Percutaneous transhepatic portal catheterization guided by ultrasound technology for islet transplantation in rhesus monkey
Feng Gao, Shao-Dong Ai, Sheng Liu, Wen-Bin Zeng and Wei Wang
Changsha, China
Percutaneous transhepatic portal catheterization guided by ultrasound technology for islet transplantation in rhesus monkey
Feng Gao, Shao-Dong Ai, Sheng Liu, Wen-Bin Zeng and Wei Wang
Changsha, China
BACKGROUND:Pig islet xenotransplantation has the potential to overcome the shortage of donated human islets for islet cell transplantation in type 1 diabetes. Testing in nonhuman primate models is necessary before clinical application in humans. Intraportal islet transplantation in monkeys is usually performed by surgical infusion during laparotomy or laparoscopy. In this paper, we describe a new method of percutaneous transhepatic portal catheterization (PTPC) as an alternative to current methods of islet transplantation in rhesus monkeys.
METHODS:We performed ultrasound-guided PTPC in five adult rhesus monkeys weighing 7-8 kg, with portal vein catheterization confirmed by digital subtraction angiography. We monitored for complications in the thoracic and abdominal cavity. To evaluate the safety of ultrasound-guided PTPC, we recorded the changes in portal pressure throughout the microbead transplantation procedure.
RESULTS:Ultrasound-guided PTPC and infusion of 16 000 microbeads/kg body weight into the portal vein was successful in all five monkeys. Differences in the hepatobiliary anatomy of rhesus monkeys compared to humans led to a higher initial complication rate. The first monkey died of abdominal hemorrhage 10 hours post-transplantation. The second suffered from a mild pneumothorax but recovered fully after taking only conservative measures. After gaining experience with the first two monkeys, we decreased both the hepatic puncture time and the number of puncture attempts required, with the remaining three monkeys experiencing no complications. Portal pressures initially increased proportionalto the number of transplanted microbeads but returned to preinfusion levels at 30 minutes post-transplantation. The changes in portal pressures occurring during the procedure were not significantly different.
CONCLUSIONS:Ultrasound-guided PTPC is an effective, convenient, and minimally invasive method suitable for use in non-human primate models of islet cell transplantation provided that care is taken with hepatic puncture. Its advantages must be weighed against the risks of procedure-related complications.
(Hepatobiliary Pancreat Dis Int 2012;11:154-159)
ultrasound-guided; portal venous catheterization; islet xenotransplantation; rhesus monkey
Introduction
In recent years, the technologies related to the isolation and purification of islet cells and immunosuppressive therapies have been improved. These, along with international multi-center studies, suggest that pancreatic islet transplantation may play a greater role in the treatment of type 1 diabetes. Islet cell transplantation is one of the most effective treatments for type 1 diabetes.[1]Unfortunately, only a subset of patients have benefitted from islet transplantation due to the limited availability of donor islets. Pig islet xenotransplantation is one of the most promising means of alleviating this shortage.[2,3]It must be tested on nonhuman primates before large-scale clinical application.[4-6]The rhesus monkey is a valuable experimental model for diabetes research, including experimental pancreatic islet transplantation.[7,8]At present, intraportal islet transplantation in non-human primates is usually performed by cannulation of a mesenteric vein during laparotomy or laparoscopy.[9]In this paper, we describe a new method of intraportal microbead injection in rhesus monkeys that can serve as an alternative tocurrent methods of islet cell transplantation. This is a minimally invasive method involving ultrasound-guided percutaneous transhepatic portal catheterization (PTPC) for delivery of transplanted alginate microbeads into the portal vein. The hepatobiliary anatomy of the rhesus monkey is significantly different from that of the human being. In addition to a much smaller size, the rhesus monkey liver is composed of six relatively separate lobes (Fig. 1).[10]Alginate microbeads are similar in size to islets and show good biocompatibility. Here, we imitated islet transplantation using alginate microbeads instead of islets in order to evaluate the safety of ultrasound-guided PTPC. It proved to be a relatively easy, convenient, and feasible way to reach the portal bloodstream in the rhesus monkey. However, these advantages have to be balanced against a potentially higher rate of procedure-related complications in this non-human primate model.
