ldentification of α7 nicotinic acetylcholine receptor on hippocampal astrocytes cultured in vitro and its role on inflammatory mediator secretion***★
Yan Wang, Ning Zhu Kewan Wang, Zhongyi Zhang Yong Wang
1 Department of Pharmacy, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China
2 Department of Pharmacy, the 458 Hospital of Chinese PLA, Guangzhou 510602, Guangdong Province, China
3 Department of Neurosurgery, Nanfang Hospital, Southern Medical University, Guangzhou 510602, Guangdong Province, China
ldentification of α7 nicotinic acetylcholine receptor on hippocampal astrocytes culturedin vitroand its role on inflammatory mediator secretion***★
Yan Wang1,2, Ning Zhu1, Kewan Wang3, Zhongyi Zhang1, Yong Wang1
1Department of Pharmacy, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China
2Department of Pharmacy, the 458 Hospital of Chinese PLA, Guangzhou 510602, Guangdong Province, China
3Department of Neurosurgery, Nanfang Hospital, Southern Medical University, Guangzhou 510602, Guangdong Province, China
The present study found expressions of α7 nicotinic acetylcholine receptor on hippocampal slices and hippocampal astrocytes using double immunofluorescence stainings. Expression of glial fibrillary acidic protein in the cultured hippocampal slices and hippocampal astrocytes significantly increased, and levels of macrophage inflammatory protein 1α, RANTES, interleukin-1β, interleukin-6, and tumor necrosis factor-α increased in the supernatant of cultured astrocytes following exposure to 200 nM amyloid β protein 1-42. Preconditioning of 10 μM nicotine, a nicotinic acetylcholine receptor agonist, could attenuate the influence of amyloid β protein 1-42 in inflammatory mediator secretion of cultured astrocytes. Experimental findings indicated that α7 nicotinic acetylcholine receptor was expressed on the surface of hippocampal astrocytes, and activated α7 nicotinic acetylcholine receptor was shown to inhibit inflammation induced by amyloid β protein 1-42.
α7 nicotinic acetylcholine receptor; astrocytes; inflammation; cytokines; chemotactic factor; amyloid β protein; hippocampus; neural regeneration
Research Highlights
(1) The present study detected the expression of α7 nicotinic acetylcholine receptor on the surface of hippocampal astrocytes.
(2) Activated α7 nicotinic acetylcholine receptor was shown to inhibit inflammation induced by amyloid β protein 1-42.
Abbreviations
AD, Alzheimer’s disease; Aβ1-42, amyloid β protein 1-42; GFAP, glial fibrillary acidic protein
lNTRODUCTlON
Previous studies have shown that cholinergic neurotransmitters released from the peripheral vagus nerve activate the α7 nicotinic acetylcholine receptor in macrophages, as well as inhibit synthesis and secretion of inflammatory mediators of macrophages, thereby resulting in decreased peripheral inflammation[1-2].
Alzheimer’s disease (AD) is associated with cerebral chronic inflammation caused by genetic and environmental factors[3]. Astrocytes and microglia, which exhibit similar characteristics to peripheral macrophages, are predominant in chronic inflammation of the central nervous system. It is difficult to ascertain the role of α7 nicotinic acetylcholine receptor on astrocytesin vivo, because of the variety of cells. Therefore,in vitrostudies areextremely helpful. Although cell culture is considered to be the best model for analyzing cellular functions, the relationships between cells are different in cultured cells compared with those in the organisms. Therefore, the use of brain slice organotypic cultures has become a powerful tool for the analysis of neural mechanisms, allowing for close-to-normal relationships between neurons, glia, and other membrane structures[4].
However, the localization of α7 nicotinic acetylcholine receptor on astrocytes in organotypically cultured hippocampal slices remains poorly understood. Previous results have shown that cholinergic drugs delay AD development[5]. However, the role of astrocytes in this process remains unclear.
