SE, van Tulder MW, Leemans CR, Kramer MH, et al. E ff ect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo) radiotherapy : a systematic review. Clin Nutr 2013;32:671-678.
179. Hashim SA, Vanltallie TB. Ketone Bodyerapy: From ketogenic diet to oral administration of ketone ester. J Lipid Res 2014;55:1818-1826.
181. L?nbro S, Dalgas U, Primdahl H, Johansen J, Nielsen JL, Overgaard J, et al. Lean body mass and muscle function in head and neck cancer patients and healthy individuals – results from the DAHANCA 25 study. Acta Oncol 2013;52:1543-1551.
Cite this article as:Klement RJ. Restricting carbohydrates to fight head and neck cancer—is this realistic? Cancer Biol Med 2014;11:145-161. doi: 10.7497/j.issn.2095-3941.2014.03.001
Rainer J. Klement
E-mail: rainer_klement@gmx.de
Received May 29, 2014; accepted July 13, 2014. Available at www.cancerbiomed.org
Copyright ? 2014 by Cancer Biology & Medicine
 Cancer Biology & Medicine2014年3期
Cancer Biology & Medicine2014年3期
- Cancer Biology & Medicine的其它文章
- Breast metastasis from lung cancer: a report of two cases and literature review
- The gene expression patterns of BMPR2, EP300, TGFβ2, and TNFAIP3 in B-Lymphoma cells
- Neoadjuvant chemoradiotherapy for resectable esophageal cancer: an in-depth study of randomized controlled trials and literature review
- Hepatitis B virus X protein accelerates the development of hepatoma
- Systemic treatment in EGFR-ALK NSCLC patients: second line therapy and beyond
- Heterogeneity and renal mass biopsy: a review of its role and reliability