Methods
Experimental animals
Five rhesus monkeys 6-8 years old, each weighing 7-8 kg, were purchased from Gaoyao Kangda Laboratory Animal Science and Technology Co. Ltd. (Gaoyao, China). The protocols were approved by the Animal Ethics Committee of the Third Xiangya Hospital, Central South University (Changsha, China). Our protocols followed the guidelines regarding the care of laboratory animals issued by the Ministry of Science and Technology of China in 2006.[11]
Preparation and counting of micro-capsules
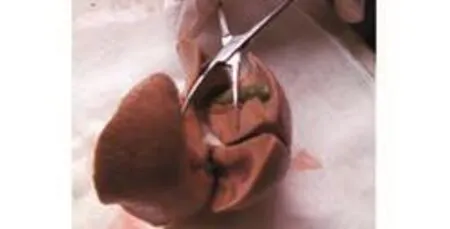
Fig. 1. Gross anatomy of the rhesus monkey hepatic portal vein, showing that it is composed of six relatively separate lobes.
Alginate/poly-1-lysine/alginate microbeads were prepared as described previously.[12]Sodium alginate (from Sigma, USA) was dissolved in water to prepare the 1.5% (W/V) solution for injection. Then methylene blue was added for staining, and the mixture was stirred overnight, sterilized at high temperature, and stored at 4 ℃. An aqueous solution of 1.1% (W/V) calcium chloride was prepared. Then the filtered solution was sterilized at high temperature and stored at 4 ℃. The stained 1.5% (W/V) sodium alginate solution was loaded into a static microcapsule generator. The small droplets that formed were collected. The droplets were put into the 1.1% (W/V) calcium chloride suspension to produce microbeads. The solution was filtered with a 400-μm filter, and these microbeads were washed with phosphate-buffered saline (PBS). The morphologic features of the microbeads before transplantation were observed under an optical microscope.
One drop of microcapsule suspension was dripped onto a counting plate along the rim and the number of microcapsules was determined under a low-power lens (×10). The number of capsules in four fields was calculated using the following formula: Number of microcapsules in 1 mL suspension=[(Number of microcapsules in four fields)/4]×10 000.
PTPC and infusion of alginate microbeads
The resulting microbeads were sized 50-300 μm in diameter: of the total beads, 26.1±6.0% were 50-150 μm, 35.5±5.7% were 151-250 μm, and 38.4±2.7% were 251-300 μm. Rhesus monkeys were slowly infused with microbeads suspended in 20 mL heparinized saline (200 U/mL) via the portal vein at 16 000 microbeads/kg. All procedures were carried out under general anesthesia induced by thiopentone sodium (15 mg/kg) and maintained with oxygen and 1%-2% halothane. Access to the portal vein was achieved using a percutaneous transhepatic technique to insert a lengthened 18G indwelling needle (1.6×170 mm; Wenzhou Lihua Corp., China) into the main portal vein under ultrasound guidance (color Doppler ultrasound; Acuson Corp., Issaquah, WA, USA) and digital subtraction angiography (DSA; Siemens, Forchheim, Germany).
Each monkey was placed in the left lateral position. A 10-mL syringe was fixed on the lengthened 18G indwelling needle. The needle was inserted into the distal end of the right central lobe branch of the portal vein (equivalent to the right anterior branch of the human portal vein), and monitored by ultrasound via a rectal ultrasound probe placed in the intercostal space. If the syringe was pulled and blood flowed freely, a guidewire was inserted into the main portal vein along the needle. Then the internal metal needle was withdrawn while the external flexible catheter was inserted into the main portal vein. DSA was performed to show the location of the catheter and the structure of the portal vein both inside and outside the liver (Fig. 2).
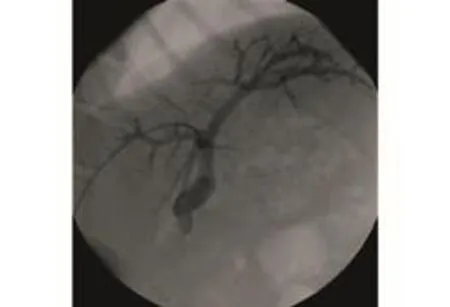
Fig. 2. DSA image of ultrasound-guided percutaneous transhepatic portal catheterization. The catheter was located properly in the portal vein.