The present study analyzed the localization of α7 nicotinic acetylcholine receptor in rat hippocampal astrocytes to determine the role of α7 nicotinic acetylcholine receptor in inflammatory factor secretion induced by amyloid β protein 1-42 (Aβ1-42).
RESULTS
Expression of α7 nicotinic acetylcholine receptor on astrocytes in cultured hippocampal slices
Expression of α7 nicotinic acetylcholine receptor on astrocytes in cultured hippocampal slices was observed using double immunofluorescence. As shown in Figure 1A, a large number of α7 nicotinic acetylcholine receptorexpressing cells were present in cultured hippocampal slices, as evident by fluorescence staining. Antibodies specific to glial fibrillary acidic protein (GFAP) were used to detect astrocytes, and Figure 1B shows astrocyte distribution. Both GFAP and α7 nicotinic acetylcholine receptor were expressed in the cells (Figure 1C).
Expression of α7 nicotinic acetylcholine receptor in cultured hippocampal astrocytes
Expression of α7 nicotinic acetylcholine receptor on astrocytes, which were purified and culturedin vitro, was detected by double immunofluorescence. Green fluorescence represented α7 nicotinic acetylcholine receptor (Figure 2A), and astrocytes exhibited red fluorescence (Figure 2B). Superimposed images revealed a co-localization of astrocytes and α7 nicotinic acetylcholine receptor (Figure 2C).
Aβ1-42 increased GFAP expression in astrocytes
Cultured hippocampal slices and astrocytes were treated with Aβ1-42(200 nM) for 48 hours, and then the expression of GFAP was detected. Results showed increased GFAP expression in cultured organic astrocytes and cultured single astrocytes (Figure 3). Astrocytes reacted to Aβ1-42, by increasing GFAP expression, which is the first stage of an inflammatory reaction in astrocytes[6].
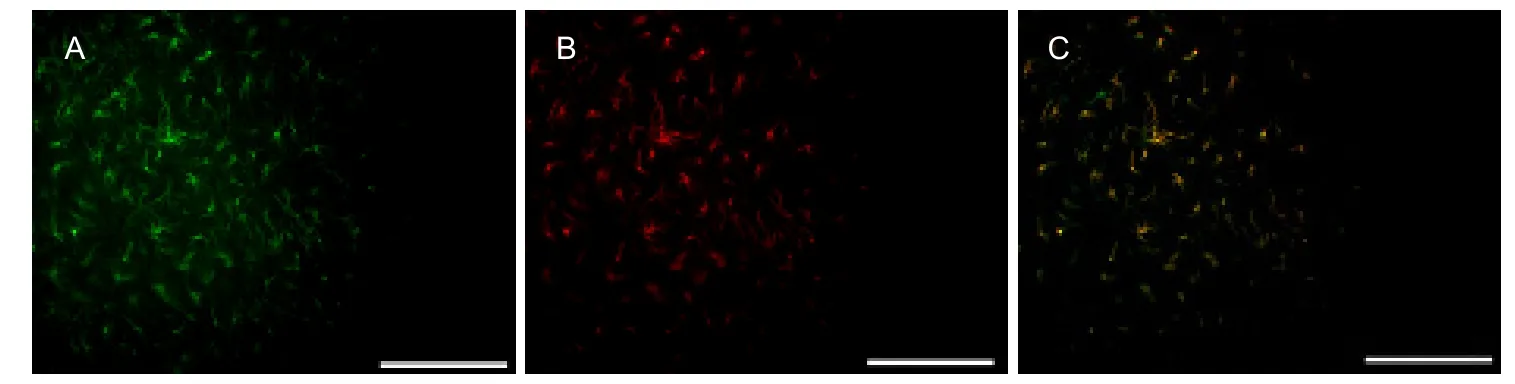
Figure 1 Expression of α7 nicotinic acetylcholine receptor on astrocytes in cultured hippocampal slices as detected by double immunofluorescence (fluorescence microscope, scale bars: 100 μm).
