Effect of Danshen on apoptosis and NF-кB protein expression of the intestinal mucosa of rats with severe acute pancreatitisor obstructive jaundice
Xi-Ping Zhang, Jun Jiang, Ya-Ping Yu, Qi-Hui Cheng and Bin Chen
Hangzhou, China
Effect of Danshen on apoptosis and NF-кB protein expression of the intestinal mucosa of rats with severe acute pancreatitis
or obstructive jaundice
Xi-Ping Zhang, Jun Jiang, Ya-Ping Yu, Qi-Hui Cheng and Bin Chen
Hangzhou, China
BACKGROUND:Intestinal mucosa injury in cases of severe acute pancreatitis (SAP) or obstructive jaundice (OJ) is one of the main reasons for the accelerated aggravation of these diseases. Besides being an organ to digest and absorb nutrients, the intestine is also a unique immune organ. When SAP and OJ develop, the destruction of the intestinal mucosa barrier is an important contributing factor for the development of bacterial translocation, systemic inflammatory response syndrome, and multiple organ dysfunction syndrome. It is important to protect the intestinal mucosa in the therapy for SAP and OJ. In this study, we determined the effect ofRadix Salviae Miltiorrhizae(Danshen) injection on apoptosis and NF-κB P65 protein expression in the intestinal mucosa of rats with SAP or OJ, and explored the protective mechanism of Danshen in their mucosa.
METHODS:Sprague-Dawley rats were used in the SAP and OJ experiments. These rats were randomly divided into shamoperated, model control, and treated groups. At various times after operation, the mortality rates were calculated. Subsequently, the rats were killed to assess the pathological changes, the expression levels of Bax and NF-κB proteins, and the apoptosis indices in the intestinal mucosa.
RESULTS:Compared to the corresponding model control group, the number of SAP or OJ rats that died in the treated group decreased but showed no statistically significant difference. At all time points after operation, there was nosignificant difference between the treated and model control groups in the staining intensity as well as the product of staining intensity and positive staining rate of Bax protein in the intestinal mucosa of SAP and OJ rats . At 3 hours after operation, the apoptosis index of the intestinal mucosa of SAP rats in the treated group was lower than that in the model control group (P<0.01). At 12 hours after operation in SAP rats and 28 days after operation in OJ rats, the staining intensity as well as the product of staining intensity and positive staining rate of NF-κB protein of the intestinal mucosa in the treated group were lower than those in the model control group (P<0.01).
CONCLUSION:Danshen exerts protective effects on the intestinal mucosa of SAP and OJ rats perhaps by inhibiting apoptosis and down-regulating NF-κB protein.
(Hepatobiliary Pancreat Dis Int 2010; 9: 537-546)
severe acute pancreatitis; obstructive jaundice;Radix Salviae Miltiorrhizae; traditional Chinese medicine; intestinal mucosa; NF-κB; apoptosis; tissue microarray
Introduction
When severe acute pancreatitis (SAP) or obstructive jaundice (OJ) develops, numerous inflammatory mediators are activated. After being transmitted through the circulatory system, these mediators may induce multiple organ dysfunctions. The intestine is one of the target organs that are vulnerable to injury in the development of multiple organ dysfunction syndromes (MODS). Some experimental studies[1,2]have shown that intestinal mucosa injury in SAP or OJ is one of the main reasons for the accelerated aggravation of these diseases.
The main active ingredients ofRadix Salviae Miltiorrhizae(Danshen) include tanshinone I, IIA and IIB, as well as isotanshinoneiand IIA. Danshen protects endothelial cells, fights against inflammation, resists lipid peroxidation, and prevents calcium overload. Some studies have shown that it can exert protective effects on the intestinal mucosa in animal models of SAP[3,4]and OJ[5-8]or patients by reducing the translocation of intestinal bacteria. However, it remains unknown whether it can reduce the mortality rates of these diseases. At present, Danshen is widely applied in the therapy of SAP in clinical or experimental studies, showing prominent efficacy. In contrast, few studies have been performed to investigate its efficacy in the treatment of OJ. The majority of studies on the effect of Danshen on OJ are in the experimental stage. Some studies have shown that Danshen improves hepatic blood flow by reducing lipid peroxidation[5]and protects the normal morphology and function of the kidney.[9,10]
This study aimed to explore the protective effects and mechanism of action of Danshen on the intestinal mucosa of SAP and OJ rats by assessing the effect of Danshen injection on apoptosis of intestinal mucosa cells and the expression levels of NF-κB proteins. The results provide new clues for clinical therapy of SAP and OJ.
Methods
Experimental animals and reagents
A total of 288 healthy male Sprague-Dawley rats of clean grade (weighing 270-330 g), were provided by the Laboratory Animal Research Center of Zhejiang University of Traditional Chinese Medicine (China). Of them, 108 were used for SAP experiments and randomly divided into sham-operated, model control, and treated groups (n=36 per group), which were further randomly subdivided into 3, 6, and 12 hour groups (n=12 per group) according to the time after operation; another 180 rats were used for OJ experiments and randomly divided into sham-operated, model control, and treated groups (n=60 per group), which were further randomly subdivided into 7, 14, 21 and 28 day groups (n=15 per group) according to the time after operation.
Sodium taurocholate and sodium pentobarbital were from Sigma Corp. (USA). Danshen injection (each 10 ml vial contained active components equivalent to 15 g of the original medicine) was from Chiatai Qingchunbao Pharmaceutical Co., Ltd. (China); anti-NF-κB P65 and anti-Bax antibodies were from Santa Cruz Biotechnology, Inc. (USA); the TUNEL assay kit was from Takara Bio Inc. (Japan).
