Anti-diabetic effects of Caulerpa lentillifera: stimulation of insulin secretion in pancreatic β-cells and enhancement of glucose uptake in adipocytes
Bhesh Raj Sharma, Dong Young Rhyu
Department of Oriental Medicine Resources and Institute of Korean Medicine Industry, Mokpo National University, Jeonnam 534-729, Republic of Korea
Anti-diabetic effects of Caulerpa lentillifera: stimulation of insulin secretion in pancreatic β-cells and enhancement of glucose uptake in adipocytes
Bhesh Raj Sharma, Dong Young Rhyu*
Department of Oriental Medicine Resources and Institute of Korean Medicine Industry, Mokpo National University, Jeonnam 534-729, Republic of Korea
PEER REVIEW
Comments
This is an ideal work to discover an alternative source for anti-diabetic agent. Authors comprehensively worked in anti-diabetic by using C. lentifera extract. The material and methods are well constructed and findings are well represented.
Details on Page 579
Peer reviewer
Ariffin Siti, Faculty of Pharmacy, Universiti Teknologi Mara, 40450 Shah Alam, Selangor, Malaysia.
E-mail: alwani229@salam.uitm.edu.my
Objective:To evaluate anti-diabetic effect of Caulerpa lentillifera (C. lentillifera).
Methods:The inhibitory effect of C. lentillifera extract on dipeptidyl peptidase-IV and α-glucosidase enzyme was measured in a cell free system. Then, interleukin-1β and interferon-γ induced cell death and insulin secretion were measured in rat insulinoma (RIN) cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and ELISA kit, respectively. Glucose uptake and glucose transporter expression were measured by fluorometry and western blotting, using 3T3-L1 adipocytes.
Results:C. lentillifera extract significantly decreased dipeptidyl peptidase-IV and α-glucosidase enzyme activities, and effectively inhibited cell death and iNOS expression in interleukin-1β and interferon-γ induced RIN cells. Furthermore, C. lentillifera extract significantly enhanced insulin secretion in RIN cells and glucose transporter expression and glucose uptake in 3T3-L1 adipocytes.
Conclusions:Thus, our results suggest that C. lentillifera could be used as a potential antidiabetic agent.
Caulerpa lentillifera, Diabetes, Glucose uptake, Insulin secretion, RIN cells, 3T3-L1 adipocytes
1. Introduction
Diabetes mellitus is caused by impaired insulin production and/or by decreased tissue response to the insulin[1]. The number of people with diabetes is increasing worldwide; 366 million people have diabetes, and 552 million people are expected to have diabetes by 2030[2]. During the pathogenesis of diabetes, mononuclear cells, infiltrating in and around the islets, generate excessive cytokines that upregulate inducible nitric oxide synthase (iNOS) expression and subsequently NO production, which impaires insulin secretion[3]. Insulin is a potent inducer of adipogenesis and enhances glucose uptake through activation of insulin signaling and glucose transporter (GLUT4) in adipocytes and myocytes[4]. So, glucose uptake in adipocytes and myocytes is decreased in insulin deficient state by defective insulin signaling. There is a wide variety of pharmacological drugs used to treat diabetes, including sulfonylureas, thiazolidinediones, biguanides, dipeptidyl peptidase (DPP)-IV inhibitors, and α-glucosidase inhibitors. However, they are all associated with various side effects which limit them for long term use[5]. Most people in developing countries depend on alternative therapies, including natural resources for their primary health care[6]. However, such alternative therapies are not yet understood for diabetes and need to be better explored[7,8]. Marine algae have been usedas a functional food since prehistoric times. They are one of the less explored sources of pharmacological candidates, and few previous studies had found anti-diabetic activities in various marine algae[9].
Caulerpa lentillifera(C. lentillifera) is native to tropical areas of the Indian and Pacific Oceans, but it is currently discovered in the coast of Korea due to abnormal temperature.C. lentilliferais a popular edible species high in minerals, dietary fibers, vitamin A, vitamin C, and several essential unsaturated fatty acids, which is eaten fresh or salted for later use[10]. Recently, anti-cancer[11], antioxidative[12], and lipid-lowering[13] activities ofC. lentilliferaextract have been documented. Also,C. lentilliferahas been used traditionally in the Philippines for the treatment of diabetes. However, there are no experimental evidences. Therefore, our hypothesis is to investigate whetherC. lentilliferaextract increases insulin secretion and glucose uptake in pancreatic-β cells and adipocytes, respectively. To test the hypothesis, we measured the effect ofC. lentilliferaextract on DPP-IV and α-glucosidase enzymes usingin vitroenzyme assay. Subsequently, we measured insulin secretion and glucose uptake in rat insulinoma (RIN) cells and 3T3-L1 adipocytes, respectively.