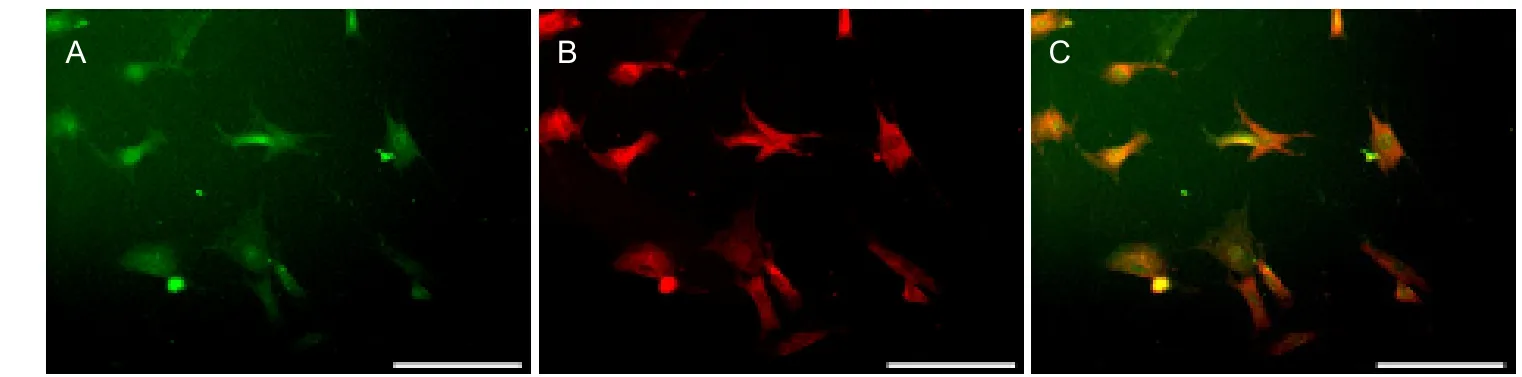
Figure 2 Double immunofluorescence of cultured hippocampal astrocytes using anti-glial fibrillary acidic protein and anti-α7 nicotinic acetylcholine receptor (fluorescence microscopy, scale bars: 100 μm).
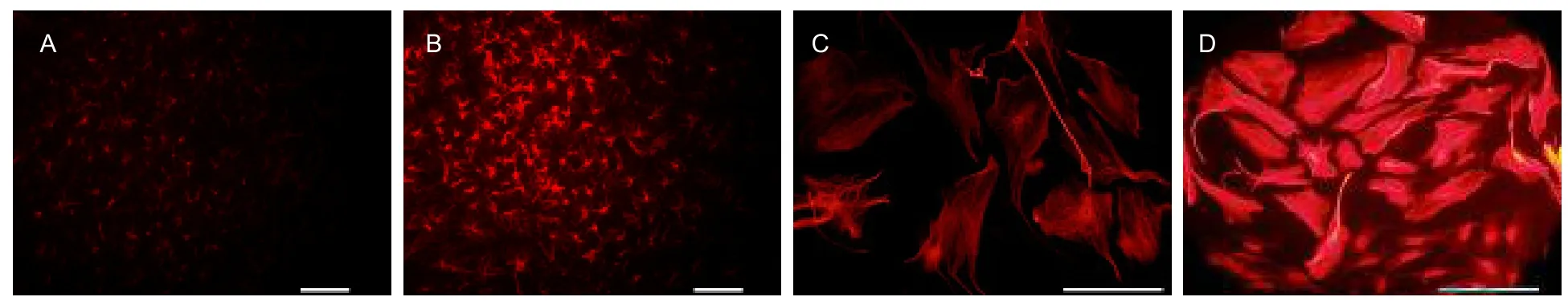
Figure 3 Expression of glial fibrillary acidic protein induced by amyloid β protein 1-42 (immunofluorescence staining, fluorescence microscopy, scale bars: 100 μm).
