Reaction Characteristics of Asymmetric Synthesis of (2S,5S)-2,5-Hexanediol Catalyzed with Baker’s Yeast Number 6*
XIAO Meitian (肖美添), YE Jing (葉靜), ZHANG Yawu (張亞武) and HUANG Yayan (黃雅燕)
?
Reaction Characteristics of Asymmetric Synthesis of (2,5)-2,5-Hexanediol Catalyzed with Baker’s Yeast Number 6*
XIAO Meitian (肖美添)1,2,**, YE Jing (葉靜)2, ZHANG Yawu (張亞武)2and HUANG Yayan (黃雅燕)2
1Institute of Pharmaceutical Engineering, Huaqiao University, Xiamen 361021, China2Department of Chemical and Pharmaceutical Engineering, Huaqiao University, Xiamen 361021, China
Baker’s yeast number 6 was selected by screening. It showed good catalytic activity and enantioselectivity for asymmetric reduction of 2,5-hexanedione to produce (2,5)-2,5-hexanediol. Gas chromatography-mass spectrometry (GC-MS) revealed that the intermediate was ()-5-hydroxyhexane-2-one. Reduction of 2,5-hexanedione proceeded in a two-step reaction. The hydroxyketone was initially formed, and this intermediate was further reduced to the diol. Factors influencing the product yield and the enantiomeric excess of the reduction of 2,5-hexandione catalyzed by baker’s yeast number 6 were investigated. Higher concentration (≤100 mmol·L-1) of 2,5-hexandione did not influence 5-hydroxyhexane-2-one production, but 2,5-hexanediol production was inhibited by excess accumulation (>30 mmol·L-1) of intermediate. The optimal conditions were glucose as the co-substrate at an initial glucose concentration of 20 g·L-1, 34°C, pH 7.0 and cell concentration 60 g·L-1(cell dry mass). Under the optimal condition and an initial substrate concentration of 30 mmol·L-1, the yield of 2,5-hexandiol was 78.7% and the enantiomeric excess of (2,5)-2,5-hexandiol was 94.4% for 24-h reduction.
baker’s yeast, asymmetric reduction, (2,5)-2,5-hexanediol, enantioselectivity, ()-5-hydroxyhexane-2-one
1 INTRODUCTION
Asymmetric reduction of ketones is one of the most important, fundamental and practical reactions for producing chiral alcohols. Chiral alcohols can be transformed into various functionalities without racemization for the synthesis of many industrially important chemicals (.., pharmaceuticals, agrochemicals, natural products) [1, 2]. Enzymatic or microbial transformation of synthetic compounds is a convenient method for preparing chiral compounds. Oxidoreductases are effective catalysts with high enantioselectivity for reduction of carbonyl groups [3]. Using microbial whole cells as biocatalysts is particularly advantageous for carrying out the desired reduction because they contain multiple dehydrogenases. Dehydrogenases can accept a broad spectrum of non-natural substrates, all the necessary cofactors and metabolic pathways for their regeneration. All the enzymes and cofactors are well protected within their natural cellular environment [4, 5].(“baker’s yeast” or “brewer’s yeast”) is the most widely used microorganism for asymmetric reduction of ketones because it is readily available, inexpensive, and has high capacity as a redox biocatalyst in various stereoselective reductions. Many attempts have been made to utilize the yeast or related strains as catalysts for asymmetric reduction of many types of carbonyl compounds (.., aldehydes, ketones, ketonic esters, keto acids and their derivatives) [6-12].
(2,5)-2,5-hexanediol is an important intermediate of the antihypertensive agents captopril and diltiazem [13]. It is a main chiral “building block” of chiral ligands such as DuPhos, which can synthesize many chiral compounds (.., chiral amino acids, ionic liquids, chiral alcohols) [14, 15]. Enantiopure alcohols can be produced by kinetic resolution or by enantioselective reduction of the corresponding prochiral oxo-function [16]. In the former, a maximal yield of 50% is possible if a catalyst with 100% selectivity is applied. In the latter, 100% yield is possible with a selective catalyst. Currently, (2, 5)-hexanediol and (2, 5)-hexanediol are synthesized on an industrial scale by lipase-catalyzed transesterification starting from the racemic/meso mixture 2,5-hexanediol [17]. Using this method, the theoretical yields are 25% for (2, 5)-hexanediol and 75% for (2, 5)-hexanediol after subsequent chemical inversion of the (,)-monoester and downstream processing.
In this study, microorganisms are screened for biotransforming 2,5-hexanedione to (2, 5)-2,5-hexanediol. Their biocatalytic kinetics is studied. Strategies beneficial to the improvement of productivity and enantioselectivity, such as types and concentration of co-substrate, temperature, pH value, cell concentration and substrate concentration, are considered.