2. Materials and methods
2.1. Reagents
2-[N-(7-nitrobenz-2-oxa-1, 3-diazol-4-yl) amino]-2-deoxyglucose (2-NBDG) was purchased from Invitrogen (Carlsbad, CA, USA). GLUT4 and iNOS antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Gly-Pro-AMC was purchased from Ana Spec, Inc. (San Jose, CA, USA). Interleukin (IL)-1β and interferon (IFN)-γ were purchased from R & D (Minneapolis, MN, USA), and rat insulin ELISA kit was purchased from Mercodia Developing diagnostics (Mercodia, Uppsala, Sweden). All reagents were purchased from Sigma (St. Louis, MO, USA), unless otherwise specified.
2.2. Plant material
C. lentilliferawas imported through Marine Global Ltd. (Okinawa, Japan), which was farmed in the Phillippines. The algae was extensively washed with tap water, and dried at room temperature for 1 h after removal of the holdfasts and epiphytes. The driedC. lentilliferawas extracted with ethanol for five times. Ethanol extract ofC. lentilliferawas then filtered, and evaporated under vacuum. The yield from the fresh dried sample was 2%.
2.3.Measurement ofDPP-IVandα-glucosidase activity
An aliquot of 10 μL serum was incubated with 25 μL of 50 mmol/L HEPES buffer (pH 7.4) containing the Gly-Pro-AMC substrate (final substrate concentration 1 mmol/L). Appropriate concentration of the sample was mixed with 25 μL of HEPES buffer. After 1 h incubation, the reaction was stopped by the addition of 70 μL of 3 mol/L acetic acid. Then, released AMC was measured using a fluorescence microplate reader, Perkin Elmer Victor3 V 1420 Multilabel Plate Counter (Perkin Elmer, USA) at an excitation wavelength of 370 nm and an emission wavelength of 440 nm.
Rat intestinal acetone powder was sonicated in normal saline (100:1, w/v), and after centrifugation at 3 000 r/min for 30 min, the supernatant was treated as a crude intestinal α-glucosidase. Ten microliters of the test samples were mixed and incubated with 50 μL of enzyme in a 96-well microplate for 5 min. The reaction mixture was further incubated another 10 min with 50 μL substrate [5 mmol/L, p-nitrophenyl-d-glucopyranoside, prepared in 100 mmol/L phosphate buffer (pH 6.8)]. Finally, the reaction was stopped by adding 50 μL of sodium carbonate (100 mmol/L), and the released nitrophenol was measured at 405 nm using a spectrophotometer (Immuno Mini NJ-2300, Japan).
2.4. Cell culture and cell viability assay
RIN and 3T3-L1 cells, as models for pancreatic β-cells and adipocytes, were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA), and grown in RPMI 1640 medium with 10% fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum, respectively. To induce differentiation, 3T3-L1 preadipocytes were cultured until confluence was reached (Day 0), and the culture medium was replaced with a fresh induction medium containing 5 μg/mL insulin, 0.5 mmol/L 3-isobutyl-1-methylxanthine, and 1 μmol/L dexamethasone in DMEM with 10% FBS for 2 d. The medium was then replaced with a differentiation medium containing 5 μg/mL insulin and DMEM with 10% FBS every 2 d for up to 8 d until the cells were harvested.
The viability of cultured cells was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, RIN (1×105cells/well) cells and 3T3-L1 (1×104cells/well) cells seeded in 96-well plates were treated with respective concentrations ofC. lentilliferaextract for 48 h. Then, MTT solution (1 mg/mL) was added to each well, and the cells were incubated at 37 °C for 1 h. Finally, dimethyl sulfoxide was added to dissolve the formazan crystals. Absorbance was measured at 540 nm using a spectrophotometer (Immuno Mini NJ-2300, Japan).
2.5. Measurement ofNOproduction
NO production was measured as a nitrite concentration in cell free culture supernatant using a colorimetric assay. Briefly, following treatment with sample and cytokines (IL-1β and IFN-γ) for 48 h, aliquots (100 μL) of culture supernatants were incubated with 100 μL of 1:1 mixture of 1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-naphthyl) ethyldiamine dihydrochloride at room temperature for 5 min, then absorbance at 540 nm was measured using a spectrophotometer (Immuno Mini NJ-2300, Japan). The concentration of NO was calculated by a linear standard curve generated from serial dilutions of sodium nitrite in working medium.
2.6. Measurement of insulin secretion
RIN cells were seeded at a density of 2×105cells/well in 24-well plates, and were treated with a sample (10 or 25 μg/ mL) and 100 μmol/L tolbutamide followed by treatment with cytokines for 48 h. Then, insulin released into the media was measured using the rat insulin ELISA kit according tomanufacturer’s instruction. The standard insulin provided in the kit was used to make the standard curve of insulin.
2.7. Oil Red O staining
Fully differentiated adipocytes were washed twice with phosphate buffer solution (PBS) and then fixed for 1 h at room temperature with 10% formalin, prepared in PBS. Then, cells were washed two times with PBS, and stained with a filtered Oil Red solution (0.5% in 60% isopropanol) for 1 h. Finally, cells were washed twice with distilled water, The fat droplets in 3T3-L1 adipocytes were observed by phase contrast microscopy.