Effects of Aβ1-42 on secretion of inflammatory mediators in astrocytes
Following exposure to 200 nM Aβ1-42, chemokine levels in the supernatants of cultured astrocytes were measured. Aβ1-42increased macrophage inflammatory protein 1α and RANTES release from astrocytes in a time-dependent manner (Figure 4).
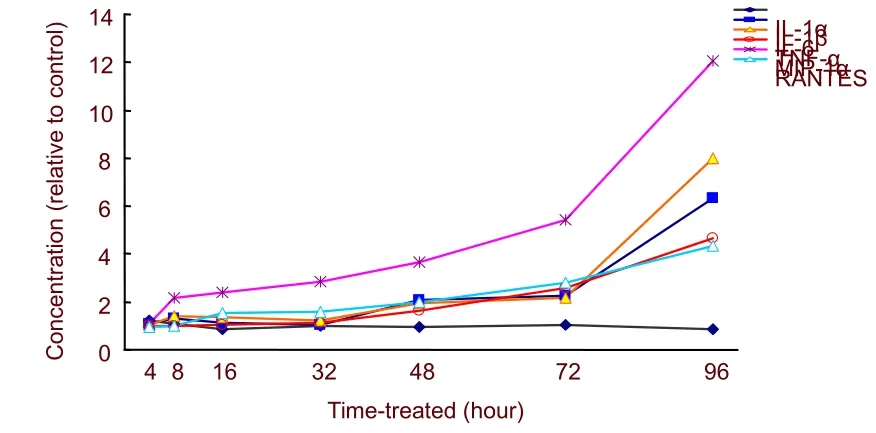
Figure 4 Effect of amyloid β protein 1-42 on secretion of astrocyte cytokines.
Concentrations of macrophage inflammatory protein 1α increased at 8 hours after treatment with Aβ1-42and reached 12-fold expression levels at 96 hours (F= 85.510,P< 0.01). Increased RANTES expression was less than macrophage inflammatory protein 1α; expression increased by 16 hours after treatment, and reached a 4.6-fold increase by 96 hours (F= 40.424,P< 0.01).
To determine whether interactions between Aβ1-42and astrocytes upregulated pro-inflammatory cytokines, supernatants were collected from cultured astrocytes treated with Aβ1-42. Concentrations of interleukin-1α, interleukin-1β, interleukin-6, and tumor necrosis factor-α were measured. As shown in Figure 4, Aβ1-42stimulated interleukin-1β, interleukin-6, and tumor necrosis factor-α release, but not interleukin-1α. The increases in cytokines profiles varied according to time. After 96 hours, changes in interleukin-6 and interleukin-1β levels were most significant, with approximately 8-fold and 6.3-fold increased expression, respectively (F= 196.317,P< 0.01;F= 368.181,P< 0.01). In addition, tumor necrosis factor-α levels were most significant at 96 hours, with approximately 4.8-fold increased expression (F= 43.955,P< 0.01).
Effects of activated α7 nicotinic acetylcholine receptor on secretion of inflammatory mediators of astrocytes mediated by Aβ1-42
To explore the role of astrocyte α7 nicotinic acetylcholine receptor in secretion of inflammatory mediators mediated by Aβ1-42, cultured astrocytes were pre-activated with nicotine (10 μM), a nicotinic acetylcholine receptor agonist, for 60 minutes, and then the concentration of inflammatory mediators was measured at 96 hours after Aβ1-42exposure. Results showed that α7 nicotinic acetylcholine receptor was pre-activated by nicotine, which decreased secretion of inflammatory mediators (except interleukin-1α) mediated by Aβ1-42(P< 0.01; Figure 5).
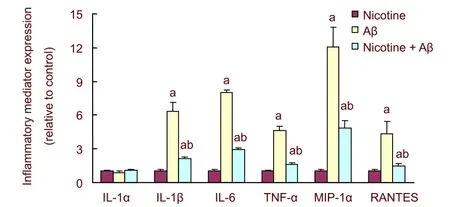
Figure 5 Effect of activated astrocytic α7 nicotinic acetylcholine receptor on secretion of inflammatory mediators mediated by amyloid β protein 1-42 (Aβ).