Infusion of the microbead suspension into the portal vein was carried out after PTPC in two separate injections. Each injection was performed at 2 mL/min to infuse a total of 10 mL microbead suspension. Each injection was followed by a 10-minute pause. Portal venous pressure (mmHg) was recorded before the two injections, immediately after each injection, and 30 minutes after the final injection using a pressure transducer (Medex, Hillard, OH, USA). The infusion route in the portal vein before and after microbead infusion was monitored by angiography (Siemens, Germany). After the operation, the intraparenchymal liver tract was sealed with gelfoam to prevent bleeding.[13]Each monkey was pressure-bandaged and remained anesthetized for 6-12 hours.
Histological evaluation
Histological analysis was performed at the end of the study, either 1 week after portal venous infusion or at autopsy, to determine whether the microbeads remained in the liver. Liver specimens were fixed in 10% formalin and processed in paraffin blocks for standard hematoxylin and eosin (HE) staining.
Statistical analysis
Data were expressed as mean±SEM. The statistical significance of differences between the groups was determined by repeated measures analysis of variance. AP<0.05 was considered statistically significant.
Results
Ultrasound-guided PTPC in rhesus monkeys
Average total puncture time was 5 minutes per monkeyand the success rate of PTPC was 100%. Complications occurred after surgery in two monkeys. One experienced a mild pneumothorax, which resolved by itself, and the other suffered an intra-abdominal hemorrhage and died (Table). For animal 1 (animals are numbered in chronological order), difficulty was encountered in trying to distinguish the intrahepatic versus the extrahepatic segments of the portal vein only by intra-operative visualization of the liver. Because the technicians were unfamiliar with the hepatobiliary anatomy of the rhesus monkey, we mistakenly punctured the right branch of the portal vein, and the entirety of which is extrahepatic in the monkey. This caused bleeding at the puncture site, which was extrahepatic and at risk for persistent bleeding due to lack of the natural tamponade effect with intrahepatic portal vein puncture. The monkey died of blood loss and shock 10 hours after surgery. Due to the slow rate of bleeding, it was hard to monitor for postprocedural bleeding using ultrasound alone, even when this was performed immediately after surgery. In animal 2, since the diaphragm of the rhesus monkey is lower than that in humans, we failed to follow the respiratory rhythm and inadvertently inserted the needle when the diaphragm was not at its highest point as it would be during maximal expiration. The thoracic cavity was injured, causing pneumothorax. No specific therapeutic measure was taken for the pneumothorax and it healed by itself. We used the lessons from the first two operations to improve our technique. Total operation time was shortened greatly and the subsequent three monkeys experienced no complications.
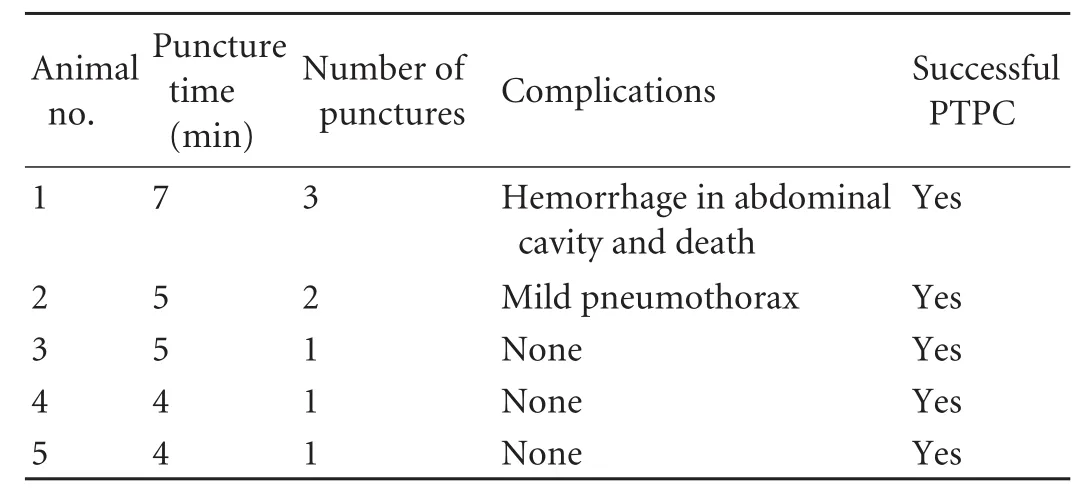
Table. PTPC in rhesus monkeys
Changes in portal venous pressure at different times during PTPC and microbead injection
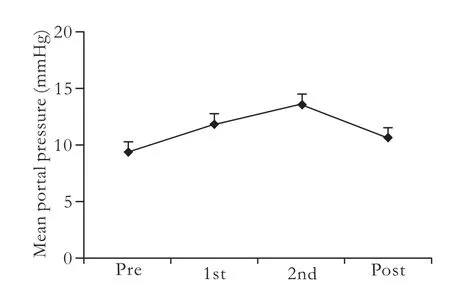
Fig. 3. Effects of infused microbeads on portal venous pressure. Portal venous pressure was measured in recipient animals infused with microbeads via the portal vein at 16 000 microbeads/kg before the infusion procedure (pre), and after the first (1st) and second (2nd) injection of microbeads, and 30 minutes after the procedure (post). The data are presented as mean±SEM (n=5) (P>0.05).
