Relationship between pancreaticobiliary maljunction and gallbladder carcinoma: a meta-analysis
Yi-Lei Deng, Nan-Sheng Cheng, Yi-Xin Lin, Rong-Xing Zhou, Chen Yang, Yan-Wen Jin and Xian-Ze Xiong
Chengdu, China
Meta-analysis
Relationship between pancreaticobiliary maljunction and gallbladder carcinoma: a meta-analysis
Yi-Lei Deng, Nan-Sheng Cheng, Yi-Xin Lin, Rong-Xing Zhou, Chen Yang, Yan-Wen Jin and Xian-Ze Xiong
Chengdu, China
BACKGROUND:Reports on the relationship between pancreaticobiliary maljunction (PBM) and gallbladder carcinoma (GBC) are conflicting. The frequency of PBM in GBC patients and the clinical features of GBC patients with PBM vary in different studies.
DATA SOURCES:English-language articles describing the association between PBM and GBC were searched in the PubMed and Web of Science databases. Nine case-control studies fulfilled the inclusion criteria and addressed the relevant clinical questions of this analysis. Data were extracted independently by two reviewers using a predefined spreadsheet.
RESULTS:The incidence of PBM was higher in GBC patients than in controls (10.60% vs 1.76%, OR: 7.41, 95% CI: 5.03 to 10.87,P<0.00001). The proportion of female patients with PBM was 1.96-fold higher than in GBC patients without PBM (80.5% vs 62.9%, OR: 1.96, 95% CI: 1.09 to 3.52,P=0.12). GBC patients with PBM were 10 years younger than those without PBM (SMD: -9.90, 95% CI: -11.70 to -8.10,P<0.00001). And a difference in the incidence of associated gallstone was found between GBC patients with and without PBM (10.8% vs 54.3%, OR: 0.09, 95% CI: 0.05 to 0.17,P<0.00001). Among the GBC patients with PBM, associated congenital dilatation of the common bile duct was present with a higher incidence ranging from 52.2% to 85.7%, and 70.0%-85.7% of them belonged to the P-C type of PBM (the main pancreatic duct enters the common bile duct). No substantial heterogeneity was found and no evidence of publication bias was observed.CONCLUSIONS:PBM is a high-risk factor for developing GBC, especially the P-C type of PBM without congenital dilatation of the common bile duct. To prevent GBC, laparoscopic cholecystectomy is highly recommended for PBM patients without congenital dilatation of the common bile duct, especially relatively young female patients without gallstones.
(Hepatobiliary Pancreat Dis Int 2011; 10: 570-580)
pancreaticobiliary maljunction; gallbladder carcinoma; congenital dilatation of the common bile duct; meta-analysis
Introduction
Gallbladder carcinoma (GBC) is the most common biliary tract cancer, accounting for 3% of all tumors.[1]GBC is hard to detect and diagnose in its early stages because it usually has very slight symptoms or is asymptomatic. But once the diagnosis is confirmed, most of these patients often have metastasis and invasion. Furthermore, GBC is not sensitive to radiotherapy and chemotherapy. All of these characteristics make GBC a highly lethal tumor with a 5-year survival rate of less than 5%.[2]Therefore, prevention is absolutely necessary before the tumor forms, while the first and most important step is to identify which patients are at high risk for GBC. This is especially important in high-incidence countries such as Japan, Korea, India, Pakistan and China. In addition, Andean-area populations, North American Indians and Mexican-Americans should also receive special attention because of a genetic susceptibility to GBC.[3]
One of the well-known risk factors is pancreaticobiliary maljunction (PBM), also know as anomalous pancreaticobiliary ductal junction or anomalous pancreaticobiliary ductal union.[4-9,18-24]PBM, a rare congenital anomaly, is defined as a junction of the bileand pancreatic ducts located outside the duodenal wall forming a long common channel and beyond the influence of the sphincter of Oddi.[7]
Although rare in Western countries,[8]PBM has been well studied and reported in Asia, especially in Japan. According to Kimura's classification, the mode of PBM can be divided into two types: P-C type, where the main pancreatic duct enters the common bile duct at a right-angle, and C-P type, where the common bile duct enters the pancreatic duct at an acute angle.[9]This classification is rough, but also has wide application. At present, the most accepted classification is that of Komi, dividing PBM into types I, II and III. Typeiresembles P-C and type II is similar to C-P of Kimura. But, based on the presence or absence of dilatation of the common channel, each type is further subdivided into two subtypes: "a" or "b". Type III has a patent accessory pancreatic duct with or without an intricate network of ducts, and is classified into types IIIa, IIIb, and IIIc (Fig. 1).[10]Regardless of the different classifications, they all share a common pathogenesis: lack of the sphincter of Oddi at the junction of the bile and pancreatic ducts, leading to regurgitation of pancreatic juice into the biliary tract. The persistent activation of pancreatic juice by bile induces long-term chronic inflammation of the gallbladder epithelium and subsequent proliferative repair, resulting in sustained epithelial hyperplasia, atypia and ultimately carcinoma.[11-13]In recent years, with the rapid improvement of imaging, and especially the widespread use of endoscopic retrograde cholangiopancreatography (ERCP), PBM has been increasingly diagnosed, and the positive correlation between PBM and GBC has drawn increasing attention.
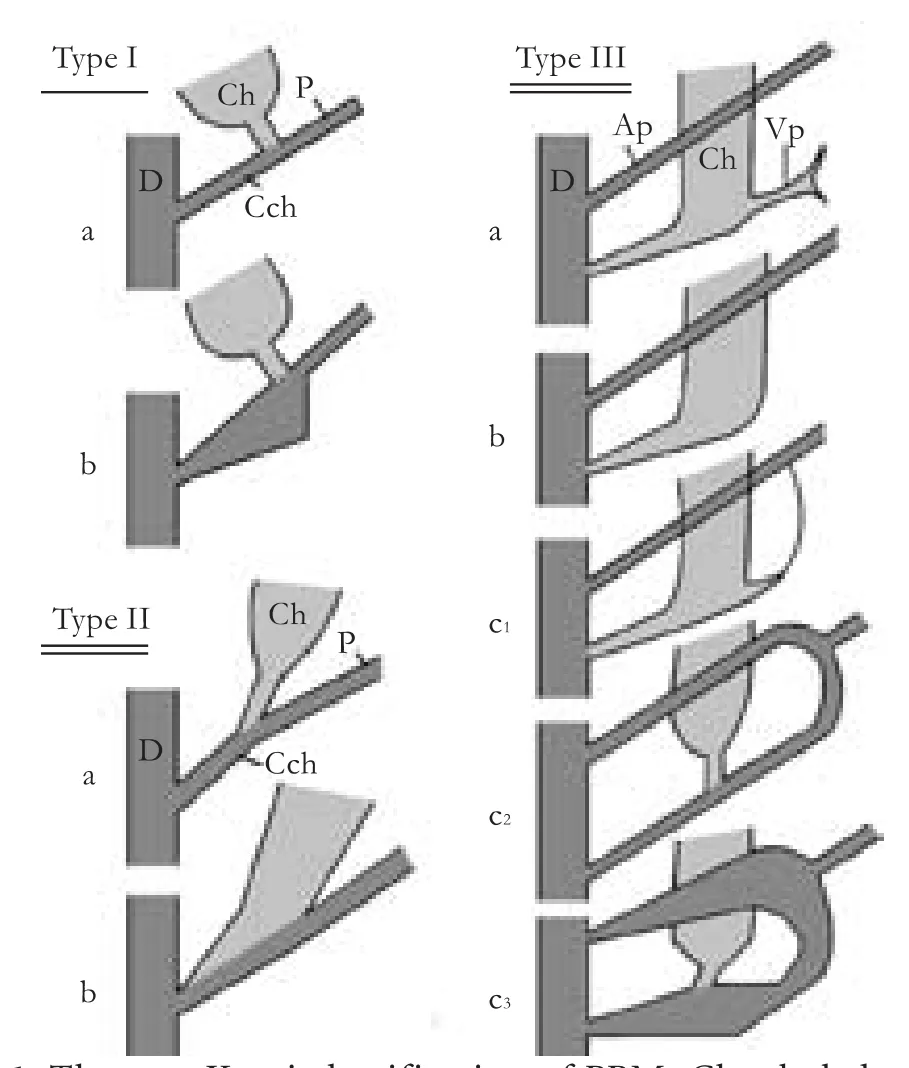
Fig. 1. The new Komi classification of PBM. Ch: choledochus; P: pancreatic duct; Cch: common channel; D: duodenum; Ap: accessory pancreatic duct; Vp: ventral pancreatic duct.