2 EXPERIMENTAL
2.1 Microorganisms and chemicals
Baker’s yeasts numbers 1-6 were purchased from Angel Yeast Company Limited (Hubei, China). Brewer’s yeast 1-4 is a preserved culture at our institute. 2,5-hexanedione, 2,5-hexanediol, (2, 5)-2,5-hexanediol (-HD), (2, 5)-2,5-hexanediol (-HD) and ()-(+)- phenethyl isocyanate [-(+)-PEIC] were purchased from Sigma (St. Louis, MO, USA), and ()-5- hydroxyhexane-2-one was from Fluka (Neu Ulm, Germany). Other chemicals used were of analytical grade.
2.2 Medium
Medium for cell growth (g·L-1): glucose 20, peptone 5.0, K2HPO41.0, MgSO4·7H2O 0.5, ZnSO40.01, KCl 0.5, Fe2(SO4)30.01, pH 6.5. Medium for reaction (g·L-1): glucose 20, (NH4)2SO45.0, K2HPO41.0, MgSO4·7H2O 0.5, ZnSO40.01, KCl 0.5, Fe2(SO4)30.01, pH 7.0.
2.3 Strain screening and cell culturing
Each stored strain was picked up in a loop to inoculate the 25-ml flask with 10-ml cell growth medium in 10 mmol·L-12,5-hexanedione. The flasks were shaken at 250 r·min-1and 32°C. Twenty-four hours after inoculation, concentrations of substrate and product were determined by gas chromatography (GC). The strain with high reduction activity and high optical selectivity was selected. Healthy monocolonies were isolated and tested for bioreduction capacity. Baker’s yeast number 6 with high yield and high enantiomeric excess of (2, 5)-2,5-hexanediol was obtained for subsequent experiments.
The stored strain baker’s yeast number 6 was inoculated in cell growth medium equipped with 500-ml triangular flasks. The flasks were shaken at 250 r·min-1and 30°C. After logarithmic growth of yeast cells, inocula were transferred into a 5-L fermenter (Braun Biotechnology International, Germany) with an inoculum size of 8.0% (by volume). Aerobic cultivation was carried out at a dissolved oxygen concentration of 2-3 mg·L-1. pH value was maintained at 6.5±0.05 by 2.0 mol·L-1NaOH. After growth for 36 h, the cells were harvested by centrifugation at 4°C and 4000 r·min-1for 10 min. The cell was washed twice with 50 mmol·L-1Tris-HCl (pH 7.0) and stored at 4°C until use.
2.4 Reduction of 2,5-hexanedione with baker’s yeast
The reduction of 2,5-haxenedione in the 100 ml reaction medium suspended 24.0 g wet cells (approximately 6.0 g cell dry mass) was conducted in a 250 ml shake flask. 2,5-haxenedione was added to the medium to the final fixed concentration. The flask was placed in a rotary incubator at 200 r·min-1and 34°C. At time intervals (every 4 h), 10 g·L-1glucose was fed in and 0.5 ml medium was sampled for GC determination.
2.5 Analytical methods
Quantification of 2,5-hexanedione, 5-hydroxyhexane-2-one and 2,5-hexanediol was carried out with gas chromatograph (Agilent 6890N, USA) equipped with an Innowax capillary column (30 m×0.25 mm×0.25 μm, Agilent, USA) with a flame ionization detector. To minimize the injection error, (1,3)-pentanediol was used as the internal standard. The GC condition was as follows: N2(99.99%) was the carrier gas, the flow rate was 25 ml·min-1, the injector temperature was 250°C, the detector temperature was 250°C, and the column temperature was 140°C.
The enantiomeric resolution was based on the derivation of 2,5-haxenedione with optically pure isocyanate. The sample (50 μl) was mixed with 50 μl toluene and 2 μl-(+)-PEIC. It was kept at 45°C for 2 h. A fused silica capillary column AE.SE-30 (30 m×0.25 mm×0.50 μm, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, China) was used. N2(99.99%) was the carrier gas (1.5 ml·min-1). The injector and the detector were kept at 250°C and 270°C, respectively. Retention times for (,)- and (,)-enantiomer were 18.8 min and 19.5 min, respectively. The enantiomeric excess (e.e.) of (2, 5)-2,5-hexanediol was calculated as follows:
where [,] and [,] were the concentrations of-HD and-HD, respectively.
A gas chromatograph-mass spectrometer (GC-6890/5973N, Agilent, USA) was used to identify the molecular structure of the intermediate in the fermentation. An Innovax capillary column (30 m×0.25 mm×0.25 μm, Agilent, USA) was used for GC separation using N2(99.999%) as the carrier gas at 1.0 ml·min-1in split-less mode. The column temperature program was as follows: initial temperature 60°C for 3 min, increased to 200°C at 10°C·min-1. Mass spectroscopy condition was as follows: ionic source temperature, 230°C; quadrupole temperature, 150°C; ionization mode, EI+; electronic energy, 70 eV; ejection current, 200 μA; and scanning quality scope, 10-200/. Data were collected and processed by Xcalibur software. Identification of volatiles was achieved by comparing the mass spectral data of samples with those of the NIST library and Wiley library.
