Tamarind seed coat extract restores reactive oxygen species through attenuation of glutathione level and antioxidant enzyme expression in human skin fibroblasts in response to oxidative stress
Oranuch Nakchat, Nonthaneth Nalinratana, Duangdeun Meksuriyen, Sunanta Pongsamart
Department of Biochemistry and Microbiology, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, 10330, Thailand
Tamarind seed coat extract restores reactive oxygen species through attenuation of glutathione level and antioxidant enzyme expression in human skin fibroblasts in response to oxidative stress
Oranuch Nakchat, Nonthaneth Nalinratana, Duangdeun Meksuriyen, Sunanta Pongsamart*
Department of Biochemistry and Microbiology, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, 10330, Thailand
PEER REVIEW
Peer reviewer
Professor Viroj Wiwanitkit, Visiting professor, Hainan Medical University, China; Surin Rajabhat University, Thailand; Joseph Ayobabalola University, Nigeria; University of Nis, Serbia.
Tel: 6624132436
E-mail: wviroj@yahoo.com
Comments
This work made an attempt to study on the local herbs collected from a country in Southeast Asia. The authors used standard method in ethnopharmacology to study the property of the collected herb. This work is a good example in ethnopharmacological studies and can be further applied for further usage in tropical medicine.
Details on Page 384
Objective:To investigate the role and mechanism of tamarind seed coat extract (TSCE) on normal human skin fibroblast CCD-1064Sk cells under normal and oxidative stress conditions induced by hydrogen peroxide (H2O2).
Tamarindus indica, Seed coat, Antioxidant, Reactive oxygen species, Glutathione, Antioxidant enzymes, CCD-1064Sk cells
1. Introduction
Tamarindus indicaL. (in family Fabaceae and subfamily Caesalpiniodeae) commonly known as tamarind, is originally native tree to Africa and is now worldwide cultivated in many tropical countries. Tamarind is widely used in various traditional medicine and food products[8]. The fruits can be consumed fresh and as an ingredient in many kinds of Thai food[9]. Tamarind seed, a by-product from food industries, have been used as a substitute for coffee for a long time[10]. The seed consists of a kernel and seed coat. The seed coat is a rich source of tannins and polyphenols which possesses antiallergic and antimicrobial[11], antibiotic[12], antityrosinase[13], and antioxidant activities[14,15]. However, non-enzymatic and enzymatic antioxidant activities of tamarind seed coat at cellular level are still unexplored. The present study aimed to investigate whether tamarind seed coat extract (TSCE) could protect against H2O2-induced oxidative stress in human foreskin fibroblast CCD-1064Sk cells through antioxidant defense mechanism. The cells have been widely used as a model to evaluate wound healing processes[16], and anti-skin aging[17].
2. Materials and methods
2.1. Plant material and preparation of tamarind seed coat extract
Ripened tamarind pods of the sour tamarind,Tamarindus indica“Priao-Yak”, were collected from Phetchabun, Thailand. Herbarium specimens were deposited at the Museum of Natural Medicine, Faculty of Pharmaceutical Sciences, Chulalongkorn University. The tamarind seeds were separated and roasted in a pre-acid washed sandbath at 150 °C for 1 h. The seeds were then quickly washed and dried in a hot air oven at 50 °C for 24 h. The seed coats were removed from the kernels, pulverized into a tamarind seed coat powder (TSCP). TSCP was extracted as previously described[18], with slightly modified. Three grams of TSCP was put in a cloth sack, dipped while shaking in 200 mL boilingwater and boiled for 2 min. The sack was pressed and the red water extract was collected. The residue was then reextracted until the extract was colorless. The extract was pooled and filtered. Before the cell analysis, the clarified extract was further partitioned with an equal volume of ethyl acetate and then concentrated by rotary evaporator (Buchi, Switzerland) at 40 °C and dried under blowing nitrogen gas. The dried TSCE was dissolved in dimethyl sulfoxide (DMSO) at 400 mg/mL stock solution and serial dilutions were prepared in the culture medium before used.
2.2. Cell culture
CCD-1064Sk cells were purchased from American Type Culture Collection (CRL-2076, VA, USA). The cells were grown and maintained in Iscove’s modified Dulbecco’s medium supplemented with 10% fetal bovine serum, 100 IU/mL penicillin and 100 μg/mL streptomycin and maintained at 37 °C in humidified 5% CO2incubator.
2.3. Determination of cell viability
Cell viability was determined using neutral red assay[19], with slightly modified. The cells were seeded in 96-well plates at a density of 1×105cells/mL and incubated for 24 h. After removal of medium, the cells were treated with 100 μL of TSCE at concentrations ranging from 0.05 to 1 mg/mL for 24 h. The TSCE was removed and then washed twice with 100 μL of phosphate buffer solution (PBS) (pH 7.4) and then H2O2was added to induce the cell damage. About 100 μL of 40 μg/mL neutral red medium was added and incubated for 3 h. After incubated the neutral red medium was removed and rapidly washed and fixed with 100 μL of fixing solution. The dye was extracted by adding 100 μL of extraction solution, incubated for 20 min, and then agitated on a rocking platform for 5 min. An absorbance was measured at 570 nm using micropalte reader (Wallac 1420, Perkin Elmer, USA). The cell viability was calculated compare with vehicle control (0.25% DMSO in medium).