2.8. Glucose uptake assay
3T3-L1 adipocytes grown in 96-black well plates were incubated with the sample (62.5 or 125 μg/mL) and 5 μmol/ L rosiglitazone (RSG) for 24 h at 37 °C in 5% CO2atmosphere. Subsequently, 250 μmol/L 2-NBDG (dissolved in PBS with 1% bovine serum albumin) was added to the cells and incubated for further 30 min. After incubation, cells were washed two times with PBS to remove excess fluorescence in the wells. Then, fluorescence retained by the cells was measured using the fluorescence microplate reader, Perkin Elmer Victor3 V 1420 Multilabel Plate Counter (Perkin Elmer, USA) at an excitation and emission wavelength of 485 nm and 535 nm, respectively.
2.9. Western blot
Cell lysates containing 30 μg of protein were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Then, it was transferred to nitrocellulose sheet in a western blot apparatus (Bio-Rad, Hercules, CA, USA). The nitrocellulose sheet was blocked with 5% skim milk for 1 h, rinsed by Tris-buffered-saline with Tween (1 mol/L Tris, 5 mol/L NaCl, and 0.1% Tween 20), and incubated for overnight with primary antibodies of iNOS and GLUT4. Nitrocellulose membrane was again washed with Trisbuffered-saline with Tween followed by incubation with secondary antibody (horseradish peroxidase-conjugated IgG 1:2 000) for 1 h. Finally, the expressed proteins were measured by analyzing the signal captured in nitrocellulose membrane using chemiluminescent substrate and VisionWorksTMLS (Analysis software, Upland, CA, USA).
2.10. Statistical analysis
SPSS (SPSS, Inc. Chicago, USA) was used for statistical data analysis. All data were presented as mean±SE. Groups were compared by using ANOVA, followed by Duncan’spost hoctest of multiple comparisons.P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Effect of C. lentillifera extract onDPP-IVandα-glucosidase enzyme inhibition
DPP-IV inhibitors stimulated insulin secretion by regulating the release of incretin hormones, whereas α-glucosidase inhibitors decreased postprandial blood glucose by reducing the digestion of carbohydrates[14]. Inhibitory activities ofC. lentilliferaextract on DPP-IV and α-glucosidase enzymes are shown in Figure 1.C. lentilliferaextract inhibited enzyme activity in a concentration-dependent manner, and 1 000 μg/ mL of the extract resulted in 46.1% and 42.9% inhibition of DPP-IV and α-glucosidase activity, respectively.
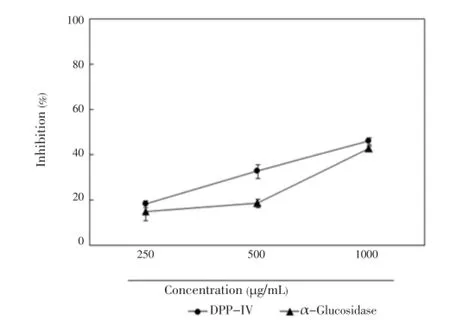
Figure 1. Effect of C. lentillifera extract on DPP-IV and α-glucosidase enzyme inhibition.Results are expressed as mean±SE of percent inhibition of three independent experiments.
3.2. Effect of C. lentillifera extract onIL-1βandIFN-γinduced cell viability inRINcells
The combination of IL-1β and IFN-γ caused pancreatic β-cell death, which is correlated with decreased insulin secretion in RIN cells.C. lentilliferaextract did not affect cell cytotoxicity at concentration of 25 μg/mL (Figure 2A). As shown in Figure 2B, IL-1β and IFN-γ decreased cell viability to 44.8%. However, the addition ofC. lentilliferaextract (10 and 25 μg/mL) increased cell viability by up to 61.3% and 65.9%, respectively.
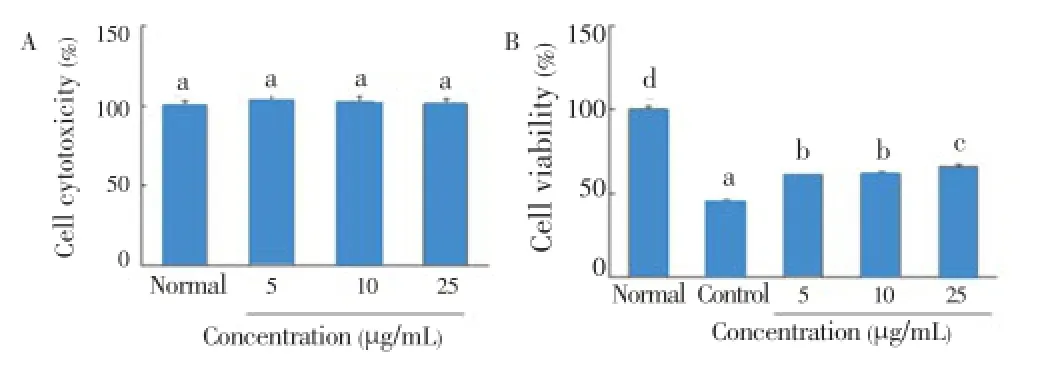
Figure 2. Effect of C. lentillifera extract on cytotoxicity (A) and IL-1β and IFN-γ induced cell viability (B) in RIN cells.RIN cells were incubated with sample for 48 h, and after 3 h of sample treatment RIN cells were exposed by IL-1β (2 ng/mL) and IFN-γ (100 IU/mL) for 48 h. Cell viability was determined by MTT assay. Each value represents the mean±SE of three independent experiments. Bars with different letters are significantly different at P<0.05 b in Duncan’s multi comparison tests.