3 RESULTS AND DISCUSSION
3.1 Screening of sp. strain with high bioreduction and catalytic activity of 2,5-hexanedione to (2S, 5S)-2,5-hexanediol
Ten strains belonging to(baker’s yeast or brewer’s yeast) were examined for their catalytic reduction activity against 2,5-hexanedione at an initial concentration of 10 mmol·L-1[18]. All 10 strains have some catalytic reduction activity, but their yields were different. The yields of baker’s yeast number 1, 3 and 6 were higher than that of the others. The enantiomeric excess of (2, 5)-2,5-haxenediol by baker’s yeast number 6 was the highest (95.8%). After repeating the reduction experiment several times, baker’s yeast number 6 showed still higher product yield and optical selectivity. In subsequent experiments, baker’s yeast number 6 was selected as the catalyst for the reduction of 2,5- hexanedione. During the reduction of 2,5-hexanedione, the reduction rate of the substrate was faster than the production rate of the product, and accumulation of undefined intermediate was continually presented.
3.2 Qualitative analysis of the intermediate and speculation of reaction mechanism
By the method described in Section 2.5, the fermentation culture for reduction 8 h was sampled to identify the molecular structure of the undefined intermediate by GC-MS. The intermediate was speculated to be 5-hydroxyhexane-2-one. The resting whole cells ofDSM 20587 were used as a biocatalyst to asymmetric deoxidize 2,5-hexanedione to-HD [19]. The reduction of 2,5-hexanedione proceeded in two-steps.cells first deoxidized 2,5-hexanedione to form the intermediate product ()-5-hydroxyhexane-2-one, which was deoxidized again to synthesize-HD. The experiment proved that the formation rate of the intermediate was significantly faster than the consumption rate. We hypothesized that the asymmetric reduction of 2,5-hexanedione by baker’s yeast followed a similar two-step process: substrate 2,5-hexanedione was first deoxidized to the intermediate ()-5-hydroxyhexane- 2-one by baker’s yeast, and the intermediate was hydrogenated again into the target product-HD. The reaction rate in the first step was faster than that in the second (Fig. 1).
3.3 Effect of co-substrate on asymmetric reduction of 2,5-hexanedione
In this work, six types of co-substrates were selected for their influence on the asymmetric reduction of 2,5-hexanedione by baker’s yeast number 6 (Fig. 2). The co-substrate (20 g·L-1) was added to the reaction medium, and the reduction carried out at 200 r·min-1and 32°C for 24 h. The yield and e.e. value were maximal when glucose was the co-substrate. Reduction activity of the strain was inhibited by using-BuOH as the co-substrate, and the yield clearly decreased. The enzyme that can reduce 2,5-hexanedione to 2,5-hexanediol is an oxidoreductase. During this process, the reduced form of nicotinamide-adenine dinucleotid (phosphate) [NAD(P)H] is required to participate in the reduction reaction. The content of NAD(P)H in yeast cells is very low, only 100 μg of NAD(P)H in 1.0 g of cells (dry mass) [20]. The co-substrate required by cofactor regeneration must therefore be added to these reactions, which can reduce NAD(P)+to NAD(P)H in the coupling enzyme system. Six types of substrates except-BuOH can be used as the sole energy source and co-substrate for the asymmetric reduction of 2,5-hexanedione by baker’s yeast number 6. An appropriate co-substrate can increase the transformation rate of the substrate, and influence the stereoselectivity of the reduction.
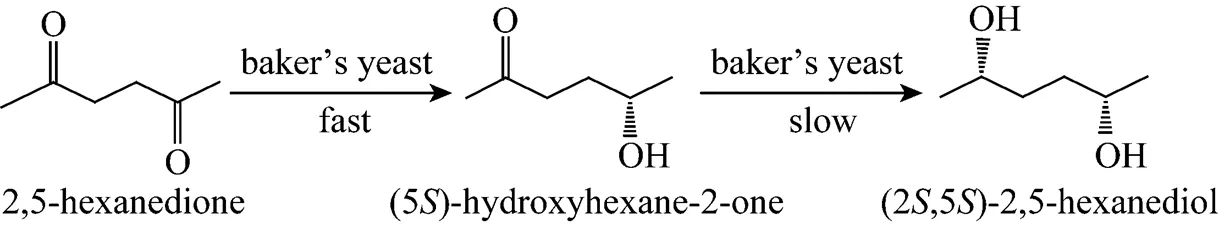
Figure 1 Asymmetric reduction of 2,5-hexanedione to (2, 5)-2,5-hexanediol with baker’s yeast number 6
Figure 2 Effect of co-substrates on asymmetric reduction of 2,5-hexanedione (Reduction conditions: initial substrate concentration 30 mmol·L-1, cell concentration about 60 g·L-1cell dry mass, pH 7.0, 34°C and 200 r·min-1for 24 h reduction)■?yield;□?e.e.