Preparation of SAP and OJ models, and associated therapeutic regimens
The rats were anesthetized with an intraperitoneal injection of 2.5% sodium pentobarbital. We have reported the procedure of the preparation of SAP and OJ models as well as the associated therapeutic regimens in previous studies.[11-14]The observation periods in the SAP experiment were 3, 6 and 12 hours in the corresponding groups, while in the OJ experiment the periods were 7, 14, 21 and 28 days.
Immunohistochemical staining of Bax and NF-κB P65 proteins in intestinal mucosa
Intestinal tissues were first prepared. The diameter of the needle was 1.5 mm, and the Envision twostep method was used to detect the expression levels of Bax and NF-κB P65 proteins in the intestine. The evaluation standards were: 1) the staining intensity was evaluated according to the extent of cell coloration: negative staining (-); mild staining, positively-stained cells showing a yellow pigment (+); moderate staining, positively-stained cells showing a brown pigment (++); intense staining, positively-stained cells showing a dark brown pigment (+++), each of which was scored as 0, 1, 2 and 3 points respectively for statistical analysis; 2) the positive staining rate was evaluated as: no positive cells (-); ≤25% positive cells (+); 26%-50% positive cells (++); and >50% positive cells (+++), each of which was scored as 0, 1, 2 and 3 points respectively for statistical analysis.
TUNEL staining in the intestinal mucosa
In tissue sections,in situDNA nick endlabeling (TUNEL) staining steps were performed as follows: Sections were baked at 60 ℃ for 30 minutes, deparaffined, and washed with Milli-Q for 5 minutes. Tissue was processed with protease K (10 μg/μl) at room temperature for 15 minutes and washed with PBS for 5 minutes. H2O2solution (3%) was used to block endogenous peroxydase for 5 minutes, then washed twice with PBS for 5 minutes each. Reaction solution (30 μl) was added in freezing conditions (TdT enzyme: labeling safe buffer=1∶10), incubated at 37 ℃ for 90 minutes, and washed twice with PBS for 5 minutes each. Anti-FITC HRP conjugate (50 μl) was added, incubated at 37 ℃ for 30 minutes, and washed twice with PBS for 5 minutes each. Staining was developed with DAB, which was terminated with a Milli-Q wash. After hematoxylin counterstaining, routine dehydration, and clearing, the tissues were mounted in neutral gum. The apoptotic index was calculated as: apoptotic index=apoptotic cell count/total cell count ×100%.
Measurements
The pathological changes of the intestinal mucosa of rats with SAP and OJ as well as those of SAP rats at 12 hours after operation were observed grossly and under light or electron microscopy. Subsequently, the mortality rates were recorded. According to the characteristics of the pathological changes in the intestinal mucosa, we established a severity score standard (Table 1) that was then used to score and compare the pathological changes in rats in each group. The changes in expression levels of Bax and NF-κB P65 proteins as well as the apoptosis index in intestinal mucosa were measured.
Statistical analysis
The compiled data were input into Excel, and then read into SPSS15.0 for further analysis. Normal data were expressed as mean±SD, while non-normal data were expressed as median (interquartile range). Analysis of variance and pairwise comparisons were used for normal data, whereas non-normal data were subjected to non-parametric tests, among which the Kruskal-Wallis test was used for pairwise comparisons and the Mann-WhitneyUtest for multiple comparisons. The Chi-square test was used for intergroup comparisons of mortality rates.
Results
SAP experiments
Mortality rate
One rat died at 3 hours and five at 12 hours after operation in the model control group; three rats died at 12 hours in the treated group, and no rats died in the other groups. There was no marked difference in mortality rate between 3 and 6 hours. At 12 hours, only the mortality rate in the model control group was higher than that in the sham-operated group (P=0.037), and no significant difference in mortality rate occurred among the other groups.
Pathological changes and pathological severity scores in intestinal mucosa
In the sham-operated group, neither enlargement of the intestinal canal nor congestion or edema of the intestinal wall was seen. The small intestinal mucosa was smooth and intact. No evident differences in pathological changes under light microscopy were observed among each time point after operation. The mucosa was normal in the majority of rats, and mild inflammatory cell infiltration was seen in the propria, submucous, and serosal layers. Goblet cells and intestinal microvilli under electron microscopy were normal. Columnar epithelial cells showed an orderly arrangement.
In the model control group, at 6 and 12 hours after operation, the intestinal canal was clearly enlarged and showed gas or fluid retention; congestion or edema of the wall was seen; small areas of ulceration in the small intestinal mucosa was observed in a few severe cases. Pathological changes under light microscopy were slightly aggravated with the increase in postoperative time. Edema and focal necrosis of the propria, submucous, and serosal layers were seen in most rats. Inflammatory cell infiltration was viewed in the various layers that constitute the mucosa. At 6 and 12 hours, the microvilli of intestinal epithelial cells were disorderly and partly shed. Defects of microvilli of the epithelial cells, collapse, shortening, and decrease of the intestinal canal, and apoptosis of lymphocytes were seen under an electron microscope.
In the treated group, the intestinal canal was slightly enlarged and showed mild gas or fluid retention; congestion and edema of the wall were evidently milder than in the model control group. Pathological changes under a light microscope were mitigated with the increase in therapeutic duration. At 3 hours after operation, the intestinal mucosa was not intact in some rats, and edema of the propria, submucous, and serosal layers was seen. At 6 hours, edema of the propria, submucous, and serosal layers was seen in the majority of rats; the mucosa showed no abnormality in some rats. At 12 hours, the mucosa showed no abnormality in most rats. The morphology and structure of the mucosa under an electron microscope were roughly normal, and the arrangement of the intestinal microvilli and columnar epithelium cells was orderly.