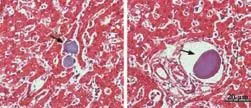
Fig. 4. HE-stained liver sections showing intact microbeads in the hepatic sinus (left) and the portal vessels (right) of all recipient rhesus monkeys.
The microbeads were transplanted via the portal vein at 16 000 microbeads/kg body weight, and the portal venous pressure before, during, and after transplantation was measured (Fig. 3). The portal venous pressure was monitored in all animals both before and after microbead injections. The changes in the pressure were found to be related to the numbers of microbeads injected. Compared to the mean basal pressures recorded before the microbead injection (10.37±5.43 mmHg), the portal vein pressure increased slightly when about 50% of the microbeads had been transplanted, reaching a peak when all of the microbeads had been transplanted. This returned to the baseline by 30 minutes after completion of the procedure. The differences between the portal venous pressure measured before, during, and after the transplantation were not statistically significant.
State of injected microbeads in hepatic vessels and hepatic sinuses of recipient animals
HE-stained liver sections showed intact microbeads in the hepatic sinuses and hepatic vessels of all recipient monkeys after the intraportal injection (Fig. 4).
Discussion
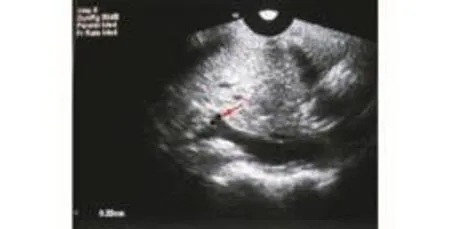
Fig. 5. Ultrasonography of the right portal vein branch of the central lobe (red arrow), showing its 3.3-mm inner diameter.
The development and application of medical imaging techniques have made it possible to perform minimally invasive punctures of the portal vein. Ultrasonographic technology has also accelerated progress in interventional medicine. Liver tissue is a good target for ultrasound imaging given that the internal and external structures can generally be visualized clearly. Selective transhepatic catheterization of the portal vein was first used as a diagnostic tool and as a means of acquiring access to bleeding esophageal varices.[14]This technique has a complication rate of <5%.[15]Percutaneous liver access for transhepatic cholangiography, biliary drainage procedures, and liver biopsy has become widely practiced, and has a proven safety record. However, portal vein puncture is difficult in rhesus monkeys because the animal is small, weighing around 6-8 kg or roughly one tenth the weight of an adult human. The anatomy of the rhesus monkey liver is significantly different from that of the human liver. It is clearly divided into six separate lobes, while the division of the lobes in humans is far more subtle, making it appear as one smooth organ. Moreover, the intercostal space in the rhesus monkey is narrow, the position of the diaphragm is low, the portal vein has a small caliber, and the common portal vein and its left and right branches are extrahepatic. The vessel suitable for puncture, the right portal vein branch of the central lobe, is short and small (inner diameter about 3 mm, length about 5 mm) (Fig. 5). It is therefore necessary to determine the safety and feasibility of ultrasound-guided PTPC in rhesus monkeys.