3.4 Effect of initial glucose concentration on asymmetric reduction of 2,5-hexanedione
During the microbial catalytic transformation process, living cells (growing or resting cells) should obtain energy to maintain activities and biocatalytic transformation by consuming fermentable carbohydrate. Glucose and ethanol could be used as electron donors for asymmetric bioreduction by yeast cells. In our experiments, glucose was supplied as the energy source. During the reduction, 10 g·L-1glucose was fed every 4 h. Increase in the initial glucose concentration clearly enhanced the reaction rate and the final yield when the initial glucose concentration was less than 20 g·L-1(Fig. 3). The e.e. of (2, 5)-2,5- hexanediol remained almost constant in the glucose concentration range from 10 g·L-1to 30 g·L-1. When glucose concentration was further increased, the yield remained almost unchanged, and the e.e. gradually decreased. When the initial glucose concentration was 20 g·L-1, the e.e. value of the target product was 95.1%, while the e.e. value was only 88.8% at 60 g·L-1.
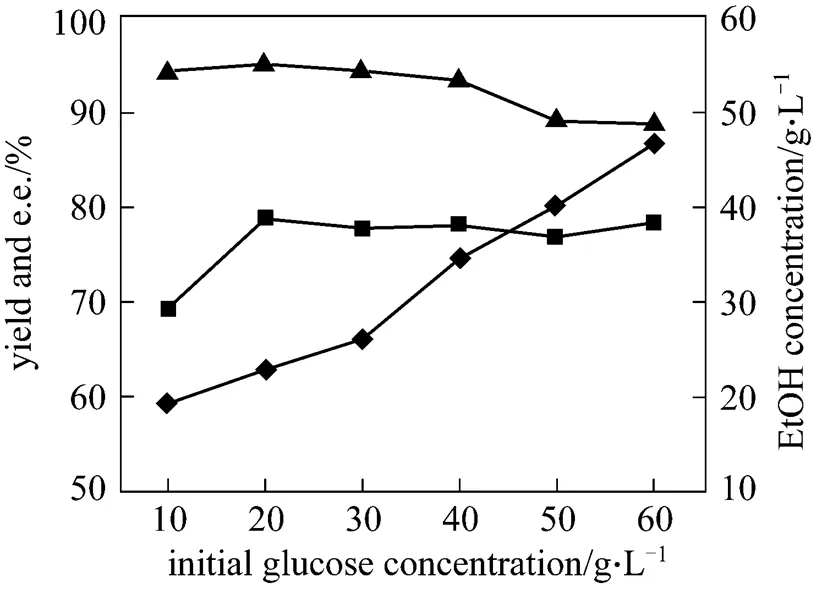
Figure 3 Effect of initial glucose concentration on asymmetric reduction of 2,5-hexanedione
(Reduction conditions: initial substrate concentration 30 mmol·L-1, cell concentration about 60 g·L-1cell dry mass, pH 7.0, 34°C and 200 r·min-1for 24 h reduction)■?yield;▲?e.e.;◆?EtOH concentration
Ethanol concentration in the reaction medium for reduction for 24 h was determined by GC. The ethanol accumulated in the reaction medium clearly increased with initial glucose concentration (Fig. 3). The accumulated ethanol concentration reached 46.5 g·L-1at an initial glucose concentration of 60 g·L-1. Glucose at high concentration is still metabolized to ethanol by certain glucose-sensitive strains such as baker’s yeast even under aerobic conditions, which is termed the “Crabtree effect” or “overflow metabolism phenomenon” [21]. In general, it is considered that this phenomenon is caused by inhibition of the aerobic respiration of the microorganism. Another theory is that it is caused by glucose inhibition in the respiratory chain enzymes.
There are many dehydrogenases (including ethanol dehydrogenase) in yeast cells. When there is no carbohydrates such as glucose in the medium and ethanol is used as a sole energy source, under ethanol dehydrogenase, ethanol is dehydrogenated to acetaldehyde, further oxidized to acetic acid then carbon dioxide, and NAD(P)+is reduced to NAD(P)H in coupling [22]. This provides NAD(P)H for the oxidoreductase and enhances the product yield. Alcohols can improve cell permeability, and are in favor of organic substrates permeating through the microorganism cells. Alcohols at higher concentration severely inhibited enzymatic activity, and had a negative influence on cell viability. Based on the consideration of the yield and e.e., the initial glucose concentration 20 g·L-1was chosen for subsequent experiments.