3.3. Effect of C. lentillifera extract onIL-1βandIFN-γinducedNOproduction and iNOSexpression inRINcells
IL-1β and IFN-γ induced pancreatic β-cell death via NO production and iNOS expression[15]. As shown in Figure 3A, incubation of RIN cells with IL-1β and IFN-γ for 48 h resulted in significant production of NO (30.7 μmol/L). However, 10and 25 μg/mL ofC. lentilliferaextract significantly decreased NO production in cytokine-induced RIN cells (from 30.7 μmol/L to 24.7 and 21.6 μmol/L, respectively). Also, the concentration of 10 and 25 μg/mL ofC. lentilliferaextract dose-dependently inhibited expression of iNOS protein (Figure 3B).
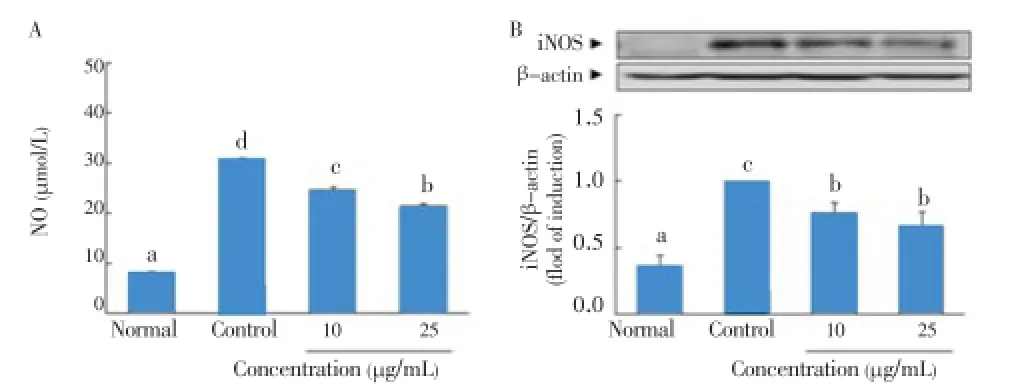
Figure 3. Effect of C. lentillifera extract on IL-1β and IFN-γ induced NO production (A) and iNOS expression (B) in RIN cells.RIN cells were pretreated with indicated concentration of sample for 3 h, followed by stimulation with IL-1β (2 ng/mL) and IFN-γ (100 IU/mL) for 48 h. Then, NO production and iNOS protein expression were determined by colorimetry and western blotting, respectively. Each value represents the mean±SE of three independent experiments. Bars with different letters are significantly different at P<0.05 in Duncan’s multi comparison tests.
3.4. Effect of C. lentillifera extract onIL-1βandIFN-γinduced insulin secretion inRINcells
IL-1β and IFN-γ dramatically decreased insulin secretion from 13.5 to 6.2 ng/mL in RIN cells (Figure 4). However, the addition of 10 and 25 μg/mL ofC. lentilliferaextract efficiently increased insulin secretion from a baseline of 6.2 to 10.7 and 12.0 ng/mL, respectively, which was comparable to the insulin secretory activity by tolbutamide as a positive control.
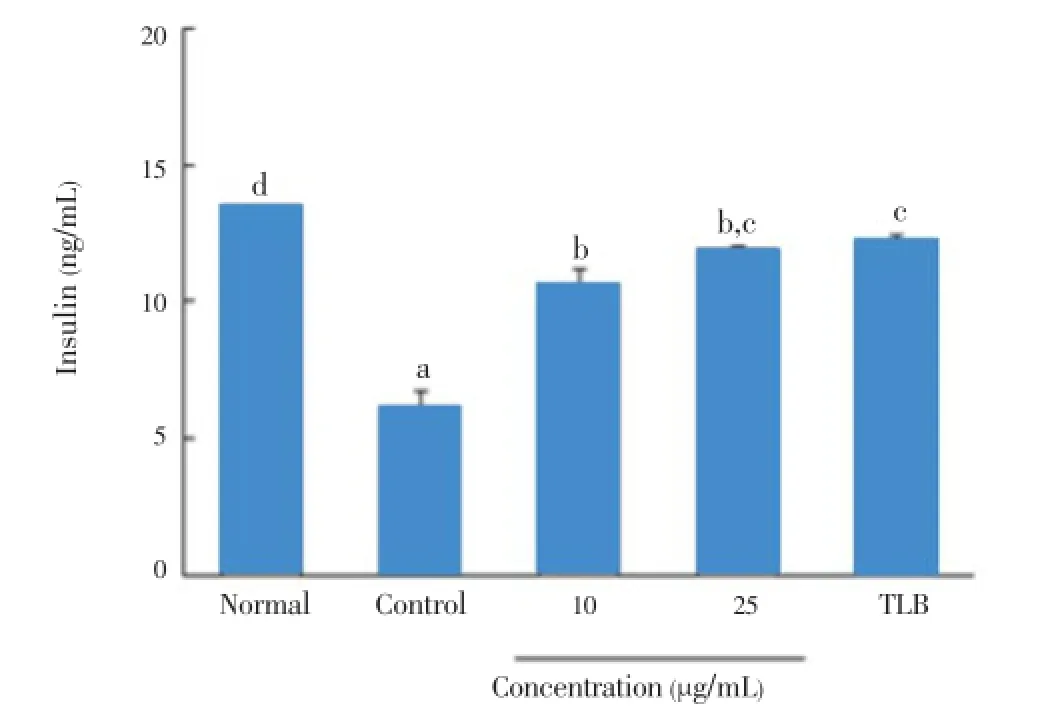
Figure 4. Effect of C. lentillifera extract on IL-1β and IFN-γ induced insulin secretion in RIN cells.RIN cells were pretreated with indicated concentrations of sample for 3 h, followed by stimulation with IL-1β (2 ng/mL) and IFN-γ (100 IU/mL) for 48 h. Then, insulin secretion was determined in cell free culture supernatant by using Rat Insulin ELISA kit. About 100 μmol/L TLB was used as a standard drug. Each value represents the mean±SE of three independent experiments. Bars with different letters are significantly different at P<0.05 in Duncan’s multi comparison tests.