At all time points after operation, the pathological severity scores for the intestinal mucosa in the modelcontrol group were significantly higher than those in the sham-operated group. At 3 hours after operation, the score in the treated group was significantly higher than that in the sham-operated group. At 12 hours, the score in the treated group was significantly lower than that in the model control group (Tables 1 and 2).
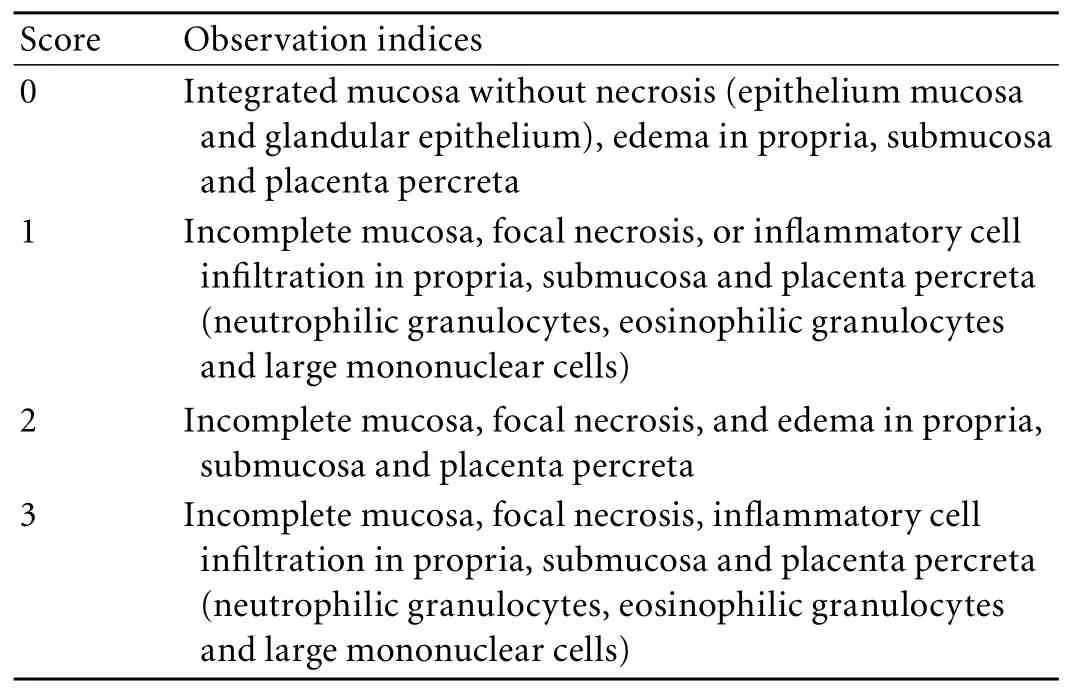
Table 1. Pathological severity score standard for intestinal mucosa
Staining intensity and positive staining rate of Bax protein
The positive signals for Bax protein were mainly localized in the cytoplasm of intestinal mucosa epithelial cells and occasionally in the cytoplasm of acinar epithelial cells and inflammatory cells. At 3 hours after operation, the staining intensity of Bax protein was higher in the model control and treated groups than in the sham-operated group (P<0.01). No significant difference was noted between the treated and model control groups (Table 2).
At 3 and 12 hours, the products of staining intensity and positive staining rate of Bax in the model control group were higher than those in the sham-operated group (P<0.01). At all time points after operation, no significant differences were noted between the treated and sham-operated groups or between the treated and model control groups (Table 2 and Fig. 1).
Apoptosis index
Apoptosis was mainly seen in intestinal mucosa epithelial cells. Very few apoptotic inflammatory cells were seen in the propria layer. At all time points after operation, no marked differences were noted between the treated and sham-operated groups or between the treated and model control groups. At 3 hours after operation, the apoptosis index was higher in the model control group than in the treated group (P<0.01) (Table 2 and Fig. 2).
Staining intensity and positive staining rate of NF-κB protein
The positive signals for NF-κB protein were mainly localized in the cytoplasm of intestinal mucosa epithelial cells and occasionally in the cytoplasm of inflammatory cells. The positive signals were mainly localized in the cytoplasm though a few were seen in the nucleus. At 6 hours after operation, the staining intensity of NF-κB was higher in the model control group than in the shamoperated group (P<0.01). No significant difference was noted between the sham-operated and treated groups. At 12 hours, the staining intensity in the treated groupwas significantly lower than that in the model control group (P<0.01) (Table 2).