3.5 Effect of pH on asymmetric reduction of 2,5-hexanedione
The effect of pH in the reaction medium on asymmetric reduction of 2,5-hexanedione was studied in the pH range 4.5 to 9.0. The yield of 2,5-hexanediol was strongly dependent on pH (Fig. 4). Under the experimental conditions, the highest yield was achieved at a pH between 6.0 and 7.5. Enantioselectivity was also affected by pH. For the pH values<6.0, the e.e. value increased with pH value, and remained at 93.0%-95.0% in the pH range 6.0 to 9.0. pH value has a crucial effect on bioreduction. The variation of pH value alters the ionic state of substrate and enzymes involved in this reaction, particularly in a reaction catalyzed by isoenzymes with different enantioselectivity at different pH, leading to changes in yield and e.e. value [23-25]. Griffin. [26] reported that for the asymmetric reduction of acetophenone with baker’s yeast entrapped in calcium alginate, there were two higher catalytic activity areas in the pH range 3.0 to 10.0, pH 3.0 and 10.0. Lou. [27] observed a similar effect of pH on immobilized baker’s yeast in the biosynthesis of optically active organosilyl alcohol via asymmetric reduction of acyl silane.

Figure 4 Effect of pH on yield and e.e. of product (Reduction conditions: initial substrate concentration 30 mmol·L-1, cell concentration about 60 g·L-1cell dry mass, 34°C and 200 r·min-1for 24 h reduction)■?yield;▲?e.e.
3.6 Effect of temperature on asymmetric reduction of 2,5-hexanedione
Seven experiments at different temperatures were carried out to test the effect of temperature on 2,5-hexanediol production (Fig. 5). When the temperature rose from 28°C to 40°C, the yield of 2,5-hexanediol increased rapidly, and the e.e. of (2, 5)-2,5-hexanediol decreased slightly. The highest yield (78.7%) was achieved at 40°C, and the highest e.e. value (95.7%) was at 28°C. Reaction temperature was one of the most important factors affecting reactions catalyzed by enzymes. Temperature increase could enhance reaction rate, but higher temperature may result in the loss of active yeast cells and decrease in reaction rate. Changes in the three-dimensional configuration in the active site of the enzyme may also affect the enantioselectivity of biocatalytic reduction [28, 29]. Yang. [30] investigated the effect of temperature on asymmetric reduction of ethyl 4-chloro-3-oxobutanoate (COBE) catalyzed by yeast cells in the aqueous phase. There was no significant influence of temperature on substrate transformation rate. At higher temperature, the yield decreased, in which the catalytic activity of ()-product reductase was enhanced and the rate of by-reaction was faster. Based on the consideration of yield and e.e.(), 34°C was chosen for subsequent experiments.

Figure 5 Effect of temperature on yield and e.e. value of product (Reduction conditions: initial substrate concentration 30 mmol·L-1, cell concentration about 60 g·L-1cell dry mass, pH 7.0 and 200 r·min-1for 24 h reduction)■?yield;▲?e.e.
3.7 Effect of cell concentration on asymmetric reduction of 2,5-hexanedione
The effect of cell concentration on the asymmetric reduction of 2,5-hexanedione was examined (Fig. 6). For cell concentrations <60 g·L-1(cell dry mass), the yield increased with cell concentration, and the e.e. remained constant. At cell concentration of 60 g·L-1(cell dry mass), the yield and e.e. were 77.9% and 95.6%, respectively. When cell concentration increased further, the yield decreased but e.e. was unaffected (93.0%-95.0%). Increase in cell concentration can enhance enzyme concentration in the reaction system and quicken the reaction rate. However, high cell concentration may make nutrients in the reaction system be consumed quickly.

Figure 6 Effect of cell concentration on yield and e.e. of product (Reduction conditions: initial substrate concentration 30 mmol·L-1, pH 7.0, 34°C and 200 r·min-1for 24 h reduction)■?yield;▲?e.e.
3.8 Effect of initial substrate concentration on asymmetric reduction of 2,5-hexanedione
At the same cell concentration, the transformation rate of the substrate declined gradually with increasing substrate concentration at 4 h, and the average accumulated rate of 2,5-hexanedione increased linearly (Table 1). This indicated that intermediate production was not inhibited by the higher substrate concentration, whereas 2,5-hexanediol production was inhibited by excess accumulated 2,5-hexanedione. For the substrate concentrations <50 mmol·L-1(.., accumulated intermediate concentration was less than 30 mmol·L-1), the average production rate of 2,5-hexanediol increased with substrate concentration at 24 h. When the accumulated concentration of the immediate was higher than 30 mmol·L-1, the production rate decreased with increasing substrate concentration. Selecting and maintaining an appropriate concentration of substrate (or immediate) increased the production rate.