2.4. Determination of intracellular ROS
The intracellular ROS level was investigated using dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay[20]. Cells were seeded into 96-well plates at a density of 1× 105cells/mL and incubated for 24 h. The volume 100 μL of TSCE at concentrations ranging from 0.05 to 1 mg/mL was added and incubated for 24 h and then washed twice with cold PBS. Thereafter, 100 μL of 5 μmol/L DCFH-DA was added and incubated for 30 min. The cells were washed and then 1 mmol/L H2O2was added and incubated for 15 min for generating intracellular ROS. The fluorescence intensity of DCF was measured by using a microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 535 nm.
2.5. Determination of intracellular GSH level
Total level of intracellular GSH was measured by using 5,5’-dithiobis(2-nitrobenzoic acid) (DTNB)-glutathione disulfide (GSSG) reductase recycling assay[21]. Cells were seeded into 6-well plates at a density of 1×105cells/mL and incubated for 24 h. Thereafter, the cells were treated with TSCE at concentrations ranging from 0.05 to 1 mg/mL for 24 h and then exposed with 1 mmol/L H2O2for 30 min. The cells were lysed, deproteinized, and centrifuged. The supernatant of cell lysates were determined. The reaction mixture contained 10 μL of cell lysates, 90 μL of 0.1 mol/L sodium phosphate buffer containing 1 mmol/L ethylene diamine tetraacetic acid (pH 7.5), 10 μL of 10 IU/mL glutathione reductase, 80 μL of 0.25 mmol/L NADPH and 10 μL of 1.5 mmol/L DTNB, respectively. The rate of TNB productionwas measured at 405 nm by using a microplate reader at 30 seconds intervals for 10 min. The total intracellular GSH level was analyzed by the kinetic method from a linear standard curve of GSH. The value was expressed in μmol/L per mg protein.
2.6. Determination of antioxidant enzymes activity
The cells were seeded into 6-well plates at a density of 1×105cells/mL and incubated for 24 h. After removal of medium, the cells were treated with TSCE at concentrations ranging from 0.05 to 1 mg/mL for 24 h and then exposed to H2O2. The cells were washed twice with cold PBS, trypsinized and centrifuged at 1 040 r/min (by using EBA 12, Hettich Zentrifugen, Tuttlingen, Germany) at 4 °C for 5 min. Cell pellets were washed twice with cold PBS and then SOD, GPx and CAT activity were determined.
2.6.1. Determination of SOD activity
The cell pellets were re-suspended in 600 μL of 50 mmol/ L NaHCO3buffer (pH 10.2). Thereafter, the cells were lysed on ice by using ultrasonic disintegrator at 5 μm of amplitude every 2 seconds for 10 seconds. Cell lysates were centrifuged at 12 500 r/min at 4 °C for 10 min. The supernatant of cell lysate was determined for SOD activity[22].
2.6.2. Determination of GPx and CAT activity
The cell pellets were re-suspended in ice-cold 0.013% sodium cholate (20×106cells/mL), sonicated on ice every 2 seconds for 10 seconds and then centrifuged at 9 660 r/min at 4°C for 10 min. The supernatant of cell lysate was determined for GPx and CAT activity[23].
2.7. Western blot analysis
The cells were seeded at a density of 1×105cells/mL into 6-well plates and incubated for 24 h. After removal of medium, the cells were treated with TSCE at concentrations ranging from 0.4 to 0.8 mg/mL for 24 h. Thereafter, the cells were exposed with 1 mmol/L H2O2for 3 h. After removal of medium, the cells were washed twice with icecold PBS, scraped, collected, and centrifuged at 1 500 r/ min at 4°C for 8 min. The protein was extracted by radioimmunoprecipitation assay lysis buffer on ice for 30 min. After centrifuged at 12 000 r/min at 4°C for 10 min, the supernatant was collected and protein concentration was measured. The protein extract was mixed with loading buffer, boiled at 95 °C for 5 min. The amount of 30 mg proteins of each sample were loaded onto 12% sodium dodecyl sulfatepolyacrylamide gel and electrophoresis at 90 V for 1.5 h. The gel was further transferred onto polyvinylidene fluoride membrane by electroblotting at 45 V for 2 h. The membrane was incubated with 5% bovine serum albumin in tris buffered saline Tween buffer for 2 h at room temperature for blocking non-specific binding protein. Blots were probed with primary rabbit antibodies (Cu,Zn-SOD or GPx or CAT from Abcam, Cambridge, England) at 4 °C overnight. The membrane was washed 6 times with tris buffered saline Tween and then blotted with a goat anti-rabbit polyclonal secondary antibody with horseradish peroxidase conjugated for 1 h at room temperature. The protein bands were detected by enhanced chemiluminascence detection reagent and exposed to Kodak X-ray films. The intensities of the bands were visualized and computed by ImageJ 1.43u software (NIH, Bethesda, MD, USA).