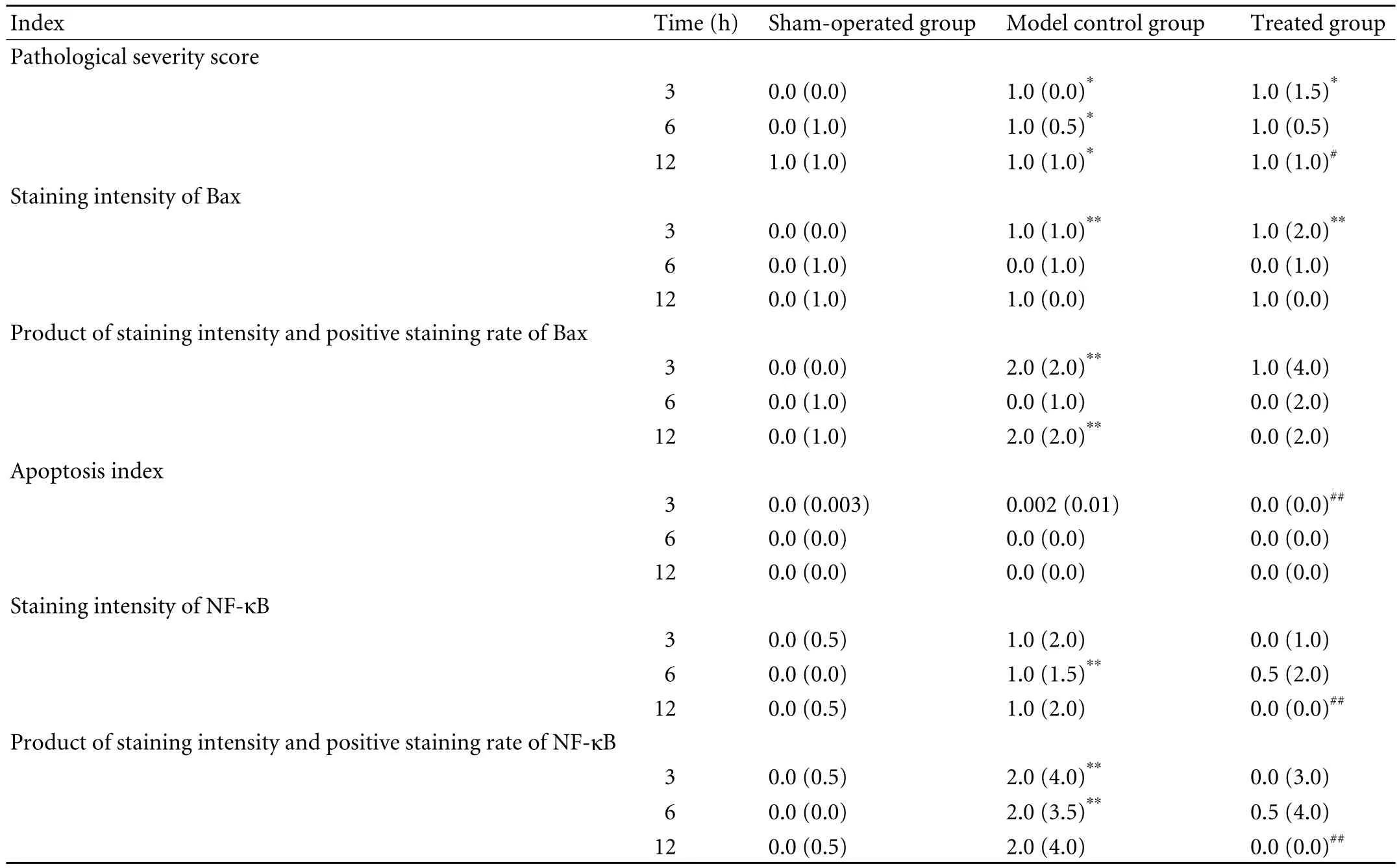
Table 2. Pathological indices in SAP groups (M(QR))
At 3 and 12 hours after operation, the staining intensity and positive staining rate of NF-κB were higher in the model control group than in the sham-operated group (P<0.01). At all time points after operation, no significant differences were observed between the treated and sham-operated groups. At 12 hours, the rates were lower in the treated group than in the model control group (P<0.01) (Table 2).
OJ experiments
Mortality rate
Two rats died on day 7, four on day 14, four on day 21, and seven on day 28 after operation in the model control group; while three died on day 14, two on day 21, and four on day 28 in the treated group. The mortality rates at 7 days showed no marked difference among the three groups. On days 14 and 21, the rates in the shamoperated group were lower than those in the model control group (P=0.032). On day 28, the rates in the sham-operated group were lower than those in both the model control (P=0.006) and treated groups (P=0.032).
Pathological changes and pathological severity scores of intestinal mucosa
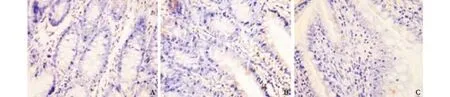
Fig. 1. Expression of Bax protein at 12 hours after operation in SAP experiment (original magnification ×200). A: sham-operated group, negative expression; B: model control group, positive staining rate (++); C: treated group, positive staining rate (+).
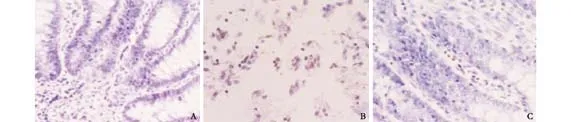
Fig. 2. Apoptotic cells at 3 hours after operation in SAP experiment (TUNEL staining, original magnification ×400). A: shamoperated group, negative expression; B: model control group, many apoptotic cells; C: treated group, a few apoptotic cells.
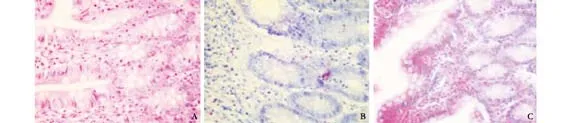
Fig. 3. Pathological changes at 28 days after operation in OJ experiment (HE staining, original magnification ×200). A: shamoperated group, normal intestinal mucosa; B: model control group, infiltration of many eosinophilic cells in intestinal lamina propria; C: treated group, infiltration of many eosinophilic cells in intestinal lamina propria.
In the sham-operated group, no obvious abnormality was seen. No differences in pathological changes undera light microscope were found at the time points after operation. The intestinal mucosa was normal in most rats. The mucosa epithelium was not intact in very few rats. Inflammatory cell infiltration was seen in the propria layer (Fig. 3A).
In the model control group, on day 7 after operation, the intestinal wall and peritoneum became jaundiced. On day 14, varying degrees of yellow staining of the intestinal wall and peritoneum were seen in the majority of rats; the intestinal canal was enlarged and showed fluid retention. On days 21 and 28, yellow staining of the wall and peritoneum was seen in all rats. No clear differences in pathological changes under a light microscope were observed among the time points after operation. On day 7, the intestinal mucosa was normal in most rats, and edema of the submucous layer was present in few rats. On day 14, the mucosa was normal in most of rats but not intact in some, and edema of the propria, submucous, and serosal layers was seen in some rats. On day 21, the mucosa was not intact in the majority of rats, edema of the propria, submucous, and serosal layers was seen in most rats, and very few showed no abnormality of the intestinal mucosa. On day 28, focal necrosis in the mucosa epithelium as well as edema of the propria, submucous, and serosal layers were seen in the majority of rats (Fig. 3B).