Table 1 also showed that the e.e. value maintained at a relatively stable and high level at a wide range of substrate concentration. When the substrate concentration was less than 100 mmol·L-1, the value of e.e. was about 93.0%-95.0%. A change in substrate concentration results in a change of apparent activity of each enzyme. At the same concentration of biomass, the yield and optical purity of the desired product are affected notably by substrate concentration. In general, decreasing the substrate concentration can enhance the yield and enantioselectivity of the product. For the microbial reduction of ethyl 2-oxo-4-phenylbutanoate (EOPB) to ethyl (R)-2-hydroxy-4-phenylbutanoate (R-EHPB), when the initial concentration of substrate (EOPB) increased from 1.4% to 1.8% (by volume), the yield and e.e. of (R)-EHPB decreased from 50.8% to 30.0%, and 71% to 55%, respectively [31]. Houng and Liau [32] demonstrated that ethyl-4-chloroacetoacetate (ECA) absorbed by XAD2 resin was released slowly into the solution so as to lessen the substrate inhibition. The yield and optical purity of product increased from 75% to 84%, and from 88% to 93%, respectively.
Note: reduction conditions (cell concentration about 60 g·L-1cell dry mass, pH 7.0, 34°C and 200 r·min-1for 24 h reduction).
3.9 Time curves of reduction reaction
Under the optimal conditions, pH 7.0, reaction temperature 34°C, cell concentration about 60 g·L-1(cell dry mass), initial glucose concentration 20 g·L-1and little aerobic cultivation, a typical time course of the reduction of 2,5-hexanedione at the substrate concentration of 30 mmol·L-1is shown in Fig. 7. At the initial reduction phase, the substrate was rapidly converted and the intermediate concentration reached a maximum at reduction time of 4 h, and the substrate conversion rate arrived at 90% for a reduction time of 7 h. The product was generated relatively slowly. At reduction time 24 h, the product yield was 78.7%, and the e.e. was 94.4%. In the reaction for 24 to 37 h, the product concentration remained unchanged and the product was not converted or degraded further into another product. The e.e. value of (2, 5)-2,5- hexanediol was maintained at a high level between 93.0% and 95.0% during the entire reaction process. This showed that the target product (2, 5)-2,5- hexanediol possessed high reaction stability. This result may provide a basis for larger scale experiments, and scaled-up production of (2, 5)-2,5-hexanediol.
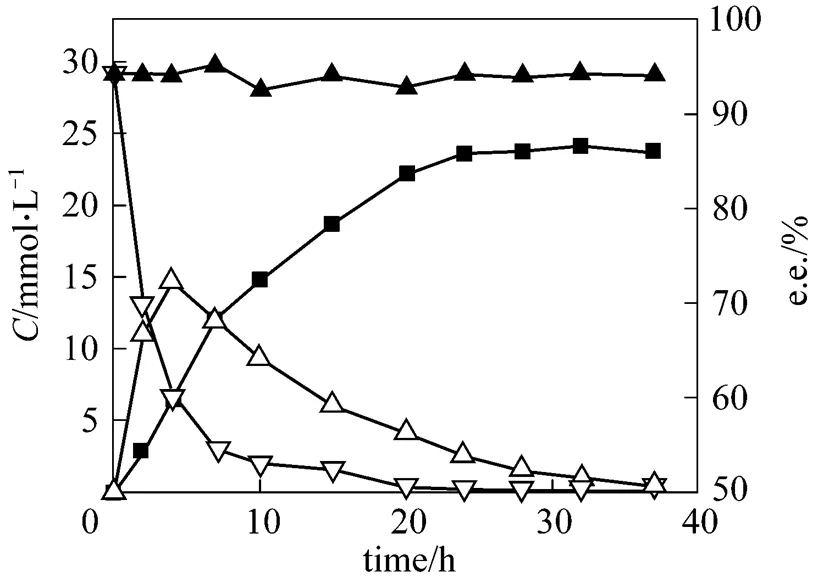
Figure 7 The time curves of conversion reaction
(Reduction conditions: initial substrate concentration 30 mmol·L-1, cell concentration about 60 g·L-1cell dry mass, pH 7.0, 34°C and 200 r·min-1for 24 h reduction)▽?2,5-hexanedione;△?5-hydroxyhexane-2-one;■?2,5-hexanediol;▲?e.e.