2.8. Statistical analysis
The SPSS program was used for statistical analysis. Data were expressed as mean±SEM obtained from each test in triplicate. Statistical analysis was performed using Oneway analysis of variance (ANOVA), followed by Turkey’spost hocmean comparison. A level ofP<0.05 was considered statistically significant.
3. Results
3.1. Effect of TSCE on intracellular ROS
Effect of the TSCE polyphenols on intracellular ROS level as compared with viability was determined using DCFH-DA and neutral red assays, respectively. The result demonstrated that TSCE alone decreased intracellular ROS in a concentration-dependent manner (Figure 1A). To optimize oxidative stress condition, the cells were treated withH2O2at various concentrations and time points. The result demonstrated that an exposure with 1 mmol/L H2O2for 15 min induced the highest oxidative stress (126.97%) without affecting cell viability (93.72%) (Figure 1B). Meanwhile, TSCE (0.2 to 1 mg/mL) significantly attenuated ROS in H2O2-treated cells (Figure 1C).
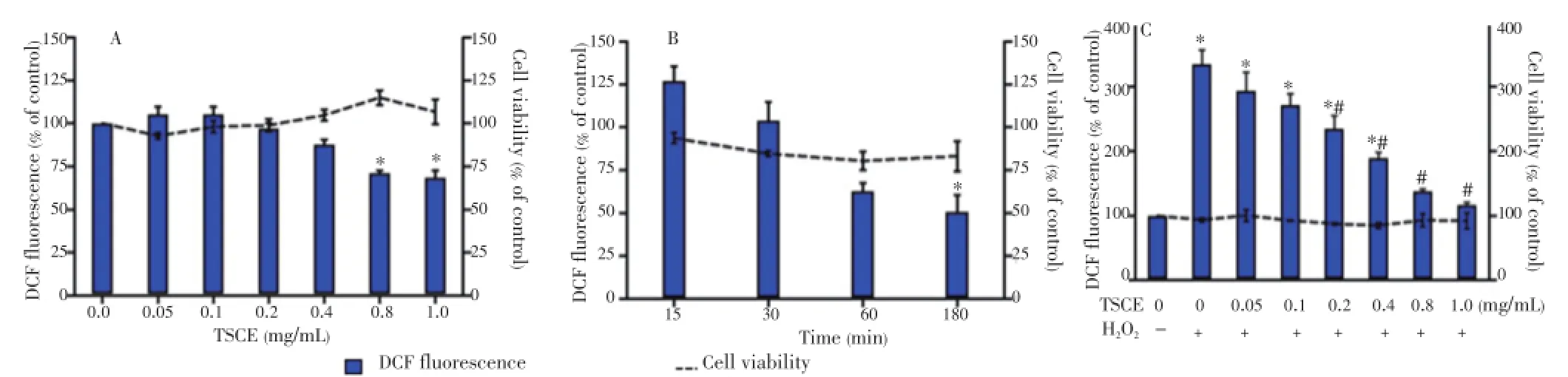
Figure 1. Effect of TSCE on the intracellular ROS generation and viability.The cells were treated with (A) TSCE (0.05-1 mg/mL) for 24 h or (B) H2O2(1 mmol/L) for 15, 30, 60 and 180 min. For oxidative stress condition, (C) treatment of cells with TSCE (0.05-1 mg/mL) for 24 h was further preloaded with DCFH-DA (5 μmol/L) for 30 min followed by exposure to H2O2(1 mmol/L) for 15 min. The intracellular ROS level was measured as compared to DCF fluorescence intensity. The cell viability was determined using neutral red assay. Data are expressed as mean±SEM of three independent experiments. Each performed in triplicate.*P<0.05 compared to vehicle control, and#P<0.05 compared to H2O2-treated cells without TSCE.
3.2. Effect of TSCE on intracellular GSH level
The effect of TSCE in the absence and presence of H2O2on intracellular GSH was investigated using DTNB-GSSG reductase recycling assay. Treatment of the cells with H2O2(1 mmol/L) for 30 min significantly decreased GSH level with low effect on cell viability (84.56%). The result showed that TSCE significantly increased total GSH level with the absence or presence of H2O2in a concentration-dependent manner (Figure 2). Noticeably, the potency of TSCE in H2O2-treated cells was 2-fold stronger than TSCE treated alone.