In the treated group, on day 7 after operation, no difference was found from the model control group. On day 14, the intestinal wall became jaundiced in half of the rats, but the intestinal canal was not enlarged and showed no fluid retention. On days 21 and 28, no obvious difference was found from the model control group. No differences in pathological changes under a light microscope were observed among time points after operation. Inflammatory cell infiltration was seen in the propria, submucous, and serosal layers in most rats. The mucosa was normal in some rats, and was not intact in very few. On day 7, the mucosa was normal in the majority of rats, and was not intact in very few. On day 14, inflammatory cell infiltration was seen in the propria, submucous, and serosal layers in the majority of rats, and very few showed no abnormality of the mucosa. On day 21, some rats showed no abnormality of the mucosa; inflammatory cell infiltration was seen in the propria layer in some; the mucosa was not intact in very few. On day 28, some rats showed no abnormality of the mucosa;inflammatory cell infiltration was seen in the propria, submucous, and serosal layers in some; and the mucosa was not intact in very few (Fig. 3C).
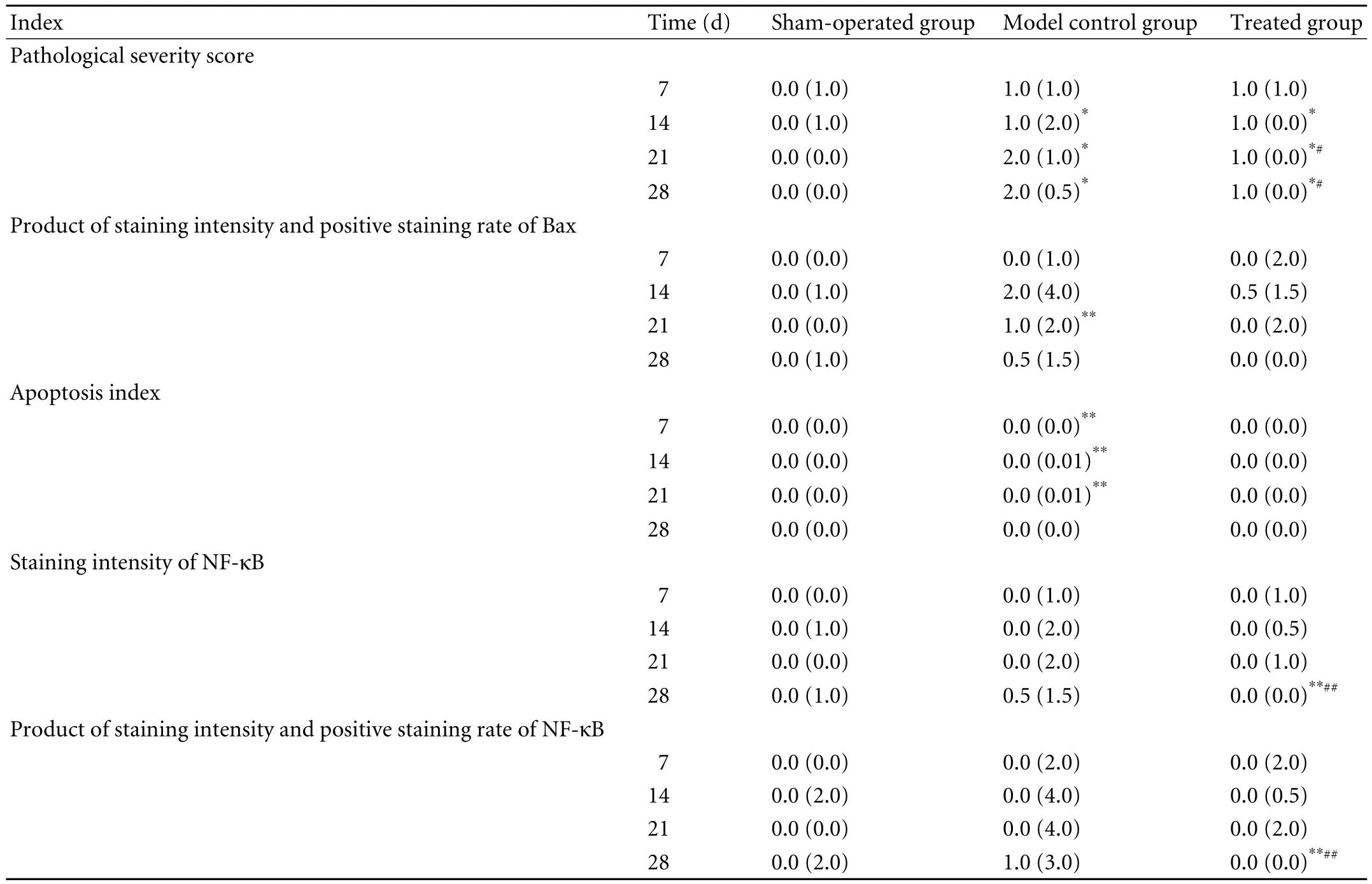
Table 3. Pathological indices in OJ groups (M(QR))
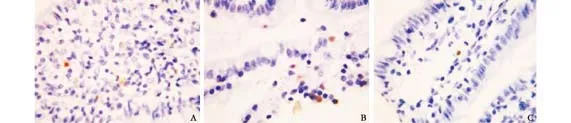
Fig. 4. Apoptotic cells in OJ experiment (TUNEL staining, original magnification ×400). A: model control group at 21 days after operation, many apoptotic cells; B: model control group at 28 days after operation, many apoptotic cells; C: treated group at 28 days after operation, a few apoptotic cells.