4 CONCLUSIONS
Baker’s yeast number 6 was selected by screening in this study. It possessed good catalytic reduction activity and enantioselectivity for the reduction of 2,5-hexanedione to produce (2, 5)-2,5-hexanediol. GC-MS revealed that the intermediate was ()-5- hydroxyhexane-2-one. Reduction of 2,5-hexanedione proceeded in two steps. First, the hydroxyketone was formed and, depending on reaction conditions, accumulated. Second, this intermediate was further reduced to the diol. Because the first step was faster than the second one, there was always an accumulation of intermediate. The conversion of substrate and the yield of product were influenced notably by environmental factors. The substrate conversion was not influenced by higher initial substrate concentration up to 100 mmol·L-1. Generation of the product was inhibited by excess accumulation (>30 mmol·L-1) of intermediate. The reduction activity of yeast cells was strongly inhibited by the high concentration of intermediate. Under the optimal conditions, pH 7.0, 34°C, cell concentration about 60 g·L-1(cell dry mass), initial glucose concentration 20 g·L-1and initial substrate concentration 30 mmol·L-1, the product yield and e.e. was 78.7% and 94.4% for biocatalytic reduction 24 h, respectively.
1 Nakamura, K., Yamanaka, R., Matsuda, T., Harada, T., “Recent developments in asymmetric reduction of ketones with biocatalysts”,:, 14, 2659-2681 (2003) .
2 Chartrain, M., Greasham, R., Moore, J., Reider, P., Robinson, D., Buckland, B., “Asymmetric bioreductions: application to the synthesis of pharmaceuticals”,...:., 11, 503-512 (2001) .
3 Homann, M.J., Vail, R.B., Previte E., Tamarez, M., Morgan, B., Dodds, D.R., Zaks, A., “Rapid identification of enantioselective ketone reductions using targeted microbial libraries”,, 60, 789-797 (2004).
4 Mandal, D., Ahmad, A., Khan, M.I., Kumar, R., “Enantioselective bioreduction of acetophenone and its analogous by the fungus Trichothecium sp.”,...:., 27, 61-63 (2004).
5 Yang, W., Xu, J.H., Xie, Y., Xu, Y., Zhao, G., Lin, G.Q., “Asymmetric reduction of ketones by employing Rhodotorula sp. AS2.2241 and synthesis of the β-blocker ()-nifenalol”,:, 17, 1769-1774 (2006).
6 Athanasiou, N., Smallridge, A.J., Trewhella, M.A., “Baker’s yeast mediated reduction of β-keto esters and β-keto amides in an organic solvent system”,...:., 11, 893-896 (2001).
7 Xiao, M.T., Huang, Y.Y., Shi, X.A., Guo, Y.H., “Bioreduction of phenylglyoxylic acid to-(-)-mandelic acid by Saccharomyces cerevisiae FD11b”,..., 37, 589-596 (2005).
8 Fardelone, L.C., Augusto, J., Rodrigues, R., Moran, P.J.S., “Baker’s yeast mediated asymmetric reduction of cinnamaldehyde derivatives”,...:., 29, 41-45 (2004).
9 Nakamura, K., Konodo, S., Nakajima, N., Ohno, A., “Mechanistic study for stereochemical control of microbial reduction ofa-keto esters in an organic solvent”,, 51, 687-694 (1995).
10 Margaret, M.K., Marko, D.M., Jeff, K., Anton, F., Stewart, J.D., “Baker’s yeast-mediated reductions ofa-keto-β-lactam. Two routes to the paclitaxel side chain”,..., 64, 6603-6608 (1999).
11 Shimizu, S., Kataoka, M., Kita, K., “Chiral alcohol synthesis with yeast carbonyl reductases”,...:., 5, 321-325 (1998).
12 Hasegawa, Y., Adachi, S., Matsuno, R., “Microbial production of 2-chloro-a-methylbenzyl alcohol and its adsorptive recovery”,..., 6, 59-64 (2000).
13 Kim, B.H., Lee, H.B., Hwang, J.K., Kim, Y.G., “Asymmetric induction in the conjugate addition of thioacetic acid to methacrylamides with chiral auxiliaries”,:, 16, 1215-1220 (2005).
14 Cobley, C.J., Lennon I.C., Mccague, R., Ramsden, J.A., Gerosa, A.Z., “On the economic application of DuPHOS rhodium(I) catalysts: A comparison of COD versus NBD precatalysts”,., 42, 7481-7483 (2001).
15 Burk, M., “Modular phospholane ligands in asymmetric catalysis”,..., 3, 363-372 (2003).
16 Schulze, B., Wubbolts, M.G., “Biocatalysis for industrial production of ?ne chemicals”,..., 10, 609-615 (1999).
17 Taylor, S.J.C., Holt, K.E., Brown, R.C., Keene, P.A., Taylor, I.N., “Choice of biocatalyst in the development of industrial biotransformations”, Stereoselective Biocatalysis, Dekker, New York, 397-404 (2000).