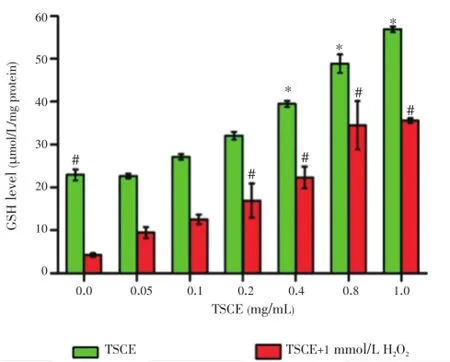
Figure 2. Effect of TSCE on total GSH level.The cells were pretreated with TSCE (0.05-1 mg/mL) for 24 h and then exposed to H2O2(1 mmol/L) for 30 min. Total GSH level was determined using DTNB-GSSG reductase recycling assay. Data are expressed as mean±SEM of three independent experiments.*P<0.05 compared to control, and#P<0.05 compared to H2O2-treated cells.
3.3. Effect of TSCE on antioxidant enzyme activity
The role of enzymatic antioxidant system was further investigated. The result showed that TSCE treated alone at the concentration to decrease intracellular ROS significantly elevated SOD and CAT activities (Figure 3A). However, TSCE did not increase the activities of SOD, GPx and CAT in H2O2-treated cells (Figure 3B).
3.4. Effect of TSCE on protein expression of antioxidant enzymes
To evaluate the role of antioxidant enzyme expression, Western blot analysis was performed. The result showed that TSCE, at the concentrations with significant increase in SOD and CAT activities (Figure 3A), did not alter the protein expression of Cu,Zn-SOD, GPx including CAT (Figure 4). However, TSCE in H2O2-treated cells significantly upregulated the protein expression of Cu,Zn-SOD and GPx but not for CAT as compared to the vehicle control (Figure 5).
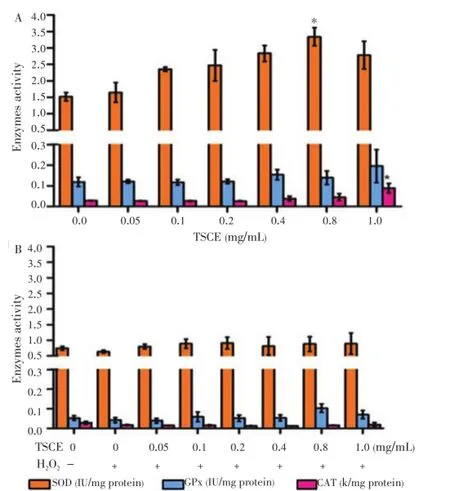
Figure 3. Effect of TSCE on SOD, GPx and CAT activities.The cells were treated with TSCE (0.05-1 mg/mL) for 24 h either TSCE alone (A) or in the presence of H2O2(2 mmol/L) for 15 min for SOD and 30 min for GPx and CAT (B). The activities of SOD, GPx and CAT were determined. Data are expressed as mean±SEM of three independent experiments.*P < 0.05 compared to vehicle control.
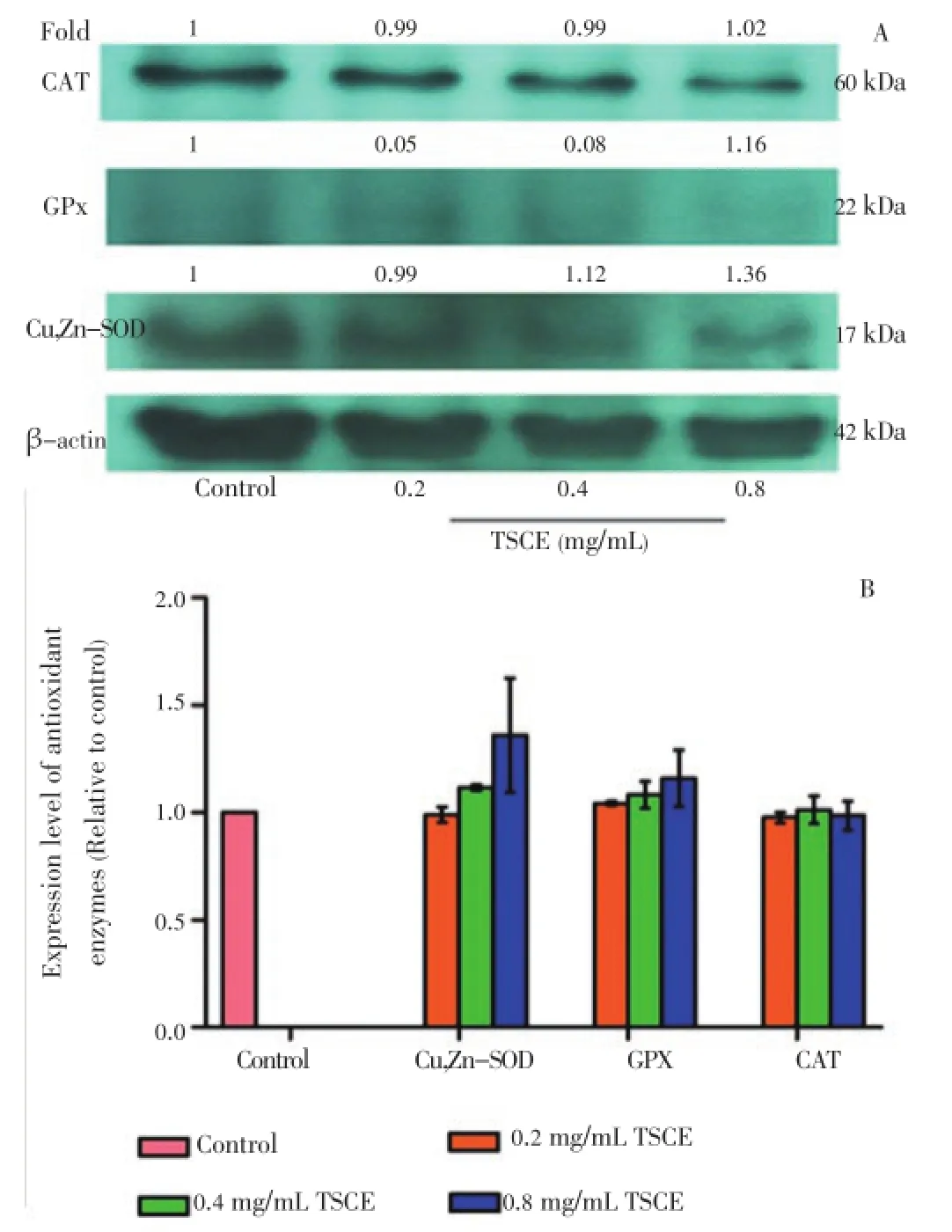
Figure 4. Effect of TSCE on protein expression of Cu,Zn-SOD, GPx and CAT.The cells were treated with TSCE (0.2-0.8 mg/mL) for 24 h. The cell lysate was subjected to electrophoresis on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis with subsequent enzyme immunoblot assay. (A) Representative immunoblot and (B) corresponding cumulative data. The intensity of each band was normalized using β-actin. Data are expressed as mean±SEM of three independent experiments.