On days 14, 21 and 28 after operation, the pathological severity scores of the intestinal mucosa in the model control and treated groups were higher than those in the sham-operated group (P<0.05). On days 21 and 28, the scores in the treated group were lower than those in model control group (P<0.05) (Tables 1 and 3).
Staining intensity and positive staining rate of Bax protein
The positive signals for Bax protein were mainly localized in the cytoplasm of intestinal mucosa epithelial cells and occasionally in the cytoplasm of acinar epithelial cells and inflammatory cells. On day 21 after operation, the staining intensity of Bax in the model control group was higher than that in the sham-operated group (P<0.01). At all time points after operation, no significant difference was noted between the treated and sham-operated groups or between the treated and model control groups.
On day 21 after operation, the staining intensity and positive staining rate of Bax in the model control group were higher than those in the sham-operated group (P<0.01). At all time points after operation, no significant difference was noted between the treated and sham-operated groups or between the treated and model control groups (Table 3).
Apoptosis index
On days 7, 14 and 12 after operation, the apoptosis index of the intestinal mucosa was higher in the model control group than in the sham-operated group (P<0.01). At all time points after operation, no significant difference was noted between the treated and shamoperated groups or between the treated and model control groups (Fig. 4).
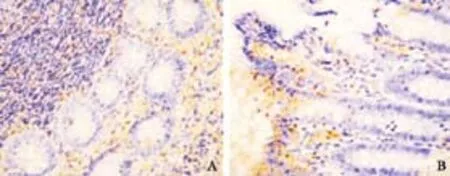
Fig. 5. Expression of NF-κB at 28 days after operation in OJ experiment (original magnification ×200). A: model control group, positive staining rate (++); B: treated group, positive staining rate (+).
Staining intensity and positive staining rate of NF-κB protein
The positive signals for NF-κB protein were mainly localized in the cytoplasm of intestinal mucosa epithelial cells and occasionally in the cytoplasm of inflammatory cells. The positive signals were mainly localized in the cytoplasm though a few were seen in the nucleus. At all time points after operation, no significant difference was noted between the treated and model control groups. On day 28 after operation, the staining intensity was lower in the treated group than in the sham-operated and model control groups (P<0.01).
At all time points after operation, no significant difference was noted between the sham-operated and model control groups. On day 21, the staining intensity and positive staining rate of NF-κB were lower in the treated group than in the sham-operated and model control groups (P<0.001) (Table 3 and Fig. 5).
Discussion
Besides being an organ to digest and absorb nutrients, the intestine is also a unique immune organ. In order to meet the needs of absorption, the intestine possesses thebody's largest mucosal surface area which separates a large number of bacteria and toxins within the intestinal cavity from the internal environment. When SAP and OJ develop, the destruction of this barrier is an important factor for the development of bacterial translocation, systemic inflammatory response syndrome (SIRS) and MODS.[1,15]Thus, it is important to protect the intestinal mucosa in the therapy of SAP and OJ.[16,17]
Apoptosis involves the activation, expression and regulation of a series of genes. Spontaneous apoptosis of intestinal epithelial cells occurs in normal humans and rodents.[18]The stability of the small intestinal mucosa depends on the equilibrium between the proliferation and apoptosis of epithelial cells. Some factors, such as ischemia/reperfusion injury, bacterial infections and nutritional deficiencies, may induce apoptosis in intestinal epithelial cells[19]and cause intestinal dysfunction.[20,21]Some studies have indicated that an increase in the apoptosis of small intestinal epithelial cells may be one of the mechanisms underlying the weakening of intestinal barrier function.[19]Bax as an apoptosis-inducing gene[22,23]induces the formation of Bcl-2[24]or Bcl-x dimers and thereby promotes apoptosis. Some studies have shown that Danshen reduces ischemia/ reperfusion-induced myocardial apoptosis[25]and ethanol-induced PC12 apoptosis[26]by downregulating the expression of Bax protein. In this study, at all time points after operation, there was no difference in the staining intensity and positive staining rate of Bax in SAP and OJ rats between the model control and treated groups, suggesting that Danshen has little or no effect on the expression of Bax protein.
We also found that, at 3 hours after operation, the apoptosis index in the intestinal mucosa in the treated group was significantly lower than that in the model control group, and mucosa injury such as inflammation, necrosis, and microvilli defects was mitigated in the treated group compared with the model control group, indicating that Danshen could protect the small intestinal mucosa of SAP rats by inhibiting the apoptosis of epithelial cells. In contrast, at all time points after operation, no marked difference in the apoptosis index in the mucosa of OJ rats was noted between the treated and sham-operated groups or between the treated and model control groups, suggesting that Danshen has a weaker effect on the apoptosis of small intestinal mucosa epithelial cells in OJ rats. Thus, we suggest that the protective effects of Danshen on the intestinal mucosa of OJ rats are unrelated to the regulation of apoptosis.