18 Lian, S.H., Xiao, M.T., Zhang, Y.W., Ye, J., Huang, Y.Y., “Asymmetric reduction of 2,5-hexanedione catalyzed by baker’s yeast”,..., 20, 45-48 (2008). (in Chinese)
19 Haberland, J., Kriegesmann, A., Wolfram, E., Hummel, W., Liese, A., “Diastereoselective synthesis of optically active (2, 5)-hexanediol”,..., 58, 595-599 (2002).
20 Windholz, M., Buhavari, S., Blumetti, R.F., The Merck Index, 10th edition, Merck &Co. Inc., Rahway, 909 (1983).
21 Barbara, M.B., Karin, M.O., Antonius, J.A., “Stoichiometry and compartmentation of NADPH metabolism in Saccharomyces cerevisiae”,.., 52, 15-37 (2001).
22 Tadashi, K., Yasufumi, M., Hideyuki, F., Hidefumi, Y., Ryuichi, M., “NAD(P)H regeneration using ethanol as an energy source in baker’s yeast-mediated bioreduction”,..., 77, 13-16 (1994).
23 Katz, M., Sarvary, I., Frejd, T., Hahn-Hagerdal, B., Gorwa-Grauslund, M.F., “An improve stereoselective reduction of a bicyclic diketone by Saccharomyces cerevisiae combining process optimization and strain engineering”,..., 59, 641-648 (2002).
24 Patel, R.N., Robison, R.S., Szarka, L.J., Kloss, J., Thottathil, J.K., Mueller, R.H., “Stereospeci?c microbial reduction of 4,5-dihydro-4- (4-methoxyphenyl)-6-(tri?uoromethyl -1H-1)-benzazepin-2-one”,..., 13, 906-912 (1991).
25 Li, Y.N., Shi X.A., Zong, M.H., Meng, C., Dong,Y.Q., Guo, Y.H., “Asymmetric reduction of 2-octanone in water/organic solvent biphasic system with Baker’s yeast FD-12”,..., 40, 1305-1311 (2007).
26 Griffin, D.R., Yang, F., Carta, G., “Asymmetric reduction of acetophenone with calcium-alginate entrapped baker’s yeast in organic solvents”,., 14, 588-593 (1998).
27 Lou, W.Y., Zong, M.H., Zhang, Y.Y., Wu, H., “Ef?cient synthesis of optically active organosilyl alcoholasymmetric reduction of acyl silane with immobilized yeast”,..., 35, 190-196 (2004).
28 Xiao, M.T., Huang, Y.Y., Ye, J., Guo, Y.H., “Study on the kinetic characterisics of the asymmetric production of-(-)-mandelic acid with immobilized.FD11b”,..., 39, 311-318 (2008).
29 Xiao, M.T., Huang, Y.Y., Meng, C., Guo, Y.H., “Kinetics of asymmetric reduction of phenylglyoxylic acid to-(-)-mandelic acid byFDllb”,...., 14, 73-80 (2006).
30 Yang, Z.H., Yao, S.J., “Asymmetric reduction of ethyl 4-chloro-3- oxobutanoate to ethyl 4-chloro-3-hydroxybutanoate by yeast cells in aqueous phase”,..., 25, 434-438 (2005). (in Chinese)
31 Oda, S., Inada, Y., Kobayashi, A., Ohta, H., “Production of ethyl ()-2-hydroxy-4-phenylbu-tanoatereduction of ethyl-2-oxo-4- phenyl-butanoate in an interface bioreactor”,..., 62, 1762-1767 (1998).
32 Houng, J.Y., Liau, J.S., “Applying slow-release biocatalysis to the asymmetric reduction of ethyl-4-chloroacetoacet”,.., 25, 17-25 (2003).
2008-09-23,
2009-03-17.
the Key Project of Science and Technology of Fujian Province (2008N0120), and the Key Discipline of Biochemical Engineering of Fujian Province (Huaqiao University).
** To whom correspondence should be addressed. E-mail: mtxiao@hqu.edu.cn
 Chinese Journal of Chemical Engineering2009年3期
Chinese Journal of Chemical Engineering2009年3期
- Chinese Journal of Chemical Engineering的其它文章
- Position Group Contribution Method for Estimation of Melting Point of Organic Compounds
- Process Intensification of VOC Removal from High Viscous Media by Rotating Packed Bed*
- Adsorption of Dye from Wastewater by Zeolites Synthesized from Fly Ash: Kinetic and Equilibrium Studies*
- Modeling of Isomerization of C8 Aromatics by Online Least Squares Support Vector Machine*
- Resolution of Ibuprofen Ester by Catalytic Antibodies in Water-miscible Organic-solvents*
- Gross Error Detection and Identification Based on Parameter Estimation for Dynamic Systems*