3.5. Effect of C. lentillifera extract on cell cytotoxicity and adipogenesis of3T3-L1cells
As shown in Figure 5A,C. lentilliferaextract did not cause cell cytotoxicity at the concentration of 125 μg/mL. Additionally,C. lentilliferaextract significantly enhanced adipogenesis in a dose-dependent manner, which was comparable to RSG as an anti-diabetic drug (Figure 5B). Also, the Oil Red O content byC. lentilliferaextract was significantly greater than that of control, and its value was comparable to RSG (Figure 5C).
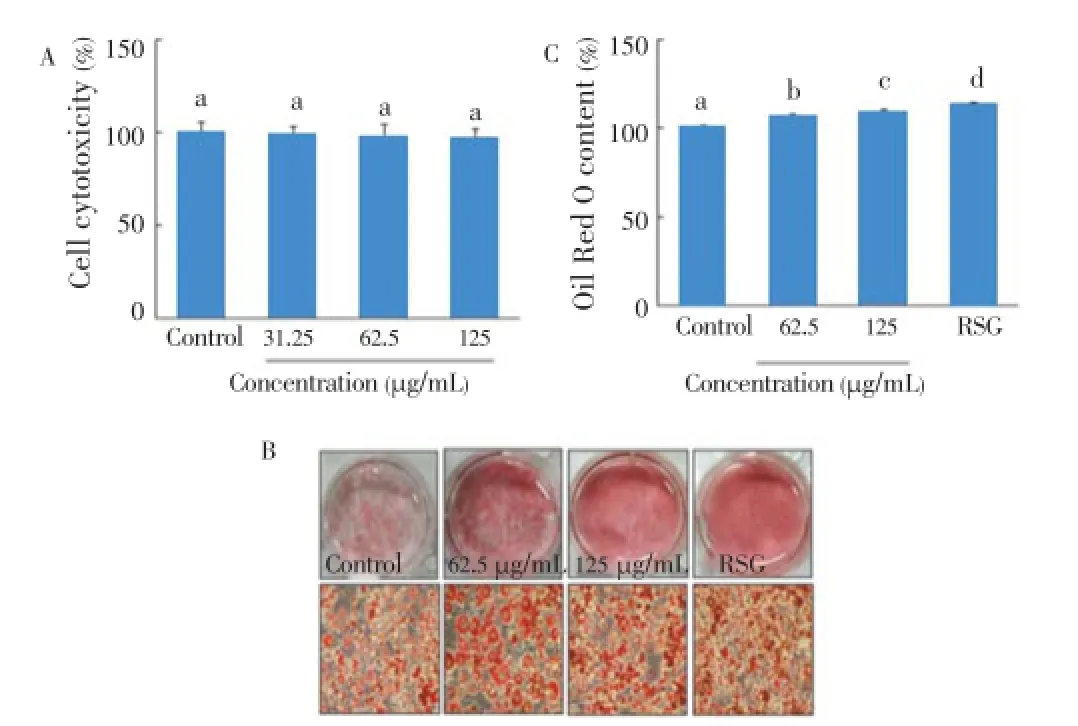
Figure 5. Effect of C. lentillifera extrac on cytotoxicity (A), adipogenesis (B), and Oil Red O content (C) in 3T3-L1 cells.Confluent 3T3-L1 preadipocytes were differentiated into adipocytes for 8 d in presence of sample, and cell viability was measured by MTT assay, adipogenesis was measured by staining with Oil Red O dye, and Oil Red O content was determined by measuring absorbance at 540 nm. RSG (5 μmol/ L) was used as a positive control. Each value represents the mean±SE of three independent experiments. Bars with different letters are significantly different at P<0.05 b in Duncan’s multi comparison tests.