In these experiments, we successfully established a rhesus monkey model of ultrasound-guided PTPC with a lengthened indwelling needle and a rectal ultrasound probe. The model has the following 5 major advantages. First, minimal invasiveness. In this model, the stress on the monkey is reduced, which makes for easier postoperative care and faster recovery. Second, single-step catheterization. After puncturing the vessel, the catheter is inserted directly with a guide wire as the needle is withdrawn. This reduces damage to the liver. After the conventional Selding puncture, the needle track mustbe expanded before the catheter can be inserted.[16]This is more complicated, requires more time, and increases both the damage to the surrounding liver tissue and the incidence of complications. Third, guidance by rectal ultrasound probe. The rectal ultrasound probe is so small that it can be easily placed into the intercostal space of rhesus monkeys, avoiding the ribs. The frequency is as high as 6-8 MHz, which is suitable for displaying the tiny structures of the portal vein. In addition, free guidance in narrow and small spaces is possible without much risk of collision between the puncture apparatus and the probe, making it possible to observe the needle’s path of insertion in a multi-angle, multi-directional manner.[17]Fourth, compatibility with percutaneous transhepatic portal vein catheterization. This method can be used with PTPC, the most common method in clinical pancreatic islet transplantation.[1,18-21]Fifth, the possibility of single-surgery treatment. Currently, an additional islet cell injection is usually required to maintain insulin independence. Our method makes multiple injections of additional islets in the same rhesus monkey possible without any need for new surgery.
A few additional issues should be acknowledged: a) High-quality ultrasound guidance is required. b) The puncture must be performed in careful coordination with the monkeys’ respiratory rhythm. c) Large rhesus monkeys are preferred because their vessels are bigger and easier to puncture.
In the above experiments, alginate microbeads, with good biocompatibility and similar sizes to islets, were used as islet substitutes for transplantation into the portal system. The safety of this method was assessed by monitoring the portal venous pressure. This rose as the number of transplanted alginate microbeads increased, but 30 minutes post-transplantation, the pressure returned to the pre-transplantation level. These variations in portal pressure are not necessarily indicative of danger. However, some clinical islet transplantations have involved much greater increases.[22]These changes in portal pressure are related to the volume of tissue injected and do not seem to be associated with the occurrence of complications or other undesirable outcomes.[23]All of the experimental animals survived except animal 1, which died of a hemorrhage, a peri-procedural complication unrelated to portal venous pressure changes. This animal’s death was secondary to operator unfamiliarity with the position and structure of the liver and portal vein. Despite the relatively small number of experimental animals, the success in the last three shows a promise for the feasibility and safety of ultrasound-guided PTPC. Our experience highlights the important differences in hepatobiliary anatomy between humans and monkeys, as well as the challenges in performing intricate procedures in animals only one tenth the size of humans. Our research could help some investigators and scientists avoid some of the pitfalls, such as those we encountered during our first 2 animals, which may pave the way for further research into xenotransplantation of pig islets into type 1 diabetes patients.
In conclusion, sufficient knowledge of the model animal’s hepatobiliary anatomy, proper ultrasound guidance, skill, and careful pre-operative preparation and postoperative care are keys to establishing the rhesus monkey model of ultrasound-guided PTPC. Ultrasound-guided PTPC is a convenient, effective, and minimally invasive method suitable for use in nonhuman primate models of islet cell transplantation provided that care is taken with hepatic puncture. Its advantages must be weighed against the risks of procedure-related complications.
Acknowledgement:We thank Rui-Zhen Li and Ping Zhou for their valuable suggestions for this work and their excellent technical assistance.
Contributors:WW proposed the study. GF and ZWB wrote the first draft. GF, ASD and LS analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. WW is the guarantor.
Funding:The study was supported by grants from the Natural Science Foundation of Hunan Province (11JJ4078) and the National Natural Science Foundation of China (30900359 and 30900377).
Ethical approval:Animal protocols were approved by the Animal Ethics Committee of the Third Xiangya Hospital, Central South University of China.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355: 1318-1330.
2 Igaz P. Xenotransplantation of islets of Langerhans: a possible therapy for diabetes mellitus? Wien Med Wochenschr 2001; 151:291-294.
3 Cozzi E, Bosio E. Islet xenotransplantation: current status of preclinical studies in the pig-to-nonhuman primate model. Curr Opin Organ Transplant 2008;13:155-158.
4 Sykes M, d'Apice A, Sandrin M; IXA Ethics Committee. Position paper of the Ethics Committee of the International Xenotransplantation Association. Xenotransplantation 2003; 10:194-203.
5 Dufrane D, Gianello P. Pig islet xenotransplantation into nonhuman primate model. Transplantation 2008;86:753-760.
6 Bottino R, Cooper DK. Islet xenotransplantation: the pig-tonon-human primate model. Xenotransplantation 2008;15:104-106.