DlSCUSSlON
Cognitive functional impairment in AD patients has been shown to be related to cholinergic deficiency in the central nervous system. However, nicotinic acetylcholine receptor excitomotors improve cognition in animals and humans[7-8]. In the clinic, cholinergic agents are the most effective and most generally used, although little is known about the mechanisms of action. It is thought that transmitters released by the vagus nerve, which activates α7 nicotinic acetylcholine receptor on macrophages, can also inhibit peripheral inflammation[2].
In contrast to macrophages, astrocytes are abundant in the central nervous system and play a crucial role in central inflammatory reaction. Therefore, α7 nicotinic acetylcholine receptor expression on hippocampal astrocytes culturedin vitrowas analyzed, as well as the role of α7 nicotinic acetylcholine receptor in inducing secretion of inflammatory mediator.
Double immunofluorescence was used to identify astrocytes and expression of α7 nicotinic acetylcholine receptor in cultured hippocampal slices. Results showed that cells other than astrocytes also expressed α7 nicotinic acetylcholine receptor, as previously reported[9]. In hippocampal cells, astrocytes are mixed with other cells in the hippocampal slice. Therefore, detailed functions of α7 nicotinic acetylcholine receptor on astrocytes remains poorly understood due to limitations with astrocyte purity. Therefore, it is important to study the mechanisms of astrocytes in astrocytic cultures. As described in a previous study[10], pooled hippocampal cells were cultured for 8-10 days and then transferred to a new culture flask to purify astrocytes from microglia and oligodendroglia. Purified astrocytes were further confirmed by immunofluorescence with GFAP-specific antibody.
Expression of α7 nicotinic acetylcholine receptor is influenced by many factors. In different encephalic regions, astrocytes can express different α7 nicotinic acetylcholine receptor expression patterns[11]. In addition, there is a significant difference in growth conditions between organotypic cultures and cultured single cells. Therefore, it is necessary to determine whether cultured single astrocytes express α7 nicotinic acetylcholine receptor.
Double immunofluorescence staining was used in the present study, and results demonstrated that α7 nicotinic acetylcholine receptor was expressed on cultured single astrocytes. These results suggested that it is possible to analyze the function of α7 nicotinic acetylcholine receptor in astrocytes using cultured single astrocyte models. In the brain, astrocytes sustain and nourish neurons, and they have been shown to play a role in intracephalic inflammatory reactions, with direct effects on the central nervous system[12]. Diffuse plaques, consisting of Aβ, results in the accumulation of astrocytes and microglia, as well as other pathological change. Previous studies have shown that Aβ directly injures neurons[13], as well as induces cytokine secretion resulting in neuronal damageviathe activation of astrocytes and microglia. To determine the effects of Aβ on astrocytes, GFAP expression was measured in cultured hippocampal slices following exposure to Aβ1-42. Results showed that Aβ1-42significantly increased GFAP expression. However, when combined with other cells, it becomes difficult to determine that the effect of Aβ1-42on astrocytes is direct or indirect. Therefore, Aβ1-42was added to cultured single astrocytes, demonstrating that Aβ1-42increased GFAP expression, enlarged astrocytic cell bodies, and created rough synapses. These results suggested that Aβ1-42directly activated astrocytes, resulting in increased GFAP expression.
Once activated, astrocytes evoked inflammatory reactions in the brain and secreted inflammatory mediators that induced neurotoxicity. The present study observed concentration changes of cytokines following treatment with Aβ1-42. All tested cytokines, with exception to interleukin-1α, exhibited expression changes in a time-dependent manner. Astrocytes have been shown to express tumor necrosis factor-α in response to an inflammatory response[14]. However, in the present study, expression increased by only 4.6 folds, compared with 8-fold increases in interleukin-6.