Although several reports have summarized the association between PBM and the risk of GBC,[8,9,18-24]none of them has provided a quantitative systematic review. In addition, the frequency of PBM in GBC patients and the clinical features of GBC patients with PBM vary in different studies.[8,9,18-24]Therefore, we carried out a meta-analysis of these studies to evaluate the association between PBM and GBC. By identifying a high-risk population, our analysis provides additional information on the primary prevention and management of GBC.
Methods
Literature search
Two researchers independently performed an electronic search to identify all relevant English-language studies describing an association between PBM and GBC published in PubMed (April 1977 to March 2011) and the Web of Science (May 1978 to April 2011).
The MeSH (medical subject heading) terms and key words for the search were as follows: pancreaticobiliary maljunction, anomalous pancreaticobiliary ductal junction, anomalous pancreaticobiliary ductal union, and gallbladder carcinoma. The search terms were combined with the Boolean operators OR and AND. All titles and abstracts were screened to determine their eligibility. If the title or abstract indicated possible relevance, their full texts were more fully examined for inclusion according to the inclusion/exclusion criteria. Any discrepancies were resolved by consensus.
Inclusion and exclusion criteria
The inclusion criteria were: (1) primary study; (2) case-control; (3) describing people with PBM and/or GBC; (4) ERCP or percutaneous transhepatic cholangiography (PTC) used as the diagnostic tool for PBM; the diagnostic criteria for PBM were an obviously long common channel, and the mean length of the contractile segment or the sphincter shorter than that of the common channel and distal to the junction; (5) diagnosis of GBC based on surgery or pathology; (6) presenting absolute numbers of true positive, false positive, false negative, and true negative cases to estimate the odds ratios (ORs) and their variance; (7) reporting one or more of the following clinical datain patients with GBC associated with PBM: age, sex, gallstone disease, associated congenital dilatation of the common bile duct (CCD) or type of PBM; and (8) in English.
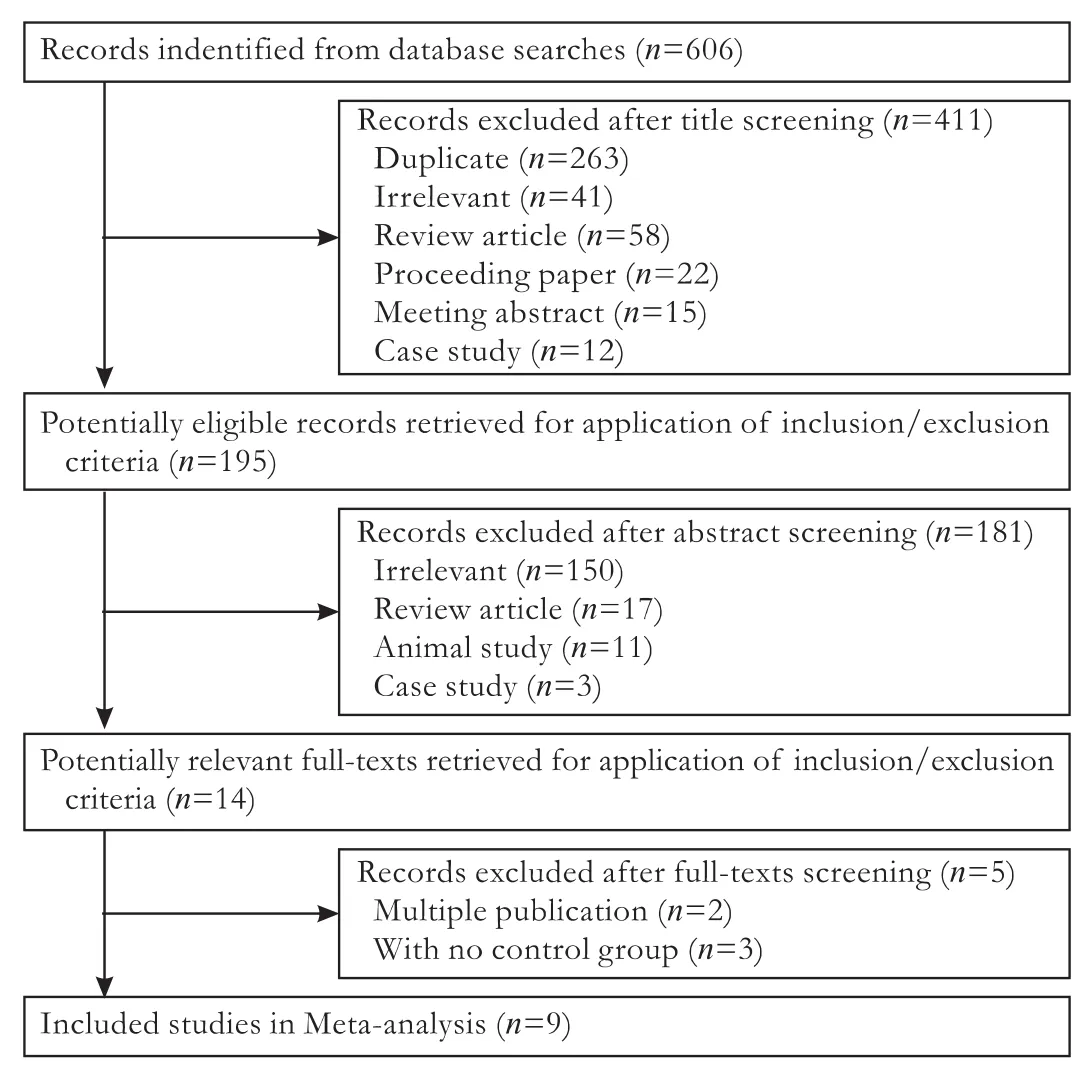
Fig. 2. Flowchart of article selection.