Figure 5. Effect of TSCE on protein expression of Cu,Zn-SOD, GPx, and CAT in H2O2-treated cells.The cells were treated with TSCE at 0.4-0.8 mg/mL for 24 h followed by 1 mmol/L H2O2for 3 h. (A) Cu,Zn-SOD, GPx, and CAT protein levels and (B) the intensity of each band after incubated with 1 mmol/L H2O2for 3 h. Data are expressed as mean±SEM (n=3).*P<0.05 compared to the vehicle control.
4. Discussion
It is well known that oxidative stress play an important role in aging and associated various diseases[1,7]. The scavenging ability of ROS, cytoprotective effects, antioxidant enzyme activity, and antioxidant mechanism of natural antioxidants are widely investigated[6,24,25]. The present study contributed to our preliminary report showed the underlying antioxidant defense mechanism of tamarind seed coat in CCD-1064Sk cells under oxidative stress. Our unpublished data also found that TSCE composed of phenolic compounds such as (+)-catechin, (-)-epicatechin and procyanidin B2, which is similar to the previous reports[13,26], with its concentrations ranging from 0.002 to 1 mg/mL, and it also possessed various antioxidant activities in cell-free system without affecting cell viability. We therefore determined that whether TSCE at the nontoxic concentrations could attenuate intracellular ROS level via an elevation of the non-enzymatic and enzymatic antioxidant system in the absence and presence of oxidative stress. H2O2, a precursor of ROS, was commonly applied as an oxidant reagent in the studies of various cell lines[24,25,27].
Therefore, H2O2was used to induce cellular stress in this study.
Our result found that TSCE possessed activity to scavenge ROS under normal and oxidative stress conditions in CCD-1064Sk cells, it may be related to the O2●-, OH●
, and H2O2scavenging ability of tamarind seed coat in cell-free system which was observed in our laboratory as well as in other published documents[15,26]. The potential ability of TSCE to scavenge ROS was due to an evaluation of GSH, which is a major intracellular non-enzymatic antioxidant in the detoxification of varieties of electrophilic compounds and peroxide via catalysis by glutathione S-transferase and GPx[28]. This result indicated that tamarind seed coat may be able to elevate GSH level in CCD-1064Sk cells as same as the study in the bark of tamarind[29].