Expression of chemotactic factors increased earlier and more dramatically than cytokines, and macrophage inflammatory protein 1α exhibited the most significant change. Chemotactic factors promote the migration and infiltration of other phagocytes, as well as enhance inflammatory reactions and prolong inflammatory processes, which can subsequently exacerbate the symptoms of AD[15].
The ion channel α7 nicotinic acetylcholine receptor exists in the peripheral and central nervous systems and exhibits a high affinity to nicotine[9]. To identify the influence of activated astrocytic α7 nicotinic acetylcholine receptor on secretion of inflammatory mediators mediated by Aβ1-42,the cultured astrocytes were pretreated with nicotine for 60 minutes prior to Aβ1-42exposure. Results suggested that once astrocytes expressed α7 nicotinic acetylcholine receptor, action of Aβ1-42in inflammatory mediators increased. Levels of cytokines and chemotactic factors significantly decreased compared with non-activated α7 nicotinic acetylcholine receptor. Therefore, results suggested that activated astrocytic α7 nicotinic acetylcholine receptor decreased secretion of inflammatory mediators and chemotactic factors, suppressed migration and infiltration of other phagocyte, and decreased formation of a self-toxic circuit loop. α7 nicotinic acetylcholine receptor activation did not completely return cytokine or chemotactic factors to normal levels, which suggested that activated α7 nicotinic acetylcholine receptor only decreased the inflammatory reaction of astrocytes.
These mechanisms could help to explain why cholinergic treatment only improves AD symptoms and postpones the pathological processes of AD, but does not cure it.
MATERlALS AND METHODS
Design
A comparative,in vitrostudy.
Time and setting
The experiment was performed at the Department of Pharmacy, Zhujiang Hospital, Southern Medical University, China from August 2006 to May 2010.
Materials
Neonatal Sprague-Dawley rats (10 days old) and
newborn rats (within 24 hours after birth) were obtained from the Experimental Animal Center of Southern Medical University, China (Certificate No. 2005A060). All experimental animal procedures complied with theGuidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[16].
Methods
Preparation of amyloid-beta peptide
Aβ1-42was prepared in fresh stock solution according to previously described methods, with slight modifications[17]. Briefly, Aβ1-42(Sigma, St. Louis, MO, USA) was dissolved in 35% acetonitrile and further diluted to 10 μM with incremental additions of PBS while vortexing between additions. The peptide solution was subsequently incubated at 37°C for 72 hours to promote fibrillization and aggregation, followed by storage at -20°C. The final working concentration used in the experiment was 200 nM Aβ1-42in culture medium[18].
Preparation of hippocampal slices
The hippocampus was isolated from 10-day-old neonatal rats and sectioned for culture. The rinsed hippocampus slices were placed onto a membrane in a solution-air culture. The culture medium comprised 50% Eagle’s-modified medium (Gibco, Carlsbad, CA, USA), 25% Earle’s balanced salt solution, and 25% horse serum containing 6.5 mg/L D-glucose. In the first 3 days, the slices were cultured at 37°C with 5% CO2, and medium was replaced every day. Afterwards, the medium was replaced every 3 days, and culture temperatures were reduced to 33°C[19].
Culture of hippocampal astrocytes
Hippocampi were dissected from the cerebrum of newborn rats within 24 hours after birth, and the tissue was digested with trypsin and centrifuged. Subsequently, the supernatant was discarded, and dissociated cells were plated in culture flasks. After 8-10 days, the culture flasks were shaken at 37°C for 4 hours in a rotator, and then the adherent cells were collected for further culture. To assess astrocytic purity, GFAP expression was detected[20].