The exclusion criteria were: (1) review articles; (2) case reports, proceedings papers and meeting abstracts; (3) studies only reported as abstracts or published information incomplete; and (4) multiple publications based on the same database.
A flowchart of the selection process is provided in Fig. 2.
Data extraction and quality assessment
Data were extracted from each study independently by both reviewers using a predefined structured spreadsheet. The details were study design, number of subjects, characteristics of cases and controls, diagnostic methods for PBM and GBC, clinical data on patients with GBC associated with PBM, ORs, and 95% confidence intervals (CIs).
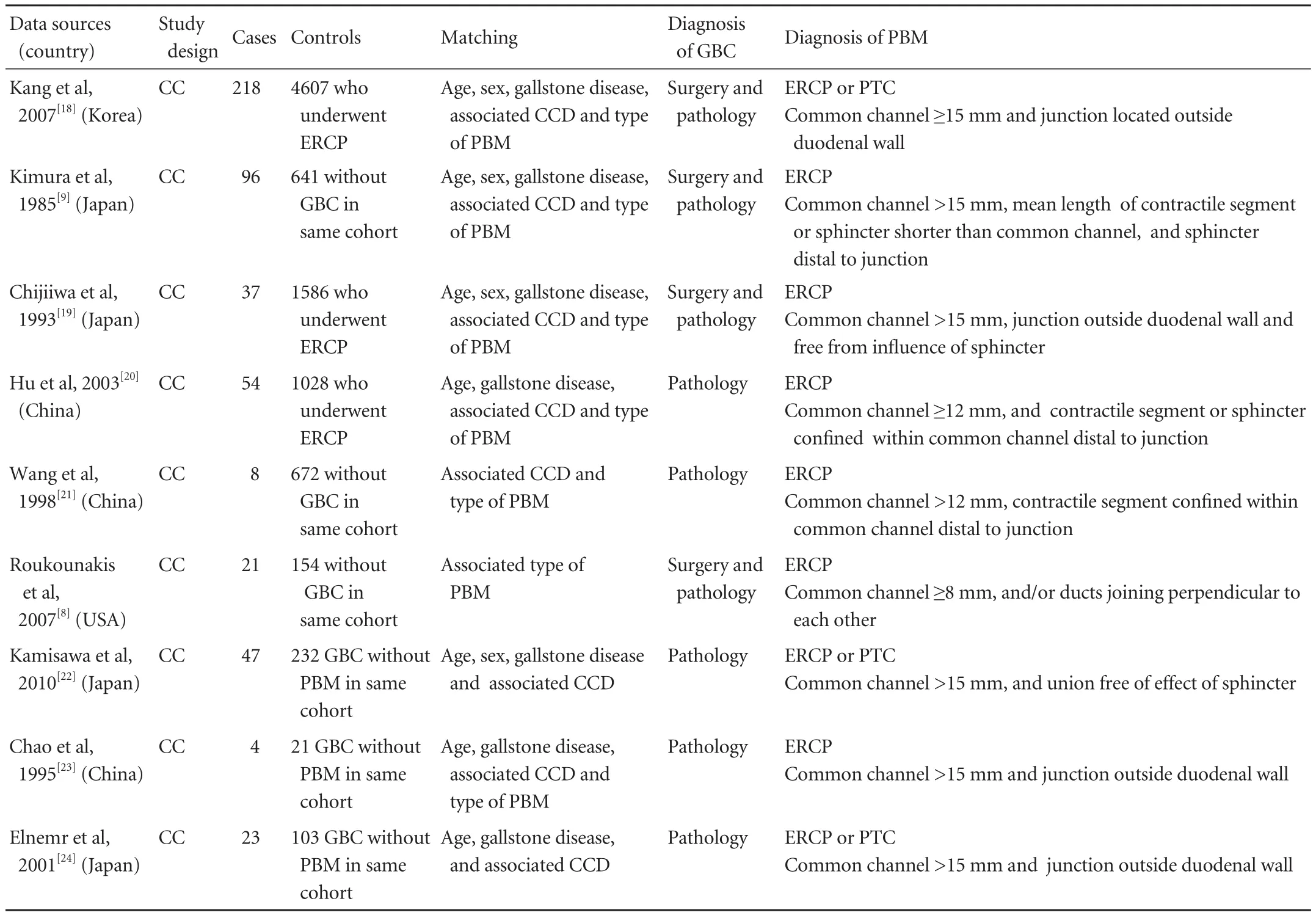
Table 1. Characteristics of studies included in the meta-analysis
The quality of each study was assessed independently by two reviewers according to criteria modified from the guidelines for reading case-control studies proposed by Lichtenstein et al.[14]These criteria include appropriate diagnostic criteria and methods for PBM and GBC, explicit inclusion and exclusion criteria, methods of data collection, investigation of bias, degree of variation, analytic methods, and sample size. However, to avoid possible subjective assessment from the reviewers, we did not generate an overall quality score,[15]but rather validity criteria were used to rank the order of the quality of studies (Table 1). For example, a study was ranked higher if cases and controls were matched by age, sex, gallstone disease, associated CCD or type of PBM, with a large sample size, and with the clear diagnoses of PBM and GBC. Disagreements were resolved by discussion and consensus between the reviewers.
Statistical analysis
First, the summary ORs and 95% CIs were calculated from the raw data using the Mantel-Haenszel method in a random effects model. Second, the statistical heterogeneity between studies was assessed using Cochran's Q test, and the Higgins I2statistic was used to measure the percentage of total variation across studies due to heterogeneity. If the I2statistic was ≤50%, there was no heterogeneity and the fixed effects model was applied. However, if heterogeneity was shown, the sources of heterogeneity were explored and subgroup or sensitivity analyses were performed. Third, funnel plots were constructed to evaluate potential publication bias. If there is no bias, the plot should resemble a symmetrical inverted funnel. Conversely, an asymmetrical and skewed shape indicates the presence of bias.[16,17]
All statistical analyses were performed with RevMan 5.0.25 (provided by the Cochrane Collaboration, Oxford, UK).
Results
Study identification
A total of 606 titles and abstracts were identified. Of these, after the primary screening of abstracts, 411 were rejected, most of which were duplicates (n=263), review articles (n=58), or irrelevant (n=41). Of the remaining 195 articles, 181 were excluded because they did not meet the inclusion criteria. Subsequently, 14 full texts were further reviewed in detail by both authors independently, and ultimately, a total of 9 articles were deemed eligible.
Study characteristics
Of the nine eligible studies in the meta-analyses, all were case-control studies. In all studies, the diagnosis of GBC was based on surgery or pathology, and ERCP or PTC was used as the diagnostic tool for PBM. Although the length of the common channel varied among different studies because of race, age and gender differences, all PBM patients met the diagnostic criterion of the junction of biliary and main pancreatic duct with an obviously long common channel (measuring ≥8, 12 or 15 mm). And the mean length of the contractile segment or the sphincter was shorter than that of the common channel and distal to the junction.