3.6. Effect of C. lentillifera extract on glucose uptake andGLUT4expression in3T3-L1cells
The effect ofC. lentilliferaextract on glucose uptake was tested using fully differentiated adipocytes. As shown in Figure 6A,C. lentilliferaextract significantly enhanced glucose uptake in a concentration-dependent manner. The results showed thatC. lentilliferaextract increased glucose uptake by 1.6 and 2 fold at 62.5 and 125 μg/mL, respectively. Similarly,C. lentilliferaextract also increased GLUT4 expression in a concentration-dependent manner (Figure 6B). The results showed that 125 μg/mL ofC. lentilliferaextract significantly increased GLUT4 expression by 1.4 fold, whereas 5 μmol/L of RSG increased GLUT4 expression by 1.6 fold.
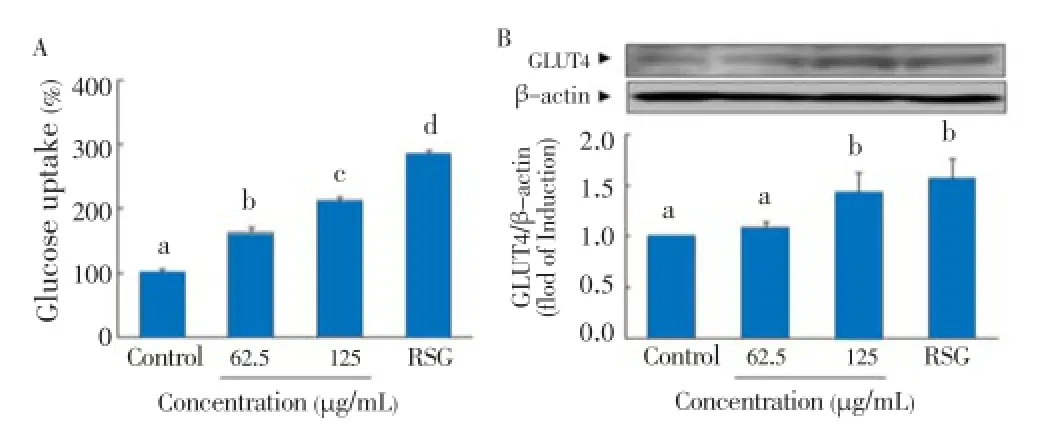
Figure 6. Effect of C. lentillifera extract on glucose uptake (A) and GLUT4 expression (B) in 3T3-L1 cells.Confluent 3T3-L1 preadipocytes were differentiated into adipocytes for 8 d. Fully differentiated 3T3-L1 adipocytes were treated with sample for 24 h, glucose uptake was measured using 2-NBDG, a fluorescent derivative of glucose as a glucose analogue by fluorometry, and GLUT4 expression was measured by western blot. RSG (5 μmol/L) was used as a positive control. Each value represents the mean±SE of three independent experiments. Bars with different letters are significantly different at P<0.05 in Duncan’s multi comparison tests.
4. Discussion
Diabetes is caused by diverse risk factors and is associated with abnormal metabolism of glucose. Ideal anti-diabetic agents not only preserve functional beta cell mass, but also enhance glucose uptake in most insulin target tissues[15]. In this study, we found thatC. lentilliferaextract significantly increased insulin secretion from RIN cells and enhanced glucose uptake in 3T3-L1 adipocytes.C. lentilliferaextract preserved functional β-cell mass from cytokine-induced injury and decreased NO and iNOS production. Additionally, this study demonstrated thatC. lentilliferaextract increased preadipocyte differentiation, GLUT4 expression, and glucose uptake in mature adipocytes.
In cell culture systems, exposure to a mixture of cytokines released excessive NO production, caused death of pancreatic β-cells, and decreased insulin secretion. This mechanism is similar to the destruction of human pancreatic β-cells in type 1 diabetic patients[16]. We found that the treatment of RIN cells with cytokines increased iNOS expression and NO production, and decreased cell viability and insulin secretion. However, the addition ofC. lentilliferaextract protected RIN cells from cytokine-induced injuries and decreased NO and iNOS activation in a dose-dependent manner. In consistent with our results, Songet al.reported that Fructus Xanthii extract increases cell viability and decreases NO and iNOS production in cytokine-treated RIN cells[17]. Recently, several algal plants with high phenolic contents have been reported to have anti-diabetic activities in cell culture system and animal model system of diabetes, thereby intensifying insulin secretion, which is also similar to our results[18].