Moreover, our result also demonstrated that TSCE elevated SOD and CAT activities but did not affect on GPx activity. Considering antioxidant enzymes activity in oxidative stress condition, H2O2at the IC50value as our study was also used to study the decrease of SOD, GPx and CAT activities in various cell lines such as osteoblast[27], Chinese hamster lung fibroblast (V79-4)[30] and human dermal fibroblast[31]. We therefore selected H2O2at the concentration of 2 mmol/ L (IC50) in our testing model. The result showed that TSCE did not increase SOD, GPx and CAT activities due to the presence of high level of H2O2. Our finding was agreed with the previous reports, demonstrating that SOD played a major role to scavenge superoxide anion but did not scavenge H2O2[2]. Surprisingly, our study revealed that TSCE increased GSH level, one of GPx substrates, but did not increase GPx activity. This result revealed that the activity of GPx is independent on GSH level in case of initial concentration of GPx is higher than initial concentration of H2O2[32]. This finding was corresponding with some documents reported that the elevation of GSH did not affect on GPx activity[33,34] while it may affect on glutathione reductase or glutathione S-transferase[33]. Moreover, various concentrations of H2O2effect on antioxidant enzymes responded differently[35]. Therefore, to gain insight into the mechanism of these enzyme systems, the effect of TSCE on antioxidant enzymes activity in oxidative stress induced by various concentration of H2O2will be further studied.
TSCE did not increase the antioxidant enzyme expression in normal condition. However, TSCE could up-regulate the protein expression of Cu,Zn-SOD and GPx under oxidative stress at sub-concentration of H2O2. This finding was corresponding with the previous reports, revealing that the expression of anti-oxidative or protective genes were observed[36,37].
In summary, this study clearly demonstrated that TSCE exhibited antioxidant activity by increasing GSH level inCCD-1064Sk cells under normal and mild oxidative stress condition induced by H2O2. Tamarind seed coat activated the antioxidant enzyme activity of SOD and CAT under normal cell condition as well as enhanced the protein expression of SOD and GPx under mild oxidative stress. Tamarind seed coat phenolic compounds can be simply extracted with boiling water, the extract may be used as a natural source of antioxidant and protective agent that may be useful as health promoting food and cosmeceutical application for skin aging.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This research was supported by a grant under the Strategic Scholarships for Frontier Research Network program for the Ph.D. Program Thai Doctoral degree from the Office of the Higher Education Commission, Thailand, (Grant No.9/2551) and the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), Chulalongkorn University, Thailand, (Grant No. 1/26).
Comments
Background
Tamarindus indicaL. is originally native tree to Africa and widely used in various traditional medicine and food products. The seed of this plant consists of a kernel and seed coat. The seed coat is a rich source of tannins and polyphenols which possesses antiallergic and antimicrobial, antibiotic, antityrosinase, and antioxidant activities. However, non-enzymatic and enzymatic antioxidant activities of tamarind seed coat at cellular level are still unexplored
Research frontiers
A new information on a local herb study center from a developing tropical countries. The information is interesting and can be further reference in ethnopharmacology.
Related reports
There are some previous reports on ethnopharmacology studies from Thailand. However, those previous ones did not focus on the present studied herbs. Hence, the work can be a good original data for followers.
Innovations and breakthroughs
The study is a proof of the property of ethnopharmaceuticals and it can be further developed for new drugs in the field that can be useful for the local and regional medicine.
Applications
As note, the result can be further references in herbal medicine and drug development. This kind of study is not widely available. The application can be warranted.
Peer review
This work described an attempt to study on the local herbs collected from a country in Southeast Asia. The authors used standard method in ethnopharmacology to study the property of the collected herb. This work is a good example in ethnopharmacological studies and can be further applied for further usage in tropical medicine.
[1] Sarma AD, Mallick AR, Ghosh AK. Free radicals and their role in different clinical conditions: an overview. Int J Pharm Sci Res 2010; 1(3): 185-192.
[2] Sen S, Chakraborty R, Sridhar C, Reddy YS, De B. Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. Int J Pharm Sci Rev Res 2010; 3(1): 91-100.
[3] Ivani?ová E, Tokár M, Mocko K, Bojňanská T, Mare?ek J, Mendelová A. Antioxidant activity of selected plant products. J Microbiol Biotechnol Food Sci 2013; 2: 1692-1703.
[4] Maizura M, Aminah A, Wan Aida WM. Total phenolic content and antioxidant activity of kesum (Polygonum minus), ginger (Zingiber officinale) and turmeric (Curcuma longa) extract. Int Food Res J 2011; 18(2): 529-534.
[5] Chidambaram U, Pachamuthu V, Natarajan S, Elango B, Suriyanarayanan, Ramkumar KM. In vitro evaluation of free radical scavenging activity of Codariocalyx motorius root extract. Asian Pac J Trop Med 2013; 6(3): 188-194.
[6] Yoo KM, Lee CH, Lee H, Moon B, Lee CY. Relative antioxidant and cytoprotective activities of common herbs. Food Chem 2008; 106(3): 929-936.