All cases and controls were matched by the relevant demographic and clinical data (Table 1). According to Komi's classification, the most accepted classification currently, PBM is divided into types I, II and III. However, all studies included in this paper were designed with Kimura's classification, types P-C and C-P.
In addition, all PBM patients included were roughly classified into two basic groups, with or without associated CCD, regardless of the type of CCD. But in fact, CCD has been classified into five types by Todani et al,[25]which is used widely at present. Typeimeans a solitary fusiform extrahepatic cyst, which is further subdivided into subtypes "a", "b" and "c" according to the shape of the affected segment; type II is an extrahepatic supraduodenal diverticulum; type III is a choledochocele; type IV comprises fusiform extraand intra-hepatic cysts (IVa), or multiple extrahepatic cysts (IVb); and type V is multiple intrahepatic cysts or Caroli's disease. Therefore, it was impossible to address the percentage of different types of CCD in PBM patients who developed GBC in our study.
Among the nine included studies, six presented sufficient data for the incidence of PBM in GBC patients and controls (healthy subjects or non-GBC patients), which were suitable for the meta-analysis. The other three studies were excluded because of the absence of effective controls (Kamisawa et al,[22]2010; Chao et al,[23]1995; Elnemr et al,[24]2001). They presented data for the incidence of PBM in GBC patients only (Table 2). Table 3 presents the demographic and clinical data (age, sex and associated gallstone) on GBC patients with or without PBM in our meta-analysis.
Data analysis
Studies comparing the incidence of PBM in GBC patients and controls
Six of the nine studies investigated the difference in theincidence of PBM between GBC patients and controls (Table 2). The results of the meta-analysis of the 6 studies are shown in Fig. 3. The pooled incidence of PBM was 10.60% (46 of 434) in cases and 1.76% (153 of 8688) in controls, giving a summary OR of 7.41 (95% CI: 5.03 to 10.87). The fixed effects model was used because the test for heterogeneity was not statistically significant (χ2=4.85, df=5, P=0.43, I2=0%). Thus, there was a statistically significant difference in the incidence of PBM in cases and controls (Z=10.25, P<0.00001), suggesting a close etiologic association between PBM and GBC.
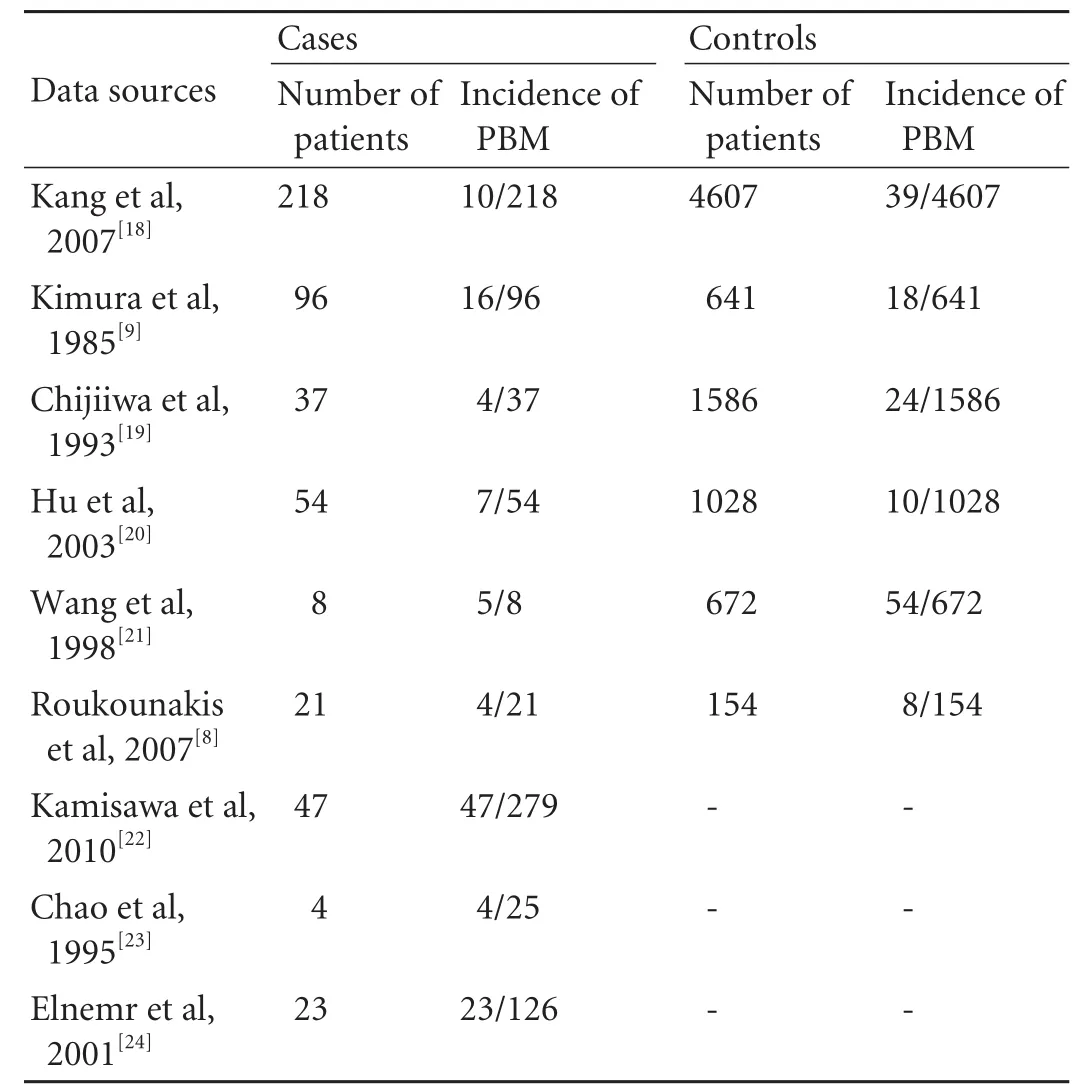
Table 2. Studies comparing the incidence of PBM in GBC patients and controls in the meta-analysis
Clinical features of patients with GBC with or without PBM
Of the nine studies, seven provided raw data on clinical features (age, sex and associated gallstone) of patients with GBC with or without PBM, whereas three did not differentiate the sex distribution between the two groups (Table 3). Only four of the seven gave information on sex distribution for both cases and controls. The proportion of female patients was 80.5% (62 of 77) in cases and 62.9% (348 of 553) in controls, giving a summary odds ratio of 1.96 (95% CI: 1.09 to 3.52).No substantial heterogeneity was found (χ2=5.84, df=3, P=0.12, I2=49%) and the fixed effects model was used (Fig. 4A). Thus, the proportion of female patients in cases was higher than that in controls (Z=2.25, P=0.02).

Fig. 3. Fixed effect model of odds ratio for incidence of PBM: cases versus controls.