Identification of astrocytes
The hippocampal slices and cultured astrocytes were fixed in 4% paraformaldehyde (pH 7.2) for 20 minutes and washed with 0.01 M PBS. Following a blocking step in PBS containing 10% goat serum and 0.3% Triton X-100, the specimens were incubated with mouse anti-GFAP polyclonal antibody (1:200; Chemicon, Santa Cruz, CA, USA) at 4°C overnight. The sections were then washed three times with PBS for 10 minutes each and incubated with Texas Red-conjugated anti-mouse IgG secondary antibody (1:100; Chemicon) at room temperature for 1 hour. Then sections were washed three times with PBS, coverslipped, and examined under a fluorescence microscope (Olympus, Tokyo, Japan).
Double immunofluorescence of α7 nicotinic acetylcholine receptor in cultured hippocampal slices and cultured astrocytes
Double immunofluorescence was used to identify expression of astrocytic α7 nicotinic acetylcholine receptor in hippocampal organotypic-cultured hippocampal slices and cultured astrocytes. Sections and cultured astrocytes were fixed with 4% paraformaldehyde, and then incubated with 0.5% Triton X-100 for 30 minutes. Rinsed sections and cultured astrocytes were pre-blocked with goat serum prior to primary antibody incubation. Mouse anti-GFAP (1:200; Chemicon) and rabbit anti-α7 nicotinic acetylcholine receptor (1:200; Chemicon) polyclonal antibodies served as the primary antibodies at 4°C overnight, respectively. Normal rabbit serum was used as the negative control. Neuronal signals served as a putative positive control. After three washes with PBS for 5 minutes each, Texas Red-conjugated anti-mouse IgG (1:100; Chemicon) and FITC-labeled anti-rabbit IgG (1:100; Chemicon) were used as secondary antibodies, respectively. Then slides were washed three times with PBS and examined under a fluorescence microscope (BX51; Olympus).
Determination of inflammatory factor expression in supernatant of astrocytes using the liquid phasetechnique
Following treatment of cultured astrocytes with 200 nM Aβ1-42for 4, 8, 16, 32, 48, 72, and 96 hours, supernatants were collected to detect concentrations of cytokines and chemokines. To determine the role of α7 nicotinic acetylcholine receptor, the cells were pretreated with 10 μM nicotine (Sigma) for 1 hour prior to Aβ1-42administration. Control cultures were from the same culture set, but were treated with media only. Cytokines/ chemokines, including interleukin-1α, interleukin-1β, interleukin-6, tumor necrosis factor-α, macrophage inflammatory protein 1α, and RANTES in the culture supernatants of astrocytes were measured simultaneously using a LiquiChip work station, which employed bead-based xMAP (flexible multi-analyte profiling) technology, according to manufacture instructions[21].
Statistical analysis
All statistical analyses were performed using SPSS 11.0 software (SPSS, Chicago, IL, USA). Data were expressed as mean ± SD or mean. One-way analysis of variance was used to compare means between groups, and multiple comparisons for further steps. With homogeneity of variance, least significant difference was adopted for each two-group comparison. Otherwise, the Welch method was utilized. Differences were considered statistically significant if thePvalue < 0.05.
Funding: This study was supported by the National Natural Science Foundation of China, No. 30471928 and No. 30973162 and the Natural Science Foundation of Guangdong Province, No. 07005203.
Author contributions: The experiment was designed by Yong Wang, Kewan Wang, performed by Yong Wang, Yan Wang, Ning Zhu and Zhongyi Zhang. Yan Wang and Yong Wang were responsible for data integrity and manuscript writing.
Conflicts of interest: None declared.
Ethical approval: The pilot was approved by Medical Animal Ethical Committee of Guangdong Province in China.
[1] Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458-462.
[2] Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384-388.
[3] Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21(3):383-421.
[4] Connelly CA, Chen LC, Colquhoun SD. Metabolic activity of cultured rat brainstem, hippocampal and spinal cord slices. J Neurosci Methods. 2000;99(1-2):1-7.
[5] Salawu FK, Umar JT, Olokoba AB. Alzheimer's disease: a review of recent developments. Ann Afr Med. 2011;10(2):73-79.
[6] Balasingam V, Tejada-Berges T, Wright E, et al. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci. 1994;14(2):846-856.
[7] Newhouse PA, Kelton M. Nicotinic systems in central nervous systems disease: degenerative disorders and beyond. Pharm Acta Helv. 2000;74(2-3):91-101.
[8] O'Neill MJ, Murray TK, Lakics V, et al. The role of neuronal nicotinic acetylcholine receptors in acute and chronic neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2002;1(4):399-411.
[9] Graham A, Court JA, Martin-Ruiz CM, et al. Immunohistochemical localisation of nicotinic acetylcholine receptor subunits in human cerebellum. Neuroscience. 2002;113(3):493-507.
[10] Mccarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85(3):890-902.
[11] Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24(50):11244-11252.
[12] Nagele RG, Wegiel J, Venkataraman V, et al. Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol Aging. 2004;25(5):663-674.
[13] Sola Vigo F, Kedikian G, Heredia L, et al. Amyloid-beta precursor protein mediates neuronal toxicity of amyloid beta through Go protein activation. Neurobiol Aging. 2009;30(9):1379-1392.
[14] Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93(1-2):182-193.
[15] Cartier L, Hartley O, Dubois-Dauphin M, et al. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48(1):16-42.
[16] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[17] Franciosi S, Choi HB, Kim SU, et al. IL-8 enhancement of amyloid-beta (Abeta 1-42)-induced expression and production of pro-inflammatory cytokines and COX-2 in cultured human microglia. J Neuroimmunol. 2005; 159(1-2):66-74.
[18] Jantaratnotai N, Ryu JK, Kim SU, et al. Amyloid beta peptide-induced corpus callosum damage and glial activation in vivo. Neuroreport. 2003;14(11):1429-1433.
[19] Wang Y, Zhu XN, Yan J, et al. Increase of β1-Adrenergic receptor gene expression induced by nicotine in hippocampal slice of rat. Acta Pharmacol Sin. 2003;24(12):1281-1286.
[20] McCarthy KD, Vellis JD. Preparation of separate astroglial and oligodendroglial ceu cultures from rat cerebral tissue. J Cell Biol. 2005;14(4):404-409.
[21] de Jager W, Te VH, Prakken BJ, et al. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10(1):133-139.
Cite this article as:Neural Regen Res. 2012;7(22):1709-1714.
Yan Wang★, Master, Pharmacist-in-charge, Department of Pharmacy, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China; Department of Pharmacy, the 458 Hospital of Chinese PLA, Guangzhou 510602, Guangdong Province, China
Yong Wang, Ph.D., Professor, Department of Pharmacy, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China
yongwh2005@163.com
2012-03-18
2012-06-13
(N20100802005/WLM)
Wang Y, Zhu N, Wang KW, Zhang ZY, Wang Y.
Identification of α7-nicotinic acetylcholine receptor on hippocampal astrocytes cultured in vitro and its role on inflammatory mediator secretion. Neural Regen
Res. 2012;7(22):1709-1714.
www.crter.cn
www.nrronline.org
10.3969/j.issn.1673-5374. 2012.22.005
(Edited by Zhu YG, Pei DS/Yang Y/Song LP)
 中國(guó)神經(jīng)再生研究(英文版)2012年22期
中國(guó)神經(jīng)再生研究(英文版)2012年22期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- 5-hydroxymethyl-2-furfural prolongs survival and inhibits oxidative stress in a mouse model of forebrain ischemia******☆
- Breaking News in Spinal Cord Injury Research FDA Approved Phase I Clinical Trial of Human, Autologous Schwann Cell Transplantation in Patients with Spinal Cord Injuries☆●
- Neuroprotective effects of bovine colostrum on intracerebral hemorrhage-induced apoptotic neuronal cell death in rats☆●
- Sonic hedgehog elevates N-myc gene expression in neural stem cells***★
- Precision radiotherapy for brain tumors A 10-year bibliometric analysis☆
- Changes in expression and secretion patterns of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules during murine neural stem/progenitor cell differentiation in vitro***☆