[7] Deore SL, Kombade S, Baviskar BA, Khadabadi SS. Photoprotective antioxidant phytochemicals. Int J Phytopharm 2012; doi: 10.7439/ijpp.v2i3.501.
[8] Bhadoriya SS, Ganeshpurkar A, Narwaria J, Rai G, Jain AP. Tamarindus indica: extent of explored potential. Pharmacogn Rev 2011; 5(9): 73-81.
[9] Jittanit W, Chantara-In M, Deying T, Ratanavong W. Productionof tamarind powder by drum dryer using maltodextrin and Arabic gum as adjuncts. Songklanakarin J Sci Technol 2011; 33(1): 33-41.
[10] Vadivel V, Pugalenthi M. Evaluation of nutritional value and protein quality of an under-utilized tribal food legume. Indian Journal of Traditional Knowledge 2010; 9(4): 791-797.
[11] Tewtrakul S, Itharat A, Thammaratwasik P, Ooraikul B. Antiallergic and anti-microbial activities of some Thai crops. Songklanakarin J Sci Technol 2008; 30(4): 467-473.
[12] Aengwanich W, Suttajit M, Srikhun T, Boonsorn T. Antibiotic effect of polyphenolic compound extracted from tamarind (Tamarindus indica L.) seed coat on productive performance of broilers. Int J Poult Sci 2009; 8(8): 749-751.
[13] Kanlayavattanakul M, Lourith N. Biologically active phenolics in seed coat of three sweet Tamarindus indica varieties grown in Thailand. Adv Sci Eng Med 2012; 4(6): 511-516.
[14] Lourith N, Kanlayavattanakul M, Chanpirom S. Free radical scavenging efficacy of tamarind seed coat and its cosmetics application. J Health Res 2009; 23: 159-162.
[15] Suksomtip M, Ukrisdawithid S, Bhusawang P, Pongsamart S. Phenolic compound content, antioxidant and radical-scavenging properties of methanolic extracts from the seed coat of certain Thai tamarind cultivars. J Food Biochem 2010; 34(5): 916-931.
[16] Ghaffari A, Li Y, Kilani RT, Ghahary A. 14-3-3 sigma associates with cell surface aminopeptidase N in the regulation of matrix metalloproteinase-1. J Cell Sci 2010; 123(17): 2996-3005.
[17] Kang SG, Li J, Tong T, Ko DO, Chung DO, Jeong WC, et al. Antioxidant and anti-skin-aging effects of abalone viscera extracts in human dermal fibroblasts. Korean J Food Preserv 2012; 19(4): 463-469.
[18] Khalid S, Shaik Mossadeq WM, Israf DA, Hashim P, Rejab S, Shaberi AM, et al. In vivo analgesic effect of aqueous extract of Tamarindus indica L. fruits. Med Princ Pract 2010; 19: 255-259.
[19] Shahzad A, Cohrs RJ. In vitro antiviral activity of honey against varicella zoster virus (VZV): a translational medicine study for potential remedy for shingles. Transl Biomed 2012; 3(2): 2.
[20] Girard-Lalancette K, Pichette A, Legault J. Sensitive cell-based assay using DCFH oxidation for the determination of pro- and antioxidant properties of compounds and mixtures: analysis of fruit and vegetable juices. Food Chem 2009; 115(2): 720-726.
[21] Chirdchupunseree H, Pramyothin P. Protective activity of phyllanthin in ethanol-treated primary culture of rat hepatocytes. J Ethnopharmacol 2010; 128(1): 172-176.
[22] Agar G, Aslan A, Sarioglu EK, Alpsoy L, Ceker S. Protective activity of the methanol extract of Usnea longissima against oxidative damage and genotoxicity caused by aflatoxin B1in vitro. Turk J Med Sci 2011; 41(6): 1043-1049.
[23] Vives-Bauza C, Starkov A, Garcia-Arumi E. Measurements of the antioxidant enzyme activities of superoxide dismutase, catalase, and glutathione peroxidase. Methods Cell Biol 2007; 80: 379-393.
[24] Zhang R, Chae S, Kang KA, Piao MJ, Ko DO, Wang ZH, et al. Protective effect of butin against hydrogen peroxide-induced apoptosis by scavenging oxygen species and activating antioxidant enzymes. Mol Cell Biochem 2008; 318(1-2): 33-42.
[25] Bayati S, Yazdanparast R. Antioxidant and free radical scavenging potential of yakuchinone B derivatives in reduction of lipofuscin formation using H2O2-treated neuroblastoma cells. Iran Biomed J 2011; 15(4): 143-142.
[26] Razali N, Mat-Junit S, Abdul-Muthalib AF, Subramaniam S, Abdul-Aziz A. Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins and skins of Tamarindus indica L. Food Chem 2012; 131(2): 441-448.