Table 3. Included studies of demographic and clinical data on GBC patients with or without associated PBM in the meta-analysis
Original data on the age difference in both cases and controls were given in 7 studies consisting of 111 cases and 724 controls, with a standardized mean difference (SMD) estimate of -9.90 (95% CI, -11.70 to -8.10). No substantial heterogeneity was found (χ2=7.11,df=6, P=0.31, I2=16%) and the fixed effects model was used (Fig. 4B). Therefore, there was a statistically significant difference in the mean age between cases and controls (Z=10.76, P<0.00001), the mean age in cases was lower than that in the controls.
In addition, a significant difference in the incidence of associated gallstone was found between these two groups of patients (Z=7.69, P<0.00001). Gallstone disease was detected in 10.8% (12 of 111) in cases and 54.3% (393 of 724) in controls, yielding an OR estimate of 0.09 (95% CI: 0.05 to 0.17). No substantial heterogeneity was found (χ2=7.44,df=6,P=0.28, I2=19%) and the fixed effects model was used (Fig. 4C).
Funnel plot analysis
Publication bias was evaluated by visual inspection of the funnel plot. The plots showed relatively symmetric distributions, suggesting no publication bias (Fig. 5).
Discussion

Fig. 4. A: Fixed effect model of odds ratio for the proportion of female patients among GBC patients: PBM (+) versus PBM (-).B:Fixed effect model of standardized mean difference for mean age in GBC patients: PBM (+) versus PBM (-).C:Fixed effect model of odds ratio for incidence of associated gallstone in GBC patients: PBM (+) versus PBM (-).
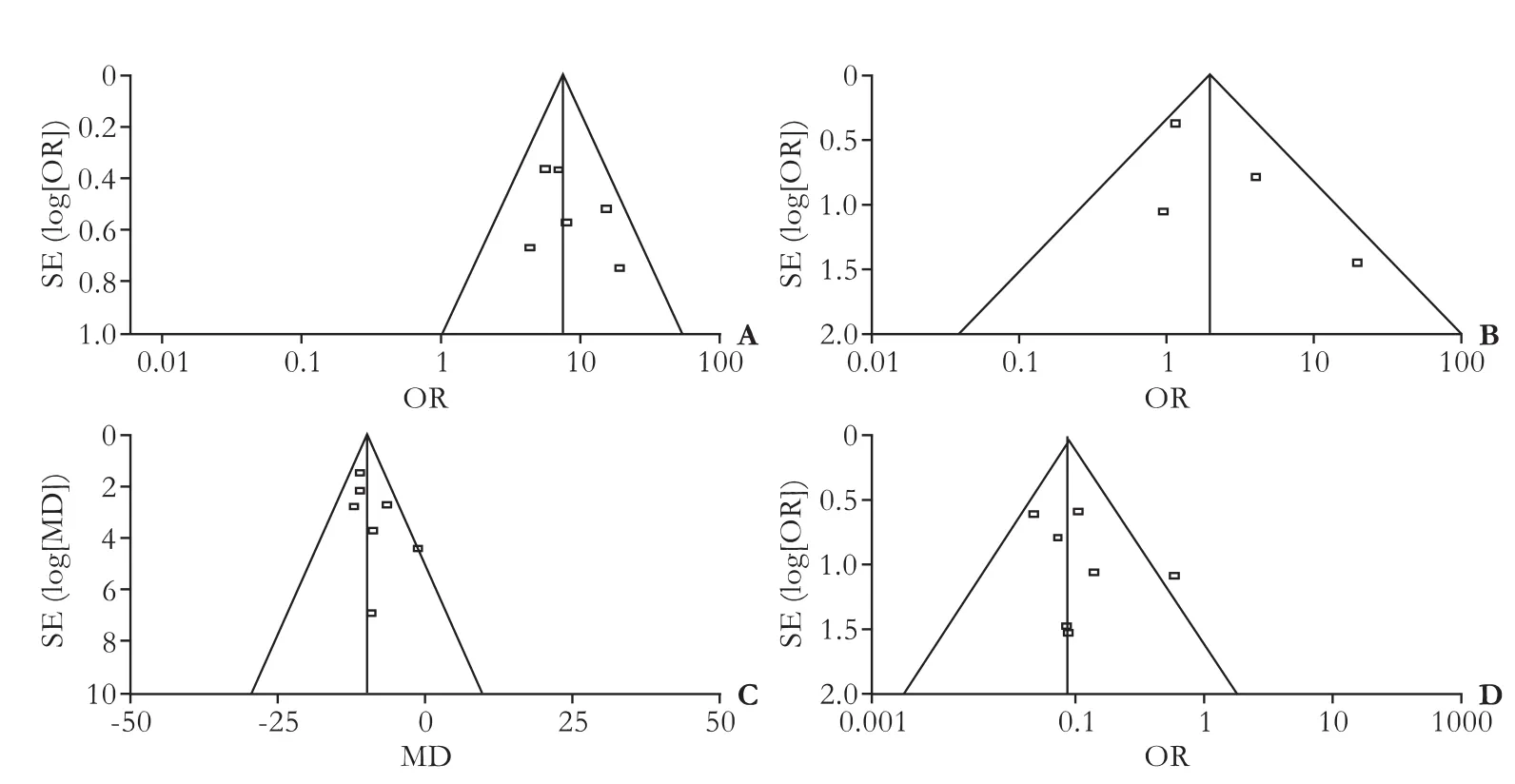
Fig. 5. A: Funnel plot of studies included in meta-analysis on the incidence of PBM in GBC(+) patients and GBC(-) patients. B: Funnel plot of studies included in meta-analysis on sex distribution in GBC(+)/ PBM(+) patients and GBC(+)/ PBM(-) patients. C: Funnel plot of studies included in meta-analysis on the difference of mean age in GBC(+)/ PBM(+) patients and GBC(+)/ PBM(-) patients. D: Funnel plot of studies included in meta-analysis on the incidence of associated gallstone in GBC(+)/ PBM(+) patients and GBC(+)/ PBM(-) patients.
PBM was not well known worldwide as a rare congenital anomaly. However, since Babbitt in 1969[26]first described PBM in three children with CCD, numerous studies and reviews have been published regarding PBM, especially in Japan, including a large number of reports on PBM without CCD in adults.[8,9,18-24]Furthermore, the view that PBM is a significant risk factor for developing GBC was gradually being confirmed. But most previous publications involved only the collection and analysis of medical records of patients with PBM, and none evaluated these reports systematically. To our knowledge, this is the first comprehensive meta-analysis focusing on the relationship between PBM and the subsequent risk of developing GBC.