NF-κB is a multi-effect regulatory factor that controls the transcription of a variety of inflammation-, immunity- and hyperplasia-related factors, such as TNF-α, IL-1β, iNOS, and ICAM-1.[27,28]Upon activation, NF-κB induces a cytokine cascade that can impair the structure and function of multiple organs,[29]thereby causing a series of pathophysiological alterations such as SIRS and MODS. Vona-Davis et al[30]found that NF-κB antagonists significantly mitigate the extent of pathological changes and control the production of numerous cytokines in SAP rats. Hietaranta et al[31]suggested that NF-κB activation induces acute injury in extrapancreatic organs such as the lung, liver, and kidney. In recent years, some experimental studies have found that NF-κB aggravates small intestinal ischemia/ reperfusion injury,[32-34]promotes intestinal epithelial cell apoptosis[35]and thereby induces intestinal damage.[36,37]
The results of this study showed that, at all time points after operation, the expression levels of NF-κB protein and the pathological severity scores of the intestinal mucosa of SAP rats in the model control group were significantly higher than those in the sham-operated group, indicating that the mucosa damage may be related to upregulation of NF-κB protein. At 12 hours after operation in SAP rats and on day 28 after operation in OJ rats, the staining intensity of NF-κB protein in the treated group was significantly lower than that in the model control group, and the pathological changes of the mucosa were also mitigated in the treated group, suggesting that Danshen injection exerts protective effects on the intestinal mucosa of SAP and OJ rats perhaps by inhibiting the expression of NF-κB protein. Although no statistical difference in the mortality rates of rats was noted between treated and model control groups in the present study, the number of rats that died in the treated group indeed decreased. This may be because the sample size was too small. Therefore, we suggest that Danshen may be able to reduce the mortality rates of SAP and OJ rats.
In conclusion, Danshen exerts protective effects on the intestinal mucosa through inhibiting the apoptosis of mucosa cells in SAP rats and the expression of NF-κB protein in the mucosa of SAP and OJ rats.
Funding:The study was supported by grants from the Technological Foundation Project of Traditional Chinese Medical Science of Zhejiang Province (No. 2003C130) and the Intensive Foundation Project for Technology of Hangzhou (No. 2004Z006).
Ethical approval:The Animal Care Committee of Hangzhou First People's Hospital approved this study.
Contributors:ZXP and YYP wrote the first draft. CB contributed to the statistical analysis. All authors contributed to the intellectual context and approved the final version. ZXP is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Zhang XP, Zhang J, Song QL, Chen HQ. Mechanism of acute pancreatitis complicated with injury of intestinal mucosa barrier. J Zhejiang Univ Sci B 2007;8:888-895.
2 Assimakopoulos SF, Vagianos CE, Patsoukis N, Georgiou C, Nikolopoulou V, Scopa CD. Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats. Acta Physiol Scand 2004;180:177-185.
3 Kim JS, Narula AS, Jobin C. Salvia miltiorrhiza water-soluble extract, but not its constituent salvianolic acid B, abrogates LPS-induced NF-kappaB signalling in intestinal epithelial cells. Clin Exp Immunol 2005;141:288-297.
4 Sha JP, Zhu BD, Guan RH, Zhi LM, Xu Y, Zhao Y, et al. Effect on Danshen root to mitochondrial electron transport chain in intestinal mucosa epithelial cells of rabbits with severe acute pancreatitis. Journal of Chengdu University of Traditional Chinese Medicine 2003;26:1-3.
5 Lin MS, Miao HL, Bao ST, Chen M, Yi X, Wei HJ. Effect of intercellular adhesion molecule-1 on enteromucosal injury in rats with obstructive jaundice. Chinese Journal of Experimental Surgery 2003;20:130-131.
6 Yuan YN, Shi HX, Chen AH. Efficacy of compound Danshen injection for auxiliary treatment of obstructive jaundice. Lishizhen Medicine and Materia Medica Research 2006;17: 1414.
7 Yang JH, Dang ZX. Observation of the protective effect of salvia miltiorrhizae injection on intestinal mucosa barrier. Journal of Capital Institute of Medicine 1995;1:148-150.
8 Miao HL, Lin MS. Mechanism of injury of enteromucosal barrier in obstructive jaundice. Journal of Guangdong Medical College 2001;19:245-246.
9 Peng B, Du J, Jia Q, Qiao A, Wu Y, Liu X, et al. The effect of salvia miltiorrhiza and shengmai on inflammatory mediator and renal function of post-operative patients with obstructive jaundice. Hua Xi Yi Ke Da Xue Xue Bao 2001;32:587-589.
10 Jin H, Wang A, Wang Y. Preventive and therapeutic effects of radix Salviae miltiorrhizae on glycerol-induced acute renal failure in rats. Zhongguo Zhong Yao Za Zhi 1997;22:236-238, 255-256.
11 Xiping Z, Dijiong W, Jianfeng L, Qihui C, Jing Y, Penghui J, et al. Effects of Salvia miltiorrhizae on ICAM-1, TLR4, NF-kappaB and Bax proteins expression in multiple organs of rats with severe acute pancreatitis or obstructive jaundice. Inflammation 2009;32:218-232.
12 Zhang XP, Feng GH, Zhang J, Cai Y, Tian H, Zhang XF, et al. Protective effects of Salvia miltiorrhizae on the hearts of rats with severe acute pancreatits or obstructive jaundice. J Zhejiang Univ Sci B 2009;10:193-202.
13 Zhang X, Chen L, Zhang J, Tian H, Zhang X, Zhou Y, et al. Effect of salvia miltiorrhizae on apoptosis and NF-kappaB p65 expression in the liver of rats with severe acute pancreatitis or obstructive jaundice. J Gastroenterol Hepatol 2009;24:841-852.
14 Xiping Z, Yang C, Dijiong W, Jie Z, Qian Y, Xinge J, et al. Effects of Salvia miltiorrhiza on intercellular adhesion molecule 1 protein expression in the lungs of rats with severe acute pancreatitis or obstructive jaundice. Pancreas 2009;38: 309-317.
15 Assimakopoulos SF, Scopa CD, Vagianos CE. Pathophysiology of increased intestinal permeability in obstructive jaundice. World J Gastroenterol 2007;13:6458-6464.
16 Gupta R, Patel K, Calder PC, Yaqoob P, Primrose JN, Johnson CD. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II >or=6). Pancreatology 2003;3:406-413
17 Gurleyik E, Coskun O, Ustundag N, Ozturk E. Prostaglandin E1 maintains structural integrity of intestinal mucosa and prevents bacterial translocation during experimental obstructive jaundice. J Invest Surg 2006;19:283-289.