7 Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transpl Immunol 2009;21:81-86.
8 Marigliano M, Casu A, Bertera S, Trucco M, Bottino R. Hemoglobin A1C Percentage in Nonhuman Primates: A Useful Tool to Monitor Diabetes before and after Porcine Pancreatic Islet Xenotransplantation. J Transplant 2011;2011: 965605.
9 Hirshberg B, Montgomery S, Wysoki MG, Xu H, Tadaki D, Lee J, et al. Pancreatic islet transplantation using the nonhuman primate (rhesus) model predicts that the portal vein is superior to the celiac artery as the islet infusion site. Diabetes 2002;51:2135-2140.
10 Chen Z, Wu D. Laboratory animal science. 1st Ed, Changsha: Hunan Science & Technology Press; 2006:91.
11 The Ministry of Science and Technology of the People's Republic of China. Guidance suggestions for the care and use of laboratory animals. 2006-09-30. Available from: http://www. most.gov.cn/fggw/zfwj/zfwj2006/200609/t20060930_54389. htm.
12 Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980;210:908-910.
13 Villiger P, Ryan EA, Owen R, O'Kelly K, Oberholzer J, Al Saif F, et al. Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant 2005;5:2992-2998.
14 Viamonte M Jr, LePage J, Lunderquist A, Pereiras R, Russell E, Viamonte M, et al. Selective catheterization of the portal vein and its tributaries. Preliminary report. Radiology 1975;114: 457-460.
15 Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg 2001;88: 165-175.
16 Lunderquist A, Vang J. Sclerosing injection of esophageal varices through transhepatic selective catheterization of the gastric coronary vein. A preliminary report. Acta Radiol Diagn (Stockh) 1974;15:546-550.
17 Shi L, Tian F, Cai Z, et al. Ultrasonic guided percutaneous trans-hepatic biliary drainage (analysis of 2156 cases). J Surg Concepts Pract 2007;12:380-381.
18 Gaglia JL, Shapiro AM, Weir GC. Islet transplantation: progress and challenge. Arch Med Res 2005;36:273-280.
19 Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med 2004;350:694-705.
20 Korsgren O, Nilsson B, Berne C, Felldin M, Foss A, Kallen R, et al. Current status of clinical islet transplantation. Transplantation 2005;79:1289-1293.
21 Owen RJ, Ryan EA, O'Kelly K, Lakey JR, McCarthy MC, Paty BW, et al. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: radiologic aspects. Radiology 2003;229:165-170.
22 Casey JJ, Lakey JR, Ryan EA, Paty BW, Owen R, O'Kelly K, et al. Portal venous pressure changes after sequential clinical islet transplantation. Transplantation 2002;74:913-915.
23 Bucher P, Mathe Z, Bosco D, Becker C, Kessler L, Greget M, et al. Morbidity associated with intraportal islet transplantation. Transplant Proc 2004;36:1119-1120.
July 13, 2011
Accepted after revision December 16, 2011
Author Affiliations: Cell Transplantation & Gene Therapy Institute (Gao F, Ai SD, Liu S and Wang W), and Department of Ultrasound (Gao F), Third Xiangya Hospital; School of Pharmaceutical Sciences (Zeng WB), Central South University, Changsha 410013, China
Wei Wang, MD, PhD, Cell Transplantation & Gene Therapy Institute, Third Xiangya Hospital, Central South University, Changsha 410013, China (Tel: 86-731-88618411; Fax: 86-731-88852936; Email: wawe01cn@yahoo.com.cn)
? 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60141-6
 Hepatobiliary & Pancreatic Diseases International2012年2期
Hepatobiliary & Pancreatic Diseases International2012年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreaticopleural fistula: etiology, treatment and long-term follow-up
- Rapamycin combined with allogenic immature dendritic cells selectively expands CD4+CD25+Foxp3+regulatory T cells in rats
- Inhibition of 12-lipoxygenase reduces proliferation and induces apoptosis of hepatocellular carcinoma cells in vitro and in vivo
- Prognostic significance and clinical relevance of Sprouty 2 protein expression in human hepatocellular carcinoma
- Management of hypersplenism in non-cirrhotic portal hypertension: a surgical series
- The impact of family history of hepatocellular carcinoma on its patients' survival