The neoplastic development of gallbladder epithelial cells in PBM patients is a multi-factor, multi-step and multi-gene pathological process, associated with many genetic mutations, such as K-ras and p53.[27-30]And the refluxing pancreatic juice is probably the most important trigger factor for the development of GBC, as it contains stable small-molecule mutagens including amino acids and peptides.[31]The persistent activation of pancreatic juice can induce gene mutations which activate the K-ras oncogene and inactivate the tumor suppressor gene p53,[27-30]resulting in gallbladder epithelial cell proliferation, metaplasia and ultimately progression to cancer.[11-13]It has been reported that the expression of proliferating cell nuclear antigen (PCNA) and Ki-67 is significantly elevated in the gallbladders of patients with PBM over those of subjects without PBM, suggesting increased gallbladder epithelial cell proliferative activity in PBM patients.[32-34]Although the precise mechanism of carcinogenesis in the gallbladder in PBM patients remains unclear, that PBM is a significant risk factor for GBC has been confirmed by many studies. However, any single study may be affected by potential confounding factors. Therefore, we systematically combined these studies using meta-analysis in order to more precisely evaluate the association between PBM and GBC.
Six of the nine studies compared the incidence of PBM in GBC patients versus controls, and found the overall OR of 7.41 (95% CI: 5.03 to 10.87), which strongly supports the conclusion that the incidence of PBM among GBC patients is significantly higher than that in the general population. Interestingly, it was also reported that GBC occurs in PBM patients with a significantly higher incidence than among those without such anomaly.[9,20]This causal relationship between PBM and GBC strongly confirms that PBM is an important risk factor for GBC.
In addition, seven of the nine studies provided raw data on the clinical features of patients with GBC with or without PBM. This allowed us to compare the age, sex and incidence of associated gallstones between these patients. The proportion of female patients in cases was 1.96-fold higher than that in controls (Fig. 4A; 95% CI: 1.09 to 3.52). This suggests that female patients with PBM are at a higher risk for developing GBC. The estimated SMD for age was -9.90 (95% CI: -11.70 to -8.10), which suggests that GBC patients with PBM are approximately 10 years younger than those without PBM (Fig. 4B).
It is now generally accepted that the presence of gallstones is the most common risk factor for developing GBC. However, based on 7 studies of 111 cases and 724 matched controls, we found that the overall OR of the incidence of gallstones in GBC patients with PBM was 0.09 (95% CI: 0.05 to 0.17) (Fig. 4C), suggesting that the presence of gallstones in these patients is much lower than that in GBC patients without PBM. Thus, we conclude that factors other than gallstones are the predominant causative factors for developing GBC in patients with PBM, who show no tendency to form simultaneous gallstones.
Table 4. Incidence of associated CCD and two types of PBM in GBC patients with PBM
A close relationship between PBM and CCD was noted early. In 1973, Babbitt et al[35]first theorized that in PBM patients, pancreatic juice refluxes into the bile duct, inducing repeated cholangitis, and thereby causes bile duct wall thickening, stenosis and dilatation. Subsequently, Kimura et al[36]described 30 cases of CCD with a very high incidence of PBM. Similar observations were made by Yamaguchi et al. He reported this occurrence in 10.5% of 1433 cases (151/1433).[37]Using an animal model of PBM, Kato et al[38]demonstrated that pancreatic juice in the common bile duct causes CCD, corroborating the theory of Babbitt. Although PBM is very frequently associated with CCD and both anomalies are thought to occur in the first ten weeks of gestation, PBM is an independent disease entity from CCD, associated with a completely different embryogenic etiology.[39]However, as with PBM, it was also reported that biliary carcinoma is associated with CCD with a relatively high frequency,[25,40,41]involving a variety of oncogenic mechanisms such as cholestasis, biliary infection and reflux of pancreatic juice.[40-43]It thus seems that there is a complex relationship between PBM, CCD and tumorigenesis in the gallbladder. And it is hard to explain which of PBM and CCD is more important and direct in facilitating GBC development and progression. However, several studies found a significantly higher incidence of GBC in cases with PBM and without CCD than those with CCD.[44,45]Eight of the studies included in our analysis compared GBC patients with PBM with CCD versus those without CCD (Table 4). Of these, six studies found that the absence of an association with CCD had a higher incidence ranging from 52.2% to 85.7% in GBC patients with PBM. Only Kang et al[18]found that seven patients (70%) with GBC associated with PBM had CCD, and the remaining three (30%) did not. Therefore, all of these studies seemingly revealed that tumorigenesis in the gallbladder is more directly associated with PBM than with CCD.
The reason for the difference in the incidence of GBC between cases of PBM with and without CCD is still unknown, but it has been speculated that anatomical differences may explain this. PBM can provide stagnant sites exposed to a mixture of bile and pancreatic juice over a prolonged period. Prolonged exposure causes persistent chronic inflammation in the biliary lining epithelium, leading to hyperplasia, atypia and ultimately carcinoma. This suggests that the stagnant site is an indispensable factor for carcinogenesis.[40,44,46,47]Such sites could be provided by cysts and the gallbladder in patients with PBM along with CCD, and a malignant process is more likely to occur within a cyst rather than within the gallbladder. This is in sharp contrast to patients with PBM and without CCD where only the gallbladder serves as a reservoir for the mixture of bile and pancreatic juice.[40,44]
In addition, the type of PBM was thought to be closely related to the occurrence of GBC. Of the nine studies in our analysis, seven provided raw data on the type of PBM (Table 4). Of these, five studies found that 70.0% to 85.7% of GBC patients with PBM belonged to the P-C type, which was much higher than the C-P type. This is consistent with previous studies that GBC is more frequently associated with the P-C type of PBM.[9,44,48]Thus, patients of the P-C type were considered to belong to the higher risk group for GBC. This was because several authors indicated that the P-C type of PBM was rarely associated with CCD, but the C-P type was usually associated with it.[9,18,44]In the above analysis, we revealed that GBC occurs more frequently in patients with PBM without CCD than in those with CCD. In other words, GBC occurs more frequently in patients with the P-C type of PBM because most cases of this type are not associated with CCD. It is postulated that the confluence of the right-angle between the pancreaticduct and the bile duct in the P-C type of PBM makes it easier for the reflux of pancreatic juice, followed by more serious injury than in the C-P type.[18]This result suggests that GBC patients with the P-C type of PBM may show a higher degree of malignancy than those with the C-P type.
All case-control studies included in this metaanalysis were retrospective, consequently addressing heterogeneity between studies because of the possibility of recall bias in such studies. But no substantial heterogeneity was found in our meta-analysis, which strongly supports our conclusion. Publication bias, a practically inevitable problem in a meta-analysis,[49]was not found in this literature, as indicated by the relatively symmetric funnel plots (Fig. 5). However, a variety of other confounding factors were not completely ruled out, such as race, gender, age, gallstones and associated with or without CCD, all of which may affect the incidence of GBC. But these concerns were remarkably alleviated because we also made a meta-analysis of the demographic and clinical data on GBC patients with or without PBM and no substantial heterogeneity was found. In addition, due to the limitations of the included studies themselves, all were designed according to Kimura's classification of PBM: type P-C and type C-P, and similarly, all PBM patients in the included studies were also divided into two groups regardless of the type of CCD: with or without. Both rough classifications are not the most accepted current classifications, which may to some extent have distorted the accuracy of our conclusions.
In summary, despite the limitations, our metaanalysis provides a set of results that reflect the relationship between PBM and GBC. Our results show a high incidence of GBC in PBM patients, especially relatively young female patients without gallstones. PBM is a high-risk factor for developing GBC, especially the P-C type of PBM without CCD.