18 Tarnawski AS, Szabo I. Apoptosis-programmed cell death and its relevance to gastrointestinal epithelium: survival signal from the matrix. Gastroenterology 2001;120:294-299.
19 Szalay L, Umar F, Khadem A, Jafarmadar M, Fürst W, Ohlinger W, et al. Increased plasma D-lactate is associated with the severity of hemorrhagic/traumatic shock in rats. Shock 2003;20:245-250.
20 Sileri P, Morini S, Sica GS, Schena S, Rastellini C, Gaspari AL, et al. Bacterial translocation and intestinal morphological findings in jaundiced rats. Dig Dis Sci 2002;47:929-934.
21 Sun Z, Wang X, Wallen R, Deng X, Du X, Hallberg E, et al. The influence of apoptosis on intestinal barrier integrity in rats. Scand J Gastroenterol 1998;33:415-422.
22 Zhang L, Xing D, Chen M. Bim(L) displacing Bcl-x(L) promotes Bax translocation during TNFalpha-induced apoptosis. Apoptosis 2008;13:950-958.
23 Gomez G, Lee HM, He Q, Englander EW, Uchida T, Greeley GH Jr. Acute pancreatitis signals activation of apoptosisassociated and survival genes in mice. Exp Biol Med (Maywood) 2001;226:692-700.
24 Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev 1999;13:1899-1911.
25 Zhao MZ, Chen YZ, Li YY. Myocardial reperfusion-induced myocardial cell apoptosis and Fas gene expression and the effect of ischemic pretreatment on them. Chinese Journal of Internal Medicine 1999,38:753-756.
26 Zou XJ, Meng XF, Yao SL. Protective effects of tanshinone IIA on ethanol-induced neurotoxicity in PC12 Cells. Chinese Pharmaceutical Journal 2006;41:1553-1556.
27 Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg 1998;175:76-83.
28 Spooner CE, Markowitz NP, Saravolatz LD. The role of tumor necrosis factor in sepsis. Clin Immunol Immunopathol 1992; 62:S11-17.
29 Ogawa M. Acute pancreatitis and cytokines: "second attack" by septic complication leads to organ failure. Pancreas 1998; 16:312-315.
30 Vona-Davis L, Yu A, Magabo K, Evans T, Jackson B, Riggs D, et al. Peptide YY attenuates transcription factor activity in tumor necrosis factor-alpha-induced pancreatitis. J Am Coll Surg 2004;199:87-95.
31 Hietaranta AJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Relationship between NF-kappaB and trypsinogen activation in rat pancreas after supramaximal caerulein stimulation. Biochem Biophys Res Commun 2001;280:388-395.
32 Yeh KY, Yeh M, Glass J, Granger DN. Rapid activation of NF-kappaB and AP-1 and target gene expression in postischemic rat intestine. Gastroenterology 2000;118:525-534.
33 Hassoun HT, Kozar RA, Kone BC, SafiHJ, Moore FA. Intraischemic hypothermia differentially modulates oxidativestress proteins during mesenteric ischemia/reperfusion. Surgery 2002;132:369-376.
34 Russell J, Epstein CJ, Grisham MB, Alexander JS, Yeh KY, Granger DN. Regulation of E-selectin expression in postischemic intestinal microvasculature. Am J Physiol Gastrointest Liver Physiol 2000;278:G878-885.
35 Giakoustidis AE, Giakoustidis DE, Koliakou K, Kaldrymidou E, Iliadis S, Antoniadis N, et al. Inhibition of intestinal ischemia/repurfusion induced apoptosis and necrosis via down-regulation of the NF-kB, c-Jun and caspace-3 expression by epigallocatechin-3-gallate administration. Free Radic Res 2008;42:180-188.
36 Kajino S, Suganuma M, Teranishi F, Takahashi N, Tetsuka T, Ohara H, et al. Evidence that de novo protein synthesis is dispensable for anti-apoptotic effects of NF-kappaB. Oncogene 2000;19:2233-2239.
37 Li X, Yuan Z, Peng Y. Recombinant heat shock protein 70 adenovirus transfection protects intestinal epithelial cells (IEC-6 cells) against hypoxia-reoxygenation in vitro. Zhonghua Yi Xue Za Zhi 2002;82:1102-1104.
Accepted after revision May 30, 2010
The sounds of wind, of rain, and of reading aloud all fall upon my ears; the affairs of the state, of the family, and of the world are all my concerns.
— Gu Xiancheng
January 25, 2010
Author Affiliations: Department of General Surgery (Zhang XP), Department of Gynecology and Obstetrics (Cheng QH), Medical Record Library (Chen B), Hangzhou First People's Hospital, Hangzhou 310006, China; Zhejiang University of Traditional Chinese Medicine, Hangzhou 310053, China (Jiang J and Yu YP)
Xi-Ping Zhang, MD, Department of General Surgery, Hangzhou First People's Hospital, Hangzhou 310006, China (Tel: 86-571-87065701; Fax: 86-571-87914773; Email: zxp99688@vip.163.com)
? 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
 Hepatobiliary & Pancreatic Diseases International2010年5期
Hepatobiliary & Pancreatic Diseases International2010年5期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- News
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Life made easy: simplifying reconstruction for dual portal veins in adult right lobe live donor liver transplantation
- Roles of Smad3 and Smad7 in rat pancreatic stellate cells activated by transforming growth factor-beta 1
- Salmonella typhi and gallbladder cancer: report from an endemic region
- Profile of hepatocyte apoptosis and bile lakes before and after bile duct decompression in severe obstructive jaundice patients