[27] Manan NA, Mohamed N, Shuid AN. Effect of low-dose versus high-dose γ-tocotrienol on the bone cells exposed to the hydrogen peroxide-induced oxidative stress and apoptosis. Evid Based Complement Alternat Med 2012; doi: 10.1155/2012/680834.
[28] Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, et al. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev 2013; doi: 10.1155/2013/972913.
[29] Bhutkar MA, Bhise SB. Anti-oxidative effect of Tamarindus indica in alloxan induced diabetic rats. Int J Res Pharm Biomed Sci 2011; 2(3): 1006-1009.
[30] Ju EM, Lee SE, Hwang HJ, Kim JH. Antioxidant and anticancer activity of extract from Betula platyphylla var. japonica. Life Sci 2004; 74(8): 1013-1026.
[31] Feng B, Ma LJ, Yao JJ, Fang Y, Mei YA, Wei SM. Protective effect of oat bran extracts on human dermal fibroblast injury induced by hydrogen peroxide. J Zhejiang Univ Sci B 2013; 14(2): 97-105.
[32] Ng CF, Schafer FQ, Buettner GR, Rodgers VG. The rate of cellular hydrogen peroxide removal shows dependency on GSH: mathematical insight into in vivo H2O2and GPx concentrations. Free Radic Res 2007; 41(11): 1201-1211.
[33] Ewertowska M, Jodynis-Liebert J, Kujawska M, Adamska T, Matlawska I, Szaufer-Hajdrych M. Effect of Aquilegia vulgaris (L.) ethyl ether extract on liver antioxidant defense system in rats. Int J Occup Med Environ Health 2009; 22(2): 115-123.
[34] Shtukmaster S, Ljubuncic P, Bomzon A. The effect of an aqueous extracts of Teucrium polium on glutathione homeostasis in vitro: a possible mechanism of its hepatoprotectant action. Adv Pharmacol Sci 2010; doi: 10.1155/2010/938324.
[35] Wijeratne SS, Cuppett SL, Schlegel V. Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J Agric Food Chem 2005; 53(22): 8768-8774.
[36] Zheng Y, Liu Y, Ge J, Wang X, Liu L, Bu Z, et al. Resveratol protects human lens epithelial cells against H2O2-induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol Vis 2010; 16: 1467-1474.
[37] Murray JI, Whitfield ML, Trinklein ND, Myers RM, Brown PO, Botstein D. Diverse and specific gene expression responses to stresses in cultured human cells. Mol Biol Cell 2004; 15(5): 2361-2374.
10.12980/APJTB.4.2014C806
*Corresponding author: Sunanta Pongsamart, Department of Biochemistry and Microbiology, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, 10330, Thailand.
Tel: +66-2-218-8372
Fax: +66-2-218-8375
E-mail: Sunanta.po@chula.ac.th
Foundation Project: Supported by a grant under the Strategic Scholarships for Frontier Research Network program (Grant No.9/2551) and the 90th Anniversary of Chulalongkorn University Fund, Chulalongkorn University, Thailand, (Grant No. 1/26).
Article history:
Received 1 Mar 2014
Received in revised form 7 Mar, 2nd revised form 13 Mar, 3rd revised form 20 Mar 2014
Accepted 30 Mar 2014
Available online 28 May 2014
Methods:Tamarind seed coats were extracted with boiling water and then partitioned with ethyl acetate before the cell analysis. Effect of TSCE on intracellular reactive oxygen species (ROS), glutathione (GSH) level, antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase activity including antioxidant protein expression was investigated.Results:TSCE significantly attenuated intracellular ROS in the absence and presence of H2O2by increasing GSH level. In the absence of H2O2, TSCE significantly enhanced SOD and catalase activity but did not affected on GPx. Meanwhile, TSCE significantly increased the protein expression of SOD and GPx in H2O2-treated cells.
Conclusions:TSCE exhibited antioxidant activities by scavenging ROS, attenuating GSH level that could protect human skin fibroblast cells from oxidative stress. Our results highlight the antioxidant mechanism of tamarind seed coat through an antioxidant enzyme system, the extract potentially benefits for health food and cosmeceutical application of tamarind seed coat.
 Asian Pacific Journal of Tropical Biomedicine2014年5期
Asian Pacific Journal of Tropical Biomedicine2014年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Ethnobotanical survey of folklore plants used in treatment of snakebite in Paschim Medinipur district, West Bengal
- Pharmacognostic studies of stem, roots and leaves of Malva parviflora L.
- Rapid detection of coliforms in drinking water of Arak city using multiplex PCR method in comparison with the standard method of culture (Most Probably Number)
- Salvia fruticosa reduces intrinsic cellular and H2O2-induced DNA oxidation in HEK 293 cells; assessment using flow cytometry
- Antisickling activity of butyl stearate isolated from Ocimum basilicum (Lamiaceae)
- Antioxidant and antimicrobial properties of Litsea elliptica Blume and Litsea resinosa Blume (Lauraceae)