In view of the above, it is most important to detect PBM before the occurrence of GBC. The general principles for the diagnosis of PBM can be divided roughly into two categories, according to whether or not it is associated with CCD. First, for PBM patients with CCD, because they are often symptomatic showing abdominal pain, jaundice and liver dysfunction,[9,46]it is easy to recommend an abdominal ultrasound examination, thereby revealing the dilated common bile duct. And a patient with suspected PBM should be confirmed on subsequent ERCP examination. Therefore, there is a relatively high rate of PBM diagnosis in such patients. Second, in contrast, for PBM patients without CCD, almost all have only very mild symptoms or are asymptomatic before overt malignancy, resulting in reduced and delayed diagnosis.[50]However, the widespread use of ultrasonography has resulted in increasing numbers of patients undergoing this examination. Once ultrasonography and/or endoscopic ultrasonography images show the diffuse thickened gallbladder wall,[51]particularly in a young female with unexplained abdominal pain, ERCP examination should be considered to confirm the existence of PBM without CCD after the exclusion of other diseases.
Consistent with the diagnosis of PBM, its treatment can also be divided into two major categories according to whether or not it is associated with CCD. First, for PBM patients with CCD, although the relative risk for developing GBC is lower than that in PBM patients without CCD, they often have cholangitis, pancreatitis[22]and even bile duct carcinoma.[8,22]In order to prevent such diseases, cholecystectomy and resection of the dilated bile duct are required.[11,25,52]However, the role of extrahepatic bile duct resection in the prevention of bile duct carcinoma remains conjectural in PBM patients with CCD, because of the still high incidence of bile duct carcinoma compared with that of the general population after surgery.[53]Second, because PBM patients without CCD have more advanced stages of GBC, they have worse outcomes than those with CCD.[24]To prevent GBC, prophylactic laparoscopic cholecystectomy rather than extrahepatic bile duct resection is highly recommended, especially in young female patients without gallstones, but long-term follow up for bile duct carcinoma is required. However, when patients continue to have repeated cholangitis and/or pancreatitis after cholecystectomy, additional resection of the extrahepatic bile duct should be considered.[54]
Funding:None.
Ethical approval:Not needed.
Contributors:DYL and XXZ proposed the study. DYL, CNS, LYX and YC collected the data. DYL, YC and XXZ analyzed and interpreted the data. DYL wrote the draft of the manuscript. All authors contributed to the design and interpretation of the study and to further drafts. XXZ is the guarantor.
Competing interest:No bene fits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin 2006;56:106-130.
2 Dowling GP, Kelly JK. The histogenesis of adenocarcinoma of the gallbladder. Cancer 1986;58:1702-1708.
3 Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int JCancer 2006;118:1591-1602.
4 Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology 2009;55:218-229.
5 Sasatomi E, Tokunaga O, Miyazaki K. Precancerous conditions of gallbladder carcinoma: overview of histopathologic characteristics and molecular genetic findings. J Hepatobiliary Pancreat Surg 2000;7:556-567.
6 Kato O, Hattori K, Suzuki T, Tachino F, Yuasa T. Clinical significance of anomalous pancreaticobiliary union. Gastrointest Endosc 1983;29:94-98.
7 Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, et al. Pancreaticobiliary maljunction: retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg 2003;10:345-351.
8 Roukounakis N, Manolakopoulos S, Tzourmakliotis D, Bethanis S, McCarty TM, Cuhn J. Biliary tract malignancy and abnormal pancreaticobiliary junction in a Western population. J Gastroenterol Hepatol 2007;22:1949-1952.
9 Kimura K, Ohto M, Saisho H, Unozawa T, Tsuchiya Y, Morita M, et al. Association of gallbladder carcinoma and anomalous pancreaticobiliary ductal union. Gastroenterology 1985;89: 1258-1265.
10 Komi N, Takehara H, Kunitomo K, Miyoshi Y, Yagi T. Does the type of anomalous arrangement of pancreaticobiliary ducts influence the surgery and prognosis of choledochal cyst? J Pediatr Surg 1992;27:728-731.
11 Matsumoto Y, Fujii H, Itakura J, Matsuda M, Yang Y, Nobukawa B, et al. Pancreaticobiliary maljunction: pathophysiological and clinical aspects and the impact on biliary carcinogenesis. Langenbecks Arch Surg 2003;388:122-131.
12 Funabiki T, Matsubara T, Miyakawa S, Ishihara S. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg 2009; 394:159-169.
13 Kamisawa T, Takuma K, Anjiki H, Egawa N, Kurata M, Honda G, et al. Pancreaticobiliary maljunction. Clin Gastroenterol Hepatol 2009;7:S84-88.
14 Lichtenstein MJ, Mulrow CD, Elwood PC. Guidelines for reading case-control studies. J Chronic Dis 1987;40:893-903.
15 Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol 1994;140:290-296.
16 Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629-634.
17 Sterne JA, Egger M. Funnel plots for detecting bias in metaanalysis: guidelines on choice of axis. J Clin Epidemiol 2001; 54:1046-1055.
18 Kang CM, Kim KS, Choi JS, Lee WJ, Kim BR. Gallbladder carcinoma associated with anomalous pancreaticobiliary duct junction. Can J Gastroenterol 2007;21:383-387.
19 Chijiiwa K, Tanaka M, Nakayama F. Adenocarcinoma of the gallbladder associated with anomalous pancreaticobiliary ductal junction. Am Surg 1993;59:430-434.
20 Hu B, Gong B, Zhou DY. Association of anomalous pancreaticobiliary ductal junction with gallbladder carcinoma in Chinese patients: an ERCP study. Gastrointest Endosc 2003;57:541-545.
21 Wang HP, Wu MS, Lin CC, Chang LY, Kao AW, Wang HH, et al. Pancreaticobiliary diseases associated with anomalous pancreaticobiliary ductal union. Gastrointest Endosc 1998; 48:184-189.
22 Kamisawa T, Honda G, Kurata M, Tokura M, Tsuruta K. Pancreatobiliary disorders associated with pancreaticobiliary maljunction. Dig Surg 2010;27:100-104.
23 Chao TC, Jan YY, Chen MF. Primary carcinoma of the gallbladder associated with anomalous pancreaticobiliary ductal junction. J Clin Gastroenterol 1995;21:306-308.
24 Elnemr A, Ohta T, Kayahara M, Kitagawa H, Yoshimoto K, Tani T, et al. Anomalous pancreaticobiliary ductal junction without bile duct dilatation in gallbladder cancer. Hepatogastroenterology 2001;48:382-386.
25 Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 1977;134:263-269.
26 Babbitt DP. Congenital choledochal cysts: new etiological concept based on anomalous relationships of the common bile duct and pancreatic bulb. Ann Radiol (Paris) 1969;12: 231-240.
27 Hanada K, Itoh M, Fujii K, Tsuchida A, Ooishi H, Kajiyama G. K-ras and p53 mutations in stageigallbladder carcinoma with an anomalous junction of the pancreaticobiliary duct. Cancer 1996;77:452-458.