Following the experiment on β-cells, we measured the effect ofC. lentilliferaextract on adipogenesis, glucose uptake, and GLUT4 expression in adipocytes as an insulin target tissue. Insulin signaling, after binding to its specific receptor in adipocytes, regulates the storage of free fatty acids in the form of triglyceride; however, massive adipocytes produce a number of adipocytokines such as tumor necrosis factor-α and IL-6 that consequently modulate insulin signaling with excess free fatty acids[19]. Therefore, adipocytes of type 2 diabetic patients are not capable of accumulating lipid, which results in insulin resistance[20]. Recently, antidiabetic activity of different seaweeds has been explored using 3T3-L1 adipocytes[21]. We discovered thatC. lentilliferaextract increased lipid accumulation, and significantly augmented glucose uptake and GLUT4 expression in 3T3-L1 adipocytes. The ideal drugs for the treatment and prevention of diabetes should be capable of enhancing both insulin secretion and glucose uptake; however, most of the antidiabetic drugs either intensify insulin secretion in pancreatic β-cells or enhance glucose uptake in insulin sensitive tissues. Interestingly, we found thatC. lentilliferaextract not only enhances insulin secretion in pancreatic β-cells but also increases insulin sensitivity and glucose uptake in 3T3-L1 adipocytes.
DPP-IV enzyme plays an important role in glucose metabolism by intensifying insulin secretion. It degrades incretin hormones, such as glucagon-like peptide-1 and gastric inhibitory polypeptide, that play an important role in insulin secretion from pancreatic β-cells[22]. Therefore, DPP-IV inhibitors had been developed as a new class of drug for the treatment of type 2 diabetes over the past few years. Guaschet al.found novel human DPP-IV inhibitors from extracts of plants known to have anti-diabetic effects[23]. In this study, we found thatC. lentilliferaextract inhibited both DPP-IV and α-glucosidase enzymes.
C. lentillifera, as a marine resource, contains a diverse type of chemical constituents, including from polyphenols and sterols to vitamins and minerals[13]. The synergistic action of such elements would augment anti-diabetic activities. However, to address the key issue of whetherC. lentilliferaextract could increasein-vivoinsulin secretion and glucose uptake, animal based studies on its anti-diabetic activity and the phytochemical analysis are required. Our current study provides an inceptive stage in exploring anti-diabetic activity ofC. lentilliferaextract. Its mechanism is associated not only with an elevation of insulin secretion by prevention of NO and iNOS production from cytokine-induced RIN cells, but also with increased glucose uptake via GLUT4 expression in 3T3-L1 adipocytes.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (Grant No. 2012R1A1A2009172).
Comments
Background
Diabetes mellitus is caused by impaired insulin production and/or by decreased tissue response to the insulin. The number of people with diabetes is increasing worldwide; 366 million people have diabetes. There is a wide variety of pharmacological drugs used to treat diabetes. However, they are all associated with various side effects which limit them for long term use.
Research frontiers
The author found thatC. lentiferaextract significantly decreased DPP-IV and alpha glucosidase enzyme activitiesand effectively inhibited cell death and iNOS expression in IL-1β and IFN-γ induced RIN cells. It was also effectively enhanced insulin secretion in RIN cells and GLUT4epression and glucose uptake in 3T3-L1 adipocytes.
Related reports
So far several works have been done on anti-diabetic activities from alga/seaweed, but for first time the present work revealed the anti-diabetics effect ofC. lentifera. Hence the present work is considered to be the first report. The result also suggested theC. lentiferaextract not only elevated insulin secretion but also increased glucose uptake in 3T3-L1 adipocytes.
Innovations and breakthroughs
Several researches were carried out on the anti-cancer, anti-oxidative and lipid-lowering properties ofC. lentifera. In the present study, authors have extensively worked on the anti-diabetic effect ofC. lentiraferaon RIN cell and 3T3-L1 adipocytes.
Applications
The present study demonstrated the anti-diabetic properties fromC. lentiferaextract and may lead to further research on determining the responsible mechanims involved.
Peer review
This is an ideal work to discover an alternative source for anti-diabetic agent. Authors comprehensively worked in anti-diabetic by usingC. lentiferaextract. The material and methods are well constructed and findings are well represented.
[1] Nath D, Heemels MT, Anson L. Obesity and diabetes. Nature 2006; 444: 839-888.
[2] Scully T. Diabetes in numbers. Nature 2012; 485: S2-S3.
[3] Csorba TR, Lyon AW, Hollenberg MD. Autoimmunity and the pathogenesis of type 1 diabetes. Crit Rev Clin Lab Sci 2010; 47: 51-71.
[4] Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, et al. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One 2009; 4(7): e6189.
[5] Rang HP, Bailey CJ. The endocrine system pharmacology. 2nd ed. London: Longman Group Ltd; 1991, p. 504-508.
[6] Hassan Z, Yam MF, Ahmad M, Yusof AP. Antidiabetic properties and mechanism of action of Gynura procumbens water extract in streptozotocin-induced diabetic rats. Molecules 2010; 15: 9008-9023.
[7] Chan JY, Leung PC, Che CT, Fung KP. Protective effects of an herbal formulation of Radix Astragali, Radix Codonopsis and Cortex Lycii on streptozotocin-induced apoptosis in pancreatic beta-cells: an implication for its treatment of diabetes mellitus. Phytother Res 2008; 22: 190-196.
[8] Pandey A, Tripathi P, Pandey R, Srivatava R, Goswami S. Alternative therapies useful in the management of diabetes: a systematic review. J Pharm Bioallied Sci 2011; 3: 504-512.
[9] Senthilkumar T, Sangeetha N, Ashokkumar N. Antihyperglycemic, antihyperlipidemic, and renoprotective effects of Chlorella pyrenoidosa in diabetic rats exposed to cadmium. Toxicol Mech Methods 2012; 22: 617-624.
[10] Matanju P, Matanjun P, Mohamed S, Mustapha NM, Muhammad K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol 2009; 21: 75-80.
[11] Maeda R, Ida T, Ihara H, Sakamoto T. Induction of apoptosis in MCF-7 cells by β-1,3-xylooligosaccharides prepared from Caulerpa lentillifera. Biosci Biotechnol Biochem 2012; 76: 1032-1034.
[12] Nguyen VT, Ueng JP, Tsai GJ. Proximate composition, total phenolic content, and antioxidant activity of Seagrape (Caulerpa lentillifera). J Food Sci 2011; 76: C950-C958.
[13] Matanjun P, Mohamed S, Muhammad K, Mustapha NM. Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polychstum on high-cholesterol/high-fat diet in rats. J Med Food 2010; 13: 792-800.
[14] Kumar S, Kumar V, Rana M, Kumar D. Enzyme inhibitors from plants: an alternative approach to treat diabetes. Phcog Commn 2012; 2: 18-33.
[15] Ansarullah, Bharucha B, Dwivedi M, Laddha NC, Begum R, Hardikar AA, et al. Antioxidant rich flavonoids from Oreocnide integrifolia enhance glucose uptake and insulin secretion and protects pancreatic β-cells from streptozotocin insult. BMC Complement Altern Med 2011; 11: 126.
[16] Ding Y, Zhang ZF, Dai XQ, Li Y. Myricetin protects against cytokine-induced cell-death in RIN-m5f β cells. J Med Food 2012; 15: 733-740.
[17] Song MY, Kim K, Lee HJ, Park JW, Ryu DG, Kwon KB, et al. Fructus Xanthii extract protects against cytokine-induced damage in pancreatic beta-cells through suppression of NF-kappaB activation. Int J Mol Med 2009; 23: 547-553.
[18] Kim HK. Ecklonia cava inhibits glucose absorption and stimulates insulin secretion in streptozotocin-induced diabetic mice. Evid Based Complement Alternat Med 2012; doi: 10.1155/2012/439294.
[19] Wilcox G. Insulin and insulin resistance. Clin Biochem Rev 2005; 26: 19-39.
[20] Murdolo G, Smith U. The dysregulated adipose tissue: a connecting link between insulin resistance, type 2 diabetes mellitus and atherosclerosis. Nutr Metab Cardiovasc Dis 2006; 16(Suppl 1): S35-S38.
[21] Seo MJ, Choi HS, Lee OH, Lee BY. Grateloupia lanceolata (Okamura) Kawaguchi, the edible red seaweed, inhibits lipid accumulation and reactive oxygen species production during differentiation in 3T3-L1 cells. Phyther Res 2013; 27: 656-663.
[22] Mclntosh CH, Demuth HU, Pospisilik JA, Pederson R. Dipeptidyl peptidase-IV inhibitors: how do they work as new anti-diabetic agents? Regul Pept 2005; 128: 159-165.
[23] Guasch L, Lala E, Ojeda MN, Valls C, Bladé C, Mulero M, et al. Identification of novel human dipeptidyl peptidase-IV inhibitors of natural origin (Part II): in silico prediction in anti-diabetic extracts. PLoS One 2012; 7(9): e44972.
10.12980/APJTB.4.2014APJTB-2014-0091
*Corresponding author: Dong Young Rhyu, Department of Oriental Medicine Resources and Institute of Korean Medicine Industry, College of Natural Science, Mokpo National University, 1666 Youngsan-ro, Muan-gun, Jeonnam 534-729, Republic of Korea.
Tel: +82614502664
Fax: +82614506643
E-mail: rhyudy@mokpo.ac.kr
Foundation Project: Supported by the Ministry of Education, Science and Technology (Grant No. 2012R1A1A2009172).
Article history:
Received 15 Mar 2014
Received in revised form 27 Mar, 2nd revised form 10 Apr, 3rd revised form 29 Apr 2014
Accepted 19 Jun 2014
Available online 28 Jul 2014
 Asian Pacific Journal of Tropical Biomedicine2014年7期
Asian Pacific Journal of Tropical Biomedicine2014年7期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Reticulo-cutaneous fistula due to the ingestion of a long metallic rod in a cow
- Clinical, radiological and molecular diagnosis correlation in serum samples from patients with osteoarticular tuberculosis
- Down-regulated expression of NPM1 in IMS-M2 cell line by (-)-epigallocatechin-3-gallate
- Knowledge, attitude, and practices related to cutaneous leishmaniasis in an endemic focus of cutaneous leishmaniasis, Southern Iran
- An initial study of insect succession on decomposing rabbit carrions in Harare, Zimbabwe
- Modified technique of Wolbachia removal from Malaysian Aedes albopictus