28 Hanada K, Tsuchida A, Iwao T, Eguchi N, Sasaki T, Morinaka K, et al. Gene mutations of K-ras in gallbladder mucosae and gallbladder carcinoma with an anomalous junction of the pancreaticobiliary duct. Am J Gastroenterol 1999;94:1638-1642.
29 Obara T, Tanno S, Fujii T, Izawa T, Mizukami Y, Yanagawa N, et al. Epithelial cell proliferation and gene mutation in the mucosa of gallbladder with pancreaticobiliary malunion and cancer. J Hepatobiliary Pancreat Surg 1999;6:229-236.
30 Tanno S, Obara T, Fujii T, Mizukami Y, Shudo R, Nishino N, et al. Proliferative potential and K-ras mutation in epithelial hyperplasia of the gallbladder in patients with anomalous pancreaticobiliary ductal union. Cancer 1998;83:267-275.
31 Mizuno M, Kato T, Koyama K. An analysis of mutagens in the contents of the biliary tract in pancreaticobiliary maljunction. Surg Today 1996;26:597-602.
32 Yang Y, Fujii H, Matsumoto Y, Suzuki K, Kawaoi A, Suda K. Carcinoma of the gallbladder and anomalous arrangement of the pancreaticobiliary ductal system: cell kinetic studies of gallbladder epithelial cells. J Gastroenterol 1997;32:801-807.
33 Isozaki H, Okajima K, Hara H, Sako S, Mabuchi H. Proliferating cell nuclear antigen expression in the gallbladder with pancreaticobiliary maljunction. J Surg Oncol 1997;65: 46-49.
34 Ono S, Tokiwa K, Iwai N. Cellular activity in the gallbladder of children with anomalous arrangement of the pancreaticobiliary duct. J Pediatr Surg 1999;34:962-966.
35 Babbitt DP, Starshak RJ, Clemett AR. Choledochal cyst: a concept of etiology. Am J Roentgenol Radium Ther Nucl Med 1973;119:57-62.
36 Kimura K, Ohto M, Ono T, Tsuchiya Y, Saisho H, Kawamura K, et al. Congenital cystic dilatation of the common bile duct: relationship to anomalous pancreaticobiliary ductal union. AJR Am J Roentgenol 1977;128:571-577.
37 Yamaguchi M. Congenital choledochal cyst. Analysis of 1,433 patients in the Japanese literature. Am J Surg 1980;140:653-657.
38 Kato T, Hebiguchi T, Kasai M. Etiology of congenital choledochal cyst. Tohoku J Exp Med 1980;131:135-142.
39 Matsumoto Y, Fujii H, Itakura J, Mogaki M, Matsuda M, Morozumi A, et al. Pancreaticobiliary maljunction: etiologic concepts based on radiologic aspects. Gastrointest Endosc 2001;53:614-619.
40 Tsuchiya R, Harada N, Ito T, Furukawa M, Yoshihiro I. Malignant tumors in choledochal cysts. Ann Surg 1977;186: 22-28.
41 Komi N, Tamura T, Miyoshi Y, Kunitomo K, Udaka H, Takehara H. Nationwide survey of cases of choledochal cyst. Analysis of coexistent anomalies, complications and surgical treatment in 645 cases. Surg Gastroenterol 1984;3:69-73.
42 Kato T, Hebiguchi T, Matsuda K, Yoshino H. Action of pancreatic juice on the bile duct: pathogenesis of congenital choledochal cyst. J Pediatr Surg 1981;16:146-151.
43 Benjamin IS. Biliary cystic disease: the risk of cancer. J Hepatobiliary Pancreat Surg 2003;10:335-339.
44 Yamauchi S, Koga A, Matsumoto S, Tanaka M, Nakayama F. Anomalous junction of pancreaticobiliary duct without congenital choledochal cyst: a possible risk factor for gallbladder cancer. Am J Gastroenterol 1987;82:20-24.
45 Sugiyama M, Atomi Y. Anomalous pancreaticobiliary junction without congenital choledochal cyst. Br J Surg 1998; 85:911-916.
46 Misra SP, Dwivedi M. Pancreaticobiliary ductal union. Gut 1990;31:1144-1149.
47 Kinoshita H, Nagata E, Hirohashi K, Sakai K, Kobayashi Y. Carcinoma of the gallbladder with an anomalous connection between the choledochus and the pancreatic duct. Report of 10 cases and review of the literature in Japan. Cancer 1984;54: 762-769.
48 Mori K, Nagakawa T, Ohta T, Nakano T, Kayahara M, Kanno M, et al. Association between gallbladder cancer and anomalous union of the pancreaticobiliary ductal system. Hepatogastroenterology 1993;40:56-60.
49 Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on metaanalyses. BMJ 2000;320:1574-1577.
50 Sameshima Y, Uchimura M, Muto Y, Maeda J, Tsuchiyama H. Coexistent carcinoma in congenital dilatation of the bile duct and anomalous arrangement of the pancreatico-bile duct. Carcinogenesis of coexistent gall bladder carcinoma. Cancer 1987;60:1883-1890.
51 Tanno S, Obara T, Maguchi H, Mizukami Y, Shudo R, Fujii T, et al. Thickened inner hypoechoic layer of the gallbladder wall in the diagnosis of anomalous pancreaticobiliary ductal union with endosonography. Gastrointest Endosc 1997;46: 520-526.
52 Funabiki T, Matsubara T, Ochiai M, Marugami Y, Sakurai Y, Hasegawa S, et al. Surgical strategy for patients with pancreaticobiliary maljunction without choledocal dilatation. Keio J Med 1997;46:169-172.
53 Kobayashi S, Asano T, Yamasaki M, Kenmochi T, Nakagohri T, Ochiai T. Risk of bile duct carcinogenesis after excision of extrahepatic bile ducts in pancreaticobiliary maljunction. Surgery 1999;126:939-944.
54 Ohuchida J, Chijiiwa K, Hiyoshi M, Kobayashi K, Konomi H, Tanaka M. Long-term results of treatment for pancreaticobiliary maljunction without bile duct dilatation. Arch Surg 2006;141:1066-1070.
Received June 4, 2011
Accepted after revision September 19, 2011
Author Affiliations: Department of Biliary Surgery, West China Hospital, Sichuan University, Chengdu 610041, China (Deng YL, Cheng NS, Lin YX, Zhou RX, Yang C, Jin YW and Xiong XZ)
Xian-Ze Xiong, MM, Department of Biliary Surgery, West China Hospital, Sichuan University, Chengdu 610041, China (Tel: 86-28-85422465; Email: Xiongxze@sina.com)
? 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60098-2
 Hepatobiliary & Pancreatic Diseases International2011年6期
Hepatobiliary & Pancreatic Diseases International2011年6期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study
- Fulminant liver failure models with subsequent encephalopathy in the mouse
- Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway
- Undifferentiated embryonal sarcoma of the liver presenting as a hemorrhagic cystic tumor in an adult
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma
